Abstract
For multiple-brooded species, the number of reproductive events per year is a major determinant of an individual's fitness. Where multiple brooding is facultative, its occurrence is likely to change with environmental conditions, and, as a consequence, the current rates of environmental change could have substantial impacts on breeding patterns. Here we examine temporal population-level trends in the proportion of female great tits (Parus major) producing two clutches per year (‘double brooding’) in four long-term study populations in The Netherlands, and show that the proportion of females that double brood has declined in all populations, with the strongest decline taking place in the last 30 years of the study. For one of the populations, for which we have data on caterpillar abundance, we show that the probability that a female produces a second clutch was related to the timing of her first clutch relative to the peak in caterpillar abundance, and that the probability of double brooding declined over the study period. We further show that the number of recruits from the second clutch decreased significantly over the period 1973–2004 in all populations. Our results indicate that adjustment to changing climatic conditions may involve shifts in life-history traits other than simply the timing of breeding.
Keywords: climate change, double brooding, great tit, laying date, multiple breeding, selection
1. Introduction
Many species differ not only in the number of offspring they produce, but also in the number of breeding attempts per season, such that multiple breeding (more than one reproductive event in a season) is a common reproductive strategy in a variety of taxa (Verhulst et al. 1997 and references therein). Whenever reproductive costs exist, life-history theory predicts that parents face a compromise between current reproduction and future reproduction in order to maximize their own fitness (Williams 1966; Stearns 1992). Long-lived species are expected to favour their own survival at the expense of their current brood of offspring, whereas short-lived species should invest more in the current breeding attempt (Drent & Daan 1980). This trade-off is often invoked not only to explain the costs of reproduction across seasons, but also holds for within-season reproductive decisions, such as how many breeding attempts to make within a year. Furthermore, it is likely that prevailing environmental conditions and hence resource availability shape these costs and hence the likelihood of double brooding. In this paper, we use long-term, individual-based data on four populations of great tits (Parus major) in The Netherlands, to test whether the current changes in environmental conditions are affecting breeding patterns in relation to double brooding.
Several studies of birds have investigated the intra-seasonal costs of multiple breeding, most of them experimentally (Lindèn 1988; Verboven & Verhulst 1996; Verhulst et al. 1997; Verhulst 1998; Brinkhof et al. 2002; Parejo & Danchin 2006), but some also using either longitudinal studies (Tinbergen et al. 1985) or a combination of the two (Verboven & Verhulst 1996; Verboven et al. 2001). Experimental studies of clutch size (e.g. Parejo & Danchin 2006) and brood size (e.g. Lindèn 1988) manipulations are normally used to investigate the determinants of multiple breeding, but experimental delay/advance of hatching date (e.g. Brinkhof et al. 2002) is also frequently used. These studies show that delaying hatching date, as well as increasing clutch size and/or brood size, commonly leads to a lower probability of initiating a second clutch. These findings suggest that differences between populations in the occurrence and extent of multiple breeding may be causally related to differences in mean laying date and/or the number of fledglings in the nest, which again might be linked to differences in habitat between populations.
In short-lived species, differences in annual fecundity play a major role in determining population growth (Sæther & Bakke 2000). Furthermore, many such species often have two or more breeding attempts per season and the variance in individual fecundity can often be better explained by the number of breeding attempts than the number of young produced from each breeding attempt (Klomp 1970; Nagy & Holmes 2005 and references therein; Weggler 2006). Understanding what factors determine the decision to initiate multiple-breeding attempts per season is therefore interesting not only from a life-history point of view, but also from a conservation perspective as it determines population growth rate and thus the future viability of a population.
In many taxa, the main variable determining reproductive success is the abundance of prey items (for a review see White 2008): for example, long-term individual-level studies of great tit populations have shown that the synchrony of breeding with the peak in caterpillar abundance is the primary determinant of reproductive success (Perrins 1970; Van Balen 1973; Verboven & Visser 1998; Visser et al. 2006). Owing to the impact increasing spring temperatures has on the timing of the peak in caterpillar abundance, and the close link between the temperature and the timing of reproduction in birds, there has recently been great interest in such systems as they provide an ideal way in which to test the impact of climate change on natural populations (e.g. Visser et al. 1998, 2003, 2006; Gienapp et al. 2005; Both et al. 2006; Charmantier et al. 2008). As a consequence of the warming spring temperatures, many bird species have advanced their laying date (Crick et al. 1997; McCleery & Perrins 1998; but see also Visser et al. 1998, 2003 and Barbraud & Weimerskirch 2006). For instance, great tits breeding in Wytham Woods near Oxford, UK, have advanced their laying date by approximately 14 days over the past 47 years (1961–2007) (Charmantier et al. 2008). Furthermore, the observed advance in laying dates in long-term studies often show a ‘broken-stick’ pattern where there is little or no change in the laying dates in the period from the 1950s to 1970s, but in the later period from the 1970s onwards there is a strong advancement. This pattern furthermore coincides with a similar pattern of increase in spring temperatures (e.g. McCleery & Perrins 1998), again emphasizing the importance spring temperature has on the timing of reproduction in birds.
Based on the negative relationship between an individual's laying date and the probability of producing a second clutch (e.g. Verboven & Verhulst 1996; Brinkhof et al. 2002), we might therefore expect that the proportion of females producing two clutches per season should increase as laying dates become increasingly earlier. This prediction is, however, in marked contrast to the observed patterns in populations of great tits in The Netherlands (Visser et al. 2003), which show a decline in the proportion of females producing a second clutch.
The aim of this paper is to understand the reasons behind this decline in double brooding in four geographically separated populations of great tits in The Netherlands, using the data from over a 50-year period (table 1). Establishing the causes of the decline is important for understanding the effects a changing environment can have on natural populations. Timing of breeding relative to the peak in caterpillar abundance is an important predictor of the likelihood of initiating a second clutch in this species, and here we examine whether changing climatic factors have caused a shift in the relationship between the likelihood of double brooding and the relative timing of breeding over the course of the study. Because spring temperature changes have been particularly pronounced in the last three decades, we analysed the time series 1955–2004 and 1973–2004 separately. Where we find a significant decline in the proportion of females double brooding, we also tested the hypothesis that the decline reflects changing selection patterns, i.e. the benefits of double brooding relative to the costs have decreased over the study period.
Table 1.
General information of the study populations including study period, sample size (number of breeding birds for each population), number of second clutches, the total number of recruits from first clutch, the total number of recruits from second clutch and years excluded (due to large-scale manipulations or low proportion of adults ringed).
| study site | full name | study period | number of breeding birds | number of second clutches | number of recruits from first clutch | number of recruits from second clutch | year(s) excluded | coordinates (long./lat.) |
|---|---|---|---|---|---|---|---|---|
| HV1 | Hoge Veluwe | 1955–1972 | 1520 | 431 | 1159 | 82 | 1955–1958 | 52°05′ N, 05°50′ E |
| HV2 | Hoge Veluwe | 1973–2004 | 3831 | 723 | 2636 | 234 | 1973 (reorganizing of study area, see §2) | 52°05′ N, 05°50′ E |
| LB | Liesbos | 1955–2004 | 1570 | 176 | 536 | 20 | 1955–1957, 1967–1975, 1982, 1991, 1996, 1998–1999 | 51°35′ N, 04°40′ E |
| OH | Oosterhout | 1955–2004 | 1205 | 96 | 799 | 16 | 1956–1966, 1971, 1988, 1990–1992, 1996–1997 | 51°55′ N, 05°50′ E |
| VL | Vlieland | 1955–2004 | 4380 | 1287 | 3540 | 488 | 1955, 1960–1965, 1967–1969, 1971–1975, 1990–2004 | 53°15′ N, 05°00′ E |
2. Material and methods
(a) Study area, field procedures and data
Data were collected at four different localities in The Netherlands, Vlieland (VL), Hoge Veluwe (HV), Oosterhout (OH) and Liesbos (LB) (table 1). Because a storm damaged the pine plantation in the Hoge Veluwe population in the winter of 1972–1973, and nest-boxes were subsequently relocated, we treated HV1 (1955–1972) and HV2 (1973–2004) as two (temporally, not spatially) separate populations. For more details about the study populations, see Van Balen (1973).
In all areas, nest-boxes were visited at least once every week during the breeding season (April–June). Population size in an area in a given year was defined as the number of first clutches. The laying date of the first egg of the clutch was calculated from the number of eggs found during the weekly checks, assuming that one egg was laid per day. The number of eggs and/or young in the nests was counted, and when the young were 7–10 days old the parents were caught on the nest using a spring trap. Parents already ringed were identified and unringed birds were given a metal ring with a unique number. Young were ringed at day 7–10 (HV1, HV2, OH, LB) or at day 10–15 (VL). Females that were unknown (i.e. females not captured) were excluded from the analysis, and we also excluded nestlings from such nests because of the missing maternity. In total, 224 recruits were produced from nests in which the female was unknown (for the entire study period and all populations). Owing to the small number (224 out of 9510, 2.3%), it is highly unlikely that excluding such nests will bias the results in any way. Laying dates are presented as the number of days after 31 March (day 1=1 April, day 31=1 May).
We used the mean of daily average temperatures from the period 1 March to 20 April from the De Bilt meteorological station of the Royal Netherlands Meteorological Institute (KNMI), for consistency with the other studies on the same study populations (e.g. Van Balen 1973; Gienapp et al. 2006).
In some population/year combinations, only a small proportion (less than 50%) of the adults were caught and ringed and this makes it difficult to estimate any reasonable survival and/or recruitment rates for these population/year combinations, hence they were excluded from the analysis. We further excluded years if large-scale experiments (more than 70% of the population manipulated) were carried out, which affected parental survival or recruitment probability. Excluded population/year combinations are given in table 1. In all years, we also excluded individual nests in which manipulations took place (except for the viability selection analysis, see below).
Several studies have emphasized the importance of the timing of breeding relative to food abundance for the probability of producing a second clutch (e.g. Verboven et al. 2001; Brinkhof et al. 2002; Nagy & Holmes 2005), hence where we had the necessary data we used the timing of an individual's first clutch relative to the peak in caterpillar abundance (or ‘mismatch’) to predict the individual probability of producing a second clutch (see below; see also Verboven et al. (2001) for a similar approach). Caterpillar peak dates have been collected during the period 1985–2004 for the HV population (Visser et al. 2006) and we used these data to estimate the temperature period (i.e. average temperatures during a given time interval) that gave the best prediction of caterpillar peak date (see also Visser et al. (1998)). The period with the highest r2 value was 8 March–17 May with a predicted caterpillar peak of 105.47−15.968×temperature (r2=0.78, Visser et al. 2006). We subsequently used this relationship to estimate the caterpillar peak in HV for years in which we had no information (see Visser et al. 2006 for more details). The difference in days between when the chicks from the first clutch are 12 days old, and demand most food, relative to the estimated peak date in caterpillar abundance, was used as an approximation to the mismatch experienced by the birds (see Verboven et al. 2001).
(b) Spatio-temporal variation in proportion of females producing second clutches
We tested for differences among populations and changes with time in the proportion of females producing two clutches by defining a second clutch as a clutch produced following a successful first clutch by the same female, i.e. only nests where at least one chick fledged from the first clutch were included in the analyses. Thus all replacement clutches (where the first clutch failed and the pair produced a new clutch) were excluded (see Verboven & Verhulst 1996; Verboven et al. 2001; Visser et al. 2003). First and second clutches were matched on the basis of female identity as in Verboven & Verhulst (1996), thus we can be absolutely certain that it was the same female who produced a second clutch.
We first analysed population-level trends in multiple breeding for the two periods 1955–2004 and 1973–2004. We defined the proportion of females producing a second clutch in a given population in a given year as the ratio of number of ringed females producing a second clutch divided by the total number of ringed females (note that females who had a replacement clutch are excluded). We included the proportion of females double brooding as a response variable in a generalized linear model, GLM (quasi-binomial family argument to correct for overdispersion, see tables 2a and 2b) with the following terms: population as a factor and year; temperature (1 March–20 April, see above); annual population density (mean centred); the population annual mean laying date of the first clutch and its quadratic (to test for a nonlinear relationship); the population annual mean clutch size of the first clutch and its quadratic; and the variance in the laying date of the first clutch as continuous covariates. We also included the two-way interaction between population and year in the model to test for spatio-temporal trends.
Table 2a.
Analysis of variance table (‘type 3’) for the minimal adequate model from a GLM for the population-level analysis of the proportion of females second brooding produced each year, all populations combined, for the period 1955–2004 and 1973–2004. (The analysis was corrected for overdispersion (φ=6.19, φ=5.85 for the period 1955–2004 and 1973–2004, respectively), and has a total of 129 (1955–2004) and 95 (1973–2004) population year combinations; see text for further details. Note that if the same model is fitted excluding temperature, significance of year increases (Χ2=23.355 and Χ2=48.425, for the 1955–2004 and 1973–2004 period, respectively).)
| deviance | p-value | β(±s.e.) | |||||
|---|---|---|---|---|---|---|---|
| parameter | 1955–2004 | 1973–2004 | d.f. | 1955–2004 | 1973–2004 | 1955–2004 | 1973–2004 |
| population | 135.501 | 86.909 | 4(3) | <0.001 | <0.001 | ||
| year | 23.200 | 37.891 | 1 | <0.001 | <0.001 | −0.066 (0.011) | |
| average laying date | 3.368 | 36.418 | 1 | 0.066 | <0.001 | 0.220 (0.123) | −0.132 (0.023) |
| average laying date2 | 8.564 | 1 | 0.003 | −0.007 (0.003) | |||
| average clutch size | 20.734 | 17.630 | 1 | <0.001 | <0.001 | 0.546 (0.122) | 0.529 (0.127) |
| population density | 18.862 | 8.155 | 1 | <0.001 | 0.004 | −0.014 (0.003) | −0.009 (0.003) |
| temperature | 18.300 | 18.581 | 1 | <0.001 | <0.001 | −0.35 (0.084) | −0.395 (0.095) |
| population×year | 16.152 | 4(3) | 0.003 | see table 2b | see table 2b | ||
Table 2b.
Population-specific GLMs for temporal change in proportion of females double brooding in each year corrected for population density (mean centred, estimates not given) for each of the two time periods. (Note that the coefficient estimates of year given here are on a logit scale and are the ones used to draw the lines in figure 1. Also note that some populations have some years within the given time periods excluded (see table 1 for full details).)
| deviance | β(±s.e.) | p-value | ||||
|---|---|---|---|---|---|---|
| population | 1955–2004 | 1973–2004 | 1955–2004 | 1973–2004 | 1955–2004 | 1973–2004 |
| Hoge Veluwe | 5.118 | 9.101 | −0.024 (0.011) | −0.061 (0.021) | 0.024 | 0.002 |
| Liesbos | 2.266 | 9.905 | −0.024 (0.016) | −0.102 (0.036) | 0.132 | 0.002 |
| Oosterhout | 1.352 | 4.277 | −0.022 (0.019) | −0.046 (0.024) | 0.245 | 0.039 |
| Vlieland | 0.092 | 0.787 | −0.012 (0.040) | −0.051 (0.058) | 0.762 | 0.375 |
(c) Individual-level analyses
As it is only for the HV2 population we have substantial data on caterpillar peak dates, we used this population to investigate in detail the variables determining the probability of an individual starting a second clutch, and whether these had changed over time. We defined whether an individual produced a second clutch or not as a binomial trait in a logistic regression mixed model (GLMM) with year and mismatch of the first clutch (see above) as continuous covariates and the two-way interaction in the model (thus testing for a temporal change in the relationship between the probability of double brooding and the amount of mismatch experienced). Female identity and year (as a factor) were included as random effects to account for repeated measures of females and repeated measures within years. It has been demonstrated previously that mismatch is a better predictor of the proportion of females starting a second clutch than absolute timing (Verboven et al. 2001), and thus we used only mismatch for this analysis.
(d) Selection analyses
As we found no indication that the rate of decline in the proportion of females double brooding differed between populations for the period 1973–2004, we quantified selection on double brooding by testing for associations with female fecundity and survival in all four populations jointly. An offspring was classified as a recruit to the breeding population if it was seen again in the population in subsequent years after its year of birth. For the fecundity selection analysis, we estimated the fitness of an individual female from her annual reproductive success, defined as the number of offspring recruiting to the breeding population from each breeding season. We then tested for differences between single versus double brooders in their annual reproductive success. For the viability selection analysis, we used the survival of a female to subsequent years to test whether double brooding affected adult female survival rates. The estimates of fecundity and viability selection were based on recapture data under the assumption that nestlings and adults not returning to the study area in subsequent years had died. The use of recruitment as a measure of fecundity selection represents a reasonable fitness measure relative to other broods in the same year as it is only those individuals who recruit that will contribute to any response to selection.
When analysing selection on adult female survival, we included females that had been manipulated (note however that females who were removed or had their partner removed were excluded from the dataset altogether (n=2, 2 and 7 individuals in the HV, LB and VL population, respectively; years as in table 1) as there is no indication that experimental manipulations, such as clutch size manipulations, influence adult survival in this species (Tinbergen & Both 1999). Consequently, sample sizes for the viability analysis (n=5468) are higher than that for the fecundity analysis (n=4475). Owing to repeated measurements of the same female over time, we included female identity and year as random effects (as factors) in a GLMM model with female survival as a response variable (0/1). Breeding category was included as a two-level factor (single versus double brooded) in the analysis in order to determine whether fitness varied between single- versus multiple-breeding individuals.
All selection models reported here are without laying date fitted as covariate, as we wanted to consider selection on double brooding without removing any of the associated variation in laying date. However, we also repeated the selection models including laying date to control for the laying date associated variation in reproductive success and survival. Although laying date had a significant effect on fecundity (b=−0.017, s.e.=0.003, Χ12=30.19, p<0.0001), as well as on viability (b=−0.009, s.e.=0.004, Χ12=4.18, p=0.041), its inclusion in the models did not qualitatively change the conclusions, and thus we do not report the results from the laying date corrected selection models here. We did, however, correct for population density (mean centred), defined as the population-specific number of breeding females, in all selection analysis reported here.
(e) Recruitment from first and second clutches
Differences in total annual reproductive success between single- and multiple-breeding females could be due to additional recruitment from the second clutch, or due to differences in recruitment from the first clutch, implying systematic differences in the type of birds that produce two clutches rather than a benefit of the second clutch per se. To test for this, and to explore possible explanations for the decline in second brooding over time, we first compared recruitment from the first clutch between females who proceeded to have a second clutch and those that did not, and second, among those that did produce a second clutch, we considered the number of recruits produced from the second clutch only.
For the analysis of recruitment from the first clutch, we fitted a GLMM with the number of recruits from the first clutch as a response variable and included breeding category (single versus double brooder) as a factor and year as a covariate as well as the interaction between breeding category and year. Female identity and year were included as random factors.
In order to determine the contribution of the second clutch to parental reproductive success in a given year, and whether this changed over the course of the study, we also analysed how the number of recruits from the second clutch had changed over time including year as continuous covariate. Again, female identity and year were included as random factors (see above).
(f) Statistical analysis
Statistical significance was estimated from the GLMMs with the appropriate error structure. Thus, we used binomial error structure for the analysis of both the probability of double brooding and the viability selection. Similarly, the Poisson error structure was applied to the fecundity selection analysis and for the analysis of the number of recruits from the first and second clutches. All models were fitted using Schall's technique (1991) and significance levels of variables were assessed from their Wald test statistics, distributed as Χ2 on the appropriate degrees of freedom (Sokal & Rohlf 1995; Gilmoure et al. 2006). We used ASReml v. 2.0 (Gilmoure et al. 2006) as a plug-in to R v. 2.7.0 (R Development Core Team 2007) for all analysis except the GLM models that were run directly in R. For the GLM models, significance of terms was assessed using the change in deviance between the reduced and complete models and tested against the F-distribution (because of overdispersion, see Crawley (2002)) with degrees of freedom equal to the difference in the number of parameters between the two models (Crawley 2002).
In general, we fitted a global model containing all explanatory variables of interest as well as their interactions. A final model was then determined by stepwise exclusion of the least significant terms, starting with the non-significant highest order interactions and then non-significant main effects. Significance of main effects was tested separately without any interaction effects fitted.
3. Results
(a) Spatio-temporal trends in proportion of females producing second broods
For the period 1955–2004, the proportion of females producing second clutches decreased in all the four study populations, but the rate varied significantly between populations, resulting in a significant interaction term between population and year (table 2a; figure 1). There was a quadratic relationship with the mean lay date of the population and a positive relationship with the mean clutch size in the first clutch (table 2a). Furthermore, the proportion of females producing second clutches declined with both increasing population density and increasing temperature. We found no indication that the proportion of females producing a second clutch was related to the variance in laying date (F1,113=2.29, p=0.13) nor of any quadratic relationship with the mean clutch size (F1,112=0.06, p=0.80).
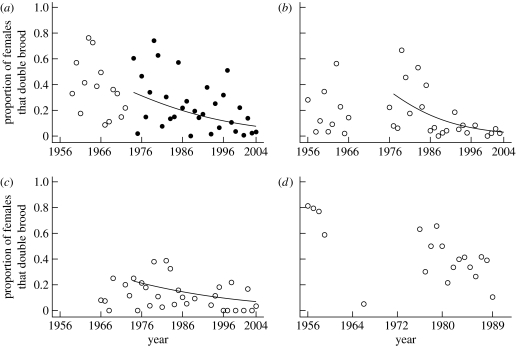
Figure 1.
Temporal trends in the proportion of females double brooding in the four study populations ((a) Hoge Veluwe 1 (open circles) and 2 (filled circles), (b) Liesbos, (c) Oosterhout, (d) Vlieland). The curves were fitted using the logistic equation from GLMs separately for each population (see table 2b for equations on a logit scale).
For the period 1973–2004, we did not find any significant interaction term between population and year (F3,84=1.514, p=0.22), suggesting that for this period all populations showed a significant negative decline (tables 2a and 2b). Again, we did not find any indication that the proportion of females producing a second clutch was related to the variance in laying date (F1,85=1.65, p=0.20) nor of any quadratic relationship with clutch size (F1,78=0.039, p=0.85). Furthermore, there was no quadratic relationship with mean laying date for this period (F1,86=3.32, p=0.07).
To investigate the significant interaction term between population and year, i.e. to study the spatio-temporal double brooding patterns in more detail, we used population-specific models (table 2b), correcting for population density, for each of the two periods. Although temporal trends were negative for all populations for both time periods, only the HV1/2 populations showed a significant temporal decline over the period 1955–2004 (table 2b). For the period 1973–2004, however, there was a significant decline in the proportion of females double brooding for the HV2, LB and OH populations (table 2b).
(b) Temporal change in the individual probability of producing a second clutch in the HV2 population
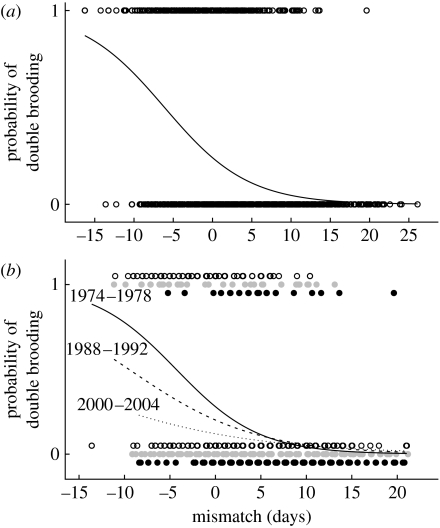
For the HV population, where we have data on caterpillar peak dates, the probability of an individual female starting a second clutch was negatively related to the difference in timing of her first clutch and the caterpillar food peak (table 3; figure 2a). The probability of producing a second clutch was also negatively related to year (table 3), indicating that the probability of double brooding had decreased over the course of the study, even after controlling for the effects of individual mismatch (figure 2b).
Table 3.
Individual probability of double brooding for the HV2 population in relation to the amount of mismatch experienced and temporal trend from a GLMM with binomial error structure (see §2). (Note that these are the Wald statistics from a full model, all main effects were significant when tested separately and main effect estimates are from these models (see main text, n=2153).)
| term | Χ2 | d.f. | p-value | estimate±s.e. |
|---|---|---|---|---|
| year | 6.92 | 1 | 0.009 | −0.073±0.029 |
| mismatch | 148.27 | 1 | <0.001 | −0.188±0.015 |
| year×mismatch | 0.01 | 1 | 0.92 | −0.001±0.002 |
Figure 2.
(a) The probability of producing a second clutch in relation to how mismatched an individual was to the food peak for the HV2 population (equation on a logit scale: −1.15−0.188×mismatch) fitted in a binomial GLMM. Negative values of mismatch indicate that the period in which the brood requires a large amount of food occurs before the seasonal peak in caterpillar abundance. Positive values indicate that the period was after the caterpillar peak. (b) The probability of producing a second clutch in relation to the amount of mismatch experienced for the first 5 (open circles and solid line, equation on logit scale: −0.944−0.219×mismatch), the mid 5 (grey circles and dashed line, equation on logit scale: −1.379−0.145×mismatch) and the last 5 (filled circles and dotted line, equation on logit scale: −1.992−0.993×mismatch) years of the study for the HV2 population. Each prediction line is restricted to the data range for the respective period. Equations are from a binomial GLMM for 5-year periods; note that predictions are back-transformed and if plotted on a logit scale the lines would be parallel.
There was, however, no indication that the slope between the probability of producing a second clutch and the degree of mismatch had changed significantly over time, as the interaction between year and mismatch was not significant (table 3). Thus, the intercepts had declined over time whereas the slopes had not (figure 2b; note that the lines are back-transformed and if plotted on a logit scale the lines would be parallel with a decline in intercepts over time). There was also no indication of any nonlinear relationship with mismatch (quadratic term for mismatch: Χ12=2.05, p=0.15).
(c) Viability selection analysis
Combining data from all four populations for 1973–2004, we did not find any significant difference in survival between females who had been single versus double brooded in a given year (Χ12=0.69, p=0.41). There was weak indication of a temporal decline in survival (Χ12=3.36, p=0.07), but no significant interaction between breeding category and year (Χ12=1.95, p=0.16), suggesting that survival between single- and multiple-breeding females had not changed differently over time. Nor did survival between single- and double-brooded females differ in different populations (population–breeding category interaction: Χ32=6.87, p=0.08). Furthermore, we did not find any differences in temporal patterns between the different populations (population–year interaction: Χ32=1.99, p=0.57), nor any indication that survival differed between single- and double-brooded individuals over time between the different populations (three-way interaction: Χ32=2.65, p=0.45). Population density had a negative effect on survival (b=−0.005, s.e.=0.002, Χ12=10.95, p<0.001).
(d) Fecundity selection analysis
Multiple-breeding females had, on average across the 1973–2004 period and the four study populations, significantly more recruits than females who produced only a single brood in a given year (1.32±0.05 s.e. versus 0.75±0.02 s.e., respectively; Χ12=50.771, p<0.001). There was a marginally significant negative temporal change in fecundity (b=−0.016, s.e.=0.008, Χ12=3.93, p=0.047), suggesting that the total number of recruits has decreased. We found no significant interaction between breeding category and year (Χ12=0.84, p=0.36), suggesting that differences in fecundity between single- and multiple-brooded females had not changed differently over time. Although there was no significant interaction, both single- and double-brooded females showed a negative trend in the number of recruits produced over time (b=−0.017, s.e.=0.008, Χ12=4.06, p=0.044 and b=−0.005, s.e.=0.01, Χ12=0.41, p=0.52 for single- and double-brooded females, respectively). We did not find any indication that the number of recruits from single- and double-brooded females had changed differently over time in the different populations (Χ32=4.98, p=0.17) or that the number of recruits produced by single- and double-brooded females differed significantly between populations (Χ32=2.25, p=0.52). Not surprisingly, we found large spatial variation in the number of recruits produced, as evident from a highly significant population×year interaction (Χ32=30.64, p<0.001). Population density had furthermore a strong negative effect on the number of recruits produced (b=−0.009, s.e.=0.001, Χ12=79.91, p<0.001).
(e) Recruitment from first clutch
As there was no indication of different temporal changes in total fecundity between double- and single-brooded individuals, we tested whether there had been any temporal change between single- and double-brooded individuals in the number of recruits produced from the first clutch during the 1973–2004 period. Because there was a significant interaction between population, year and breeding category (Χ32=13.93, p=0.003), suggesting that patterns differed between single- and double-brooded females over time in the different populations, we analysed each population separately for the ease of interpretation.
We did not find any indication that the number of recruits from the first clutch changed differently over time for any of the populations except in HV2 (Χ12=9.96, p=0.002), where single-brooded individuals showed a stronger decline (b=−0.021, s.e.=0.01) than did double-brooded individuals (b=0.003, s.e.=0.01). However, overall, there was no significant difference in the number of recruits from the first clutch produced by single- or double-brooded females for this population (Χ12=1.53, p=0.22, main effect tested separately). Also for the LB and OH populations (p=0.15 and p=0.79, respectively), there was no difference in the number of recruits produced from the first clutch between single- and double-brooded females, suggesting that in these populations the observed difference in the total number of recruits is due to a fitness benefit of having a second clutch. For the VL population, however, double-brooded females had significantly less recruits from the first clutch than did single-brooded females (Χ12=4.52, p=0.03). Apart from the different temporal pattern in the number of recruits from the first clutch between single- and double-brooded individuals found in the HV2 population, only LB showed a weak temporal trend (b=−0.027, s.e.=0.014, Χ12=4.36, p=0.04) in the number of recruits produced from the first clutch. For HV, LB and VL, there was a strong negative effect of increased population density on the number of recruits produced from the first clutch, but there was no such trend for OH (Χ12=0.07, p=0.79).
(f) Recruitment from second clutch
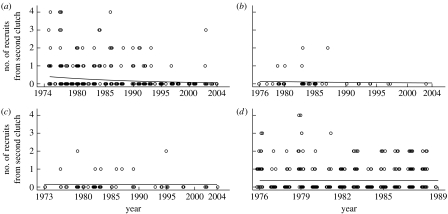
The decline in the proportion of females producing a second clutch suggests a decline in the fitness benefits of producing a second clutch, but these were not apparent in any of our analyses of female survival, fecundity or recruitment from the first clutch as described above. However, we did find a significant decline in the number of recruits produced from the second clutch during the 1973–2004 period (b=−0.053, s.e.=0.023, Χ12=5.88, p=0.01, figure 3). Not surprisingly, the number of second-clutch recruits produced from the different populations also varied considerably (Χ32=17.45, p<0.01). However, there was no interaction between population and year (Χ32=5.11, p=0.17), suggesting that the fitness benefits of producing a second clutch have declined at a similar rate in all four populations during this period. The number of recruits produced from the second clutch was furthermore negatively related to the population density (b=−0.007, s.e.=0.003, Χ12=4.78, p=0.03).
Figure 3.
Temporal trend in the number of recruits from the second clutch for each population, fitted from the equations from the population-specific GLMMs with Poisson error structure (see text for further details). The equations fitted were (all on a log scale): −0.858−0.062×year, −1.865−0.037×year, −1.891−0.004×year, −0.999−0.002×year for the (a) HV2, (b) LB, (c) OH and (d) VL populations, respectively. Note that some population–year combinations are missing (see table 1 for details).
4. Discussion
We have shown here that the proportion of females double brooding has declined over a 50-year period in all four main study populations of great tits in The Netherlands (figure 1; table 2b), although a significant decline was found only for the HV2 population for the whole period. However, for the period 1973–2004, the HV2, LB and OH populations showed a decline in the proportion of females producing a second clutch, indicating that the decline has been the strongest in the later part of the study. This decline coincides with the increasing spring temperatures during the same period, and with the analyses of changes in lay date in great tits, which have reported more rapid changes in the last three decades (e.g. McCleery & Perrins 1998).
For the HV2 population, the probability of producing a second clutch was closely related to the degree of mismatch (difference in days between when chicks from the first clutch are 12 days old and the peak in caterpillar abundance) experienced by the female (figure 2a). As predicted from the population-level analyses, this probability had changed over the study period, with individuals breeding in the later years of the study having a significantly lower probability of starting a second clutch than individuals in the earlier part of the study (figure 2b), but there was no evidence of a temporal change in how mismatch influenced the probability of double brooding (table 3). Furthermore, we found no indication of viability selection operating differentially on single- versus double-brooded individuals. Although double-brooded individuals did, on average across the whole study period in the four populations, produce more recruits than single-brooded individuals, the number of recruits from the second clutch alone declined over time for all four populations during the period 1973–2004 (figure 3); suggesting that the benefits of double brooding per se have changed over time.
Surprisingly, given the importance of multiple breeding on reproductive success, this is, to our knowledge, the only study to have considered temporal trends in the occurrence of multiple brooding and associated patterns of selection. Hence, we have very limited information about whether other populations of facultative multiple-brooded bird species are experiencing a similar decline in the number of clutches produced during the breeding season. We have been able to find only two studies mentioning temporal trends in proportion of females double brooding. Visser et al. (2003) compared large-scale responses with climate change on laying dates across a Europe-wide range of great tit and blue tit (Cyanistes caeruleus) populations and showed a decrease in the proportion of second clutches produced in both species; our results confirm and extend these analyses. Additionally, Møller (2007) studied the inter-clutch interval in a population of barn swallows (Hirundo rustica) in Denmark in relation to climate change, and briefly noted that the proportion of birds producing a second clutch had not changed over the study period (1975–2005).
In many bird species, it is commonly found that early breeding individuals have a higher probability of producing a second clutch than late breeding individuals (e.g. Brinkhof et al. 2002), and it is thought that this is because of a decline in food abundance throughout the season rather than a seasonal change in the quality of breeders, at least in the great tit (Verboven & Verhulst 1996). The importance of timing relative to the food peak for determining the probability of producing a second clutch has been demonstrated previously in great tits (Verboven et al. 2001) as well as other species (Simons & Martin 1990; Nagy & Holmes 2005), and our findings support this (figure 2a). For instance, Nagy & Holmes (2005) provided food supplements to females after the time of laying of the clutch in the Neotropical black-throated blue warbler (Dendroica caerulescens) and demonstrated that food-supplemented females produced significantly more second clutches compared with the control females who did not receive food supplementation. It is clear from this experiment, as well as similar studies (Simons & Martin 1990), that food limitation during the breeding season can be a constraint for the production of multiple clutches.
The observed relationship between the probability of double brooding and the mistiming also suggests that if the mistiming increases (i.e. a shift towards larger, more positive, mismatch values), birds will be less likely to initiate a second clutch. Based on previous work in the HV population, we know that there has been an increase in mistiming over the course of the study (Visser et al. 1998). While hatching date of caterpillars has advanced by 0.74 days per year in the period 1985–2004 (Visser et al. 2006), great tits in the same population have only advanced their laying date by 0.18 days per year over a 30-year period 1973–2003 (Gienapp et al. 2006). The close relationship between the probability of producing a second clutch and the amount of mismatch experienced (figure 2a), together with an increase in mismatch because of climate change, will lead to a decline in the probability of producing a second clutch and subsequently to a decline in the proportion of females double brooding (figure 1; table 2b).
In support of this, we found that there had been a temporal decline in the probability to produce a second clutch (figure 2b). We did not find that the relationship between the probability to produce a second clutch and mismatch had changed over the study period however. The temporal decline in the overall probability of double brooding suggests that individuals from the early part of the study experiencing a given mismatch were more likely to produce a second clutch than individuals experiencing the same amount of mismatch in the later part of the study (figure 2b).
This change suggests the possibility of changing selection patterns for birds producing a second clutch. We found, however, no indication of any temporal change in survival over the course of the study. Moreover, there was also no detectable survival difference between single- and double-brooded females. Differences in survival between single- and multiple-breeding individuals have been investigated in several different bird species before with little consensus (e.g. Bryant 1979; Verhulst 1998; Brinkhof et al. 2002; Nagy & Holmes 2005). Although we did not find any difference in survival, reproductive costs are often obscured in empirical studies by confounding effects such as territory quality (Reznick 1985).
Changes in selection pressure can also be brought about through changes in fecundity selection. In common with other studies (e.g.Weggler 2006), we found that individuals who produced two clutches per season did have higher reproductive success (measured as total number of recruits produced) than individuals who produced only a single clutch, although this is the mean across the years 1973–2004 for the four study populations. The higher reproductive success of multiple-brooded females was the same for all the four populations, suggesting that a second clutch has been an important component of reproductive output. We also found an indication that the number of recruits produced, by both single- and double-brooded females, had declined, although this was only significant for the single-brooded females. More importantly, however, we found a strong decline in the number of recruits produced from the second clutch (figure 3) for all four populations during the years 1973–2004. Although the reason for this decline is not clear, it is likely that it is related to an increase in mistiming (Visser et al. 1998). The increasing mistiming with the main food source has large implications for the reproductive success of the great tits as has been previously demonstrated in this species (Nussey et al. 2005; Visser et al. 2006), and is also suggested by the negative trend in the total number of recruits produced, as well as the decline in the number of recruits from the second clutch, found in this study. Unfortunately, data on caterpillar peak dates have been collected to a much lesser extent in the VL and OH populations than in HV, and is unavailable for the LB population, so it is difficult to say whether the increase in mistiming is also happening here, but given that the pattern of temperature increase is similar in all populations this is quite likely. Nevertheless, the observed decline in the number of recruits produced from the second clutch clearly illustrates the decreasing fitness benefit of double brooding (figure 3).
To summarize, we show here that the proportion of females producing a second clutch in four populations of great tits in The Netherlands declined over a 50-year period, and that this decline was particularly strong during the later part (1973–2004) of the study (table 2b). The reasons for this decline were twofold: first, it is likely that due to the strong negative relationship between the probability of producing a second clutch and the amount of mismatch experienced (figure 2a), observed in the HV population, and a simultaneous increase in mismatch over the study period for this population (Visser et al. 1998), birds are less likely to initiate a second clutch. The observed temporal decline in the probability of double brooding over the study period (figure 2b) supports this view. Second, there was a temporal decline in the number of recruits produced from the second clutch (figure 3), which can be one of the reasons behind the temporal decline in the probability of double brooding (figure 2b). Taken together, this suggests that changing environmental conditions are important in determining the number of clutches a female produces, and that drastic environmental changes have the potential to change an important life-history trait in this species. The observed decline in proportion of females producing a second clutch can have important consequences for population dynamics, as the number of clutches produced during the breeding season is a major component of reproductive success in this as well as in other multiple-brooded species. Other studies investigating temporal trends in multiple breeding and associated patterns of selection would be very valuable as it is becoming increasingly clear that adjustment to changing climatic conditions might involve more than simply a change of timing of breeding.
Acknowledgments
This research was carried out under licenses of the Animal Experimental Committee of the KNAW (DEC protocol no. CTE 02-06 and 07-04).
The long-term population studies have been conducted under the directorships of H. N. Kluyver (1955–1968), J. H. Van Balen (1968–1991) and A. J. Van Noordwijk (1991–2002). The database has been managed by J. Visser. We also acknowledge the large number of field assistants involved in collecting the data, in particular H. Van Eck, J. Visser, H. Bouwmeester, L. Holleman and H. Van Vugt. We would also like to thank Elina Immonen, Per Terje Smiseth and three anonymous reviewers for providing very useful comments on an earlier version of this manuscript. This work was conducted as part of a GENACT project studentship to A.H., funded by the Marie Curie Host fellowships for Early Stage training, as part of the Sixth Framework Programme of the European Commission; L.E.B.K. is funded by the Royal Society, London. M.E.V. is supported by a NWO-VICI grant.
References
- Barbraud C., Weimerskirch H. Antarctic birds breed later in response to climate change. Proc. Natl Acad. Sci. USA. 2006;103:6248–6251. doi: 10.1073/pnas.0510397103. doi:10.1073/pnas.0510397103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both C., Bouwhuis S., Lessells C.M., Visser M.E. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441:81–83. doi: 10.1038/nature04539. doi:10.1038/nature04539 [DOI] [PubMed] [Google Scholar]
- Brinkhof M.W.G., Cave A.J., Daan S., Perdeck A.C. Timing of current reproduction directly affects future reproductive output in European coots. Evolution. 2002;56:400–411. doi: 10.1111/j.0014-3820.2002.tb01349.x. doi:10.1554/0014-3820(2002)056[0400:TOCRDA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bryant D.M. Reproductive costs in the house martin (Delichon-Urbica) J. Anim. Ecol. 1979;48:655–675. doi:10.2307/4185 [Google Scholar]
- Charmantier A., McCleery R.H., Cole L.R., Perrins C., Kruuk L.E.B., Sheldon B.C. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science. 2008;320:800–803. doi: 10.1126/science.1157174. doi:10.1126/science.1157174 [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; Sussex, UK: 2002. Statistical computing. An introduction to data analysis using S-Plus. [Google Scholar]
- Crick H.Q.P., Dudley C., Glue D.E., Thomson D.L. UK birds are laying eggs earlier. Nature. 1997;388:526. doi:10.1038/41453 [Google Scholar]
- Drent R.H., Daan S. The prudent parent: energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Gienapp P., Hemerik L., Visser M.E. A new statistical tool to predict phenology under climate change scenarios. Glob. Chang. Biol. 2005;11:600–606. doi:10.1111/j.1365-2486.2005.00925.x [Google Scholar]
- Gienapp P., Postma E., Visser M.E. Why breeding time has not responded to selection for earlier breeding in a songbird population. Evolution. 2006;60:2381–2388. doi:10.1554/06-235.1 [PubMed] [Google Scholar]
- Gilmoure A.R., Gogel B.J., Cullis B.R., Welham S.J., Thompson R. VSN International; Hemel Hempstead, UK: 2006. ASReml user guide. v. 2.0. [Google Scholar]
- Klomp H. Determination of clutch-size in birds: a review. Ardea. 1970;58:1–124. [Google Scholar]
- Lindèn M. Reproductive trade-off between 1st and 2nd clutches in the great tit Parus major: an experimental study. Oikos. 1988;51:285–290. doi:10.2307/3565309 [Google Scholar]
- McCleery R.H., Perrins C.M. …temperature and egg-laying trends. Nature. 1998;391:30–31. doi:10.1038/34073 [Google Scholar]
- Møller A.P. Interval between clutches, fitness, and climate change. Behav. Ecol. 2007;18:62–70. doi:10.1093/beheco/arl051 [Google Scholar]
- Nagy L.R., Holmes R.T. Food limits annual fecundity of a migratory songbird: an experimental study. Ecology. 2005;86:675–681. doi:10.1890/04-0155 [Google Scholar]
- Nussey D.H., Postma E., Gienapp P., Visser M.E. Selection on heritable phenotypic plasticity in a wild bird population. Science. 2005;310:304–306. doi: 10.1126/science.1117004. doi:10.1126/science.1117004 [DOI] [PubMed] [Google Scholar]
- Parejo D., Danchin E. Brood size manipulation affects frequency of second clutches in the blue tit. Behav. Ecol. Sociobiol. 2006;60:184–194. doi:10.1007/s00265-005-0155-z [Google Scholar]
- Perrins C.M. Timing of birds breeding seasons. Ibis. 1970;112:242–255. doi:10.1111/j.1474-919X.1970.tb00096.x [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2007. R: a language and environment for statistical computing. ISBN 3-900051-07-0, URL: http://www.R-project.org. [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. doi:10.2307/3544698 [Google Scholar]
- Sæther B.E., Bakke O. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology. 2000;81:642–653. doi:10.2307/177366 [Google Scholar]
- Schall R. Estimation in generalized linear models with random effects. Biometrika. 1991;78:719–727. doi:10.1093/biomet/78.4.719 [Google Scholar]
- Simons L.S., Martin T.E. Food limitation of avian reproduction: an experiment with the cactus wren. Ecology. 1990;71:869–876. doi:10.2307/1937358 [Google Scholar]
- Sokal R.R., Rohlf F.J. W. H. Freeman and Company; New York, NY: 1995. Biometry. [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Tinbergen J.M., Both C. Is clutch size individually optimized? Behav. Ecol. 1999;10:504–509. doi:10.1093/beheco/10.5.504 [Google Scholar]
- Tinbergen J.M., Van Balen J.H., Van Eck H.M. Density dependent survival in an isolated great tit population: Kluyvers data reanalysed. Ardea. 1985;73:38–48. [Google Scholar]
- Van Balen J.H. Comparative study of breeding ecology of great tit (Parus major) in different habitats. Ardea. 1973;61:1–93. [Google Scholar]
- Verboven N., Verhulst S. Seasonal variation in the incidence of double broods: the date hypothesis fits better than the quality hypothesis. J. Anim. Ecol. 1996;65:264–273. doi:10.2307/5873 [Google Scholar]
- Verboven N., Visser M.E. Seasonal variation in local recruitment of great tits: the importance of being early. Oikos. 1998;81:511–524. doi:10.2307/3546771 [Google Scholar]
- Verboven N., Tinbergen J.M., Verhulst S. Food, reproductive success and multiple breeding in the great tit Parus major. Ardea. 2001;89:387–406. [Google Scholar]
- Verhulst S. Multiple breeding in the great tit. II. The costs of rearing a second clutch. Funct. Ecol. 1998;12:132–140. doi:10.1046/j.1365-2435.1998.00165.x [Google Scholar]
- Verhulst S., Tinbergen J.M., Daan S. Multiple breeding in the great tit. A trade-off between successive reproductive attempts? Funct. Ecol. 1997;11:714–722. doi:10.1046/j.1365-2435.1997.00145.x [Google Scholar]
- Visser M.E., Van Noordwijk A.J., Tinbergen J.M., Lessells C.M. Warmer springs lead to mistimed reproduction in great tits (Parus major) Proc. R. Soc. B. 1998;265:1867–1870. doi:10.1098/rspb.1998.0514 [Google Scholar]
- Visser M.E., et al. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. B. 2003;270:367–372. doi: 10.1098/rspb.2002.2244. doi:10.1098/rspb.2002.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M.E., Holleman L.J.M., Gienapp P. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia. 2006;147:164–172. doi: 10.1007/s00442-005-0299-6. doi:10.1007/s00442-005-0299-6 [DOI] [PubMed] [Google Scholar]
- Weggler M. Constraints on, and determinants of, the annual number of breeding attempts in the multi-brooded Black Redstart Phoenicurus ochruros. Ibis. 2006;148:273–284. [Google Scholar]
- White T.C.R. The role of food, weather and climate in limiting the abundance of animals. Biol. Rev. 2008;83:227–248. doi: 10.1111/j.1469-185X.2008.00041.x. doi:10.1111/j.1469-185X.2008.00041.x [DOI] [PubMed] [Google Scholar]
- Williams G.C. Natural selection costs of reproduction and a refinement of Lacks principle. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]