Abstract
Objective
Saliva is a biofluid that can be obtained from individuals without supervision by health care providers. To maximize this clinical advantage, it is highly desirable to have a global salivary analyte stabilizer for proteins, RNA and DNA at ambient temperature.
Design
Whole saliva, saliva supernatant and saliva filtrate (5.0 μm) were treated with RPS at room temperature (RT) for up to 6 days and then subjected to SDS-PAGE. Immunoblotting of β-actin and cystatin C were used to evaluate protein stability. For salivary DNA/RNA, whole saliva was incubated with RPS at RT for up to 10 weeks. After extracting total DNA/RNA in samples at week 0, 2, 6 and 10, DNA stability was assayed by chromosome 18 DNA qPCR and RNA stability by β-actin mRNA RT-qPCR.
Results
β-actin completely degraded in all types of saliva samples after 6-day incubation at RT. However, 24.0%, 91.4% and 89.3% of β-actin remained intact with RPS for whole saliva, saliva supernatant and filtrate, respectively. Similarly, 70.3% of cystatin C in supernatant remained intact in the presence of RPS. For salivary DNA/RNA, the cycle threshold (Ct) values showed no significant change for chromosome 18 DNA and β-actin mRNA in RPS-incubated saliva during the 10-week time course while significant increase in Ct values were observed in controls without RPS for both β-actin mRNA and DNA.
Conclusions
RPS provided effective concurrent stabilization to salivary DNA/RNA in whole saliva for up to 10 weeks and proteins in saliva filtrate for 6 days at RT. We also achieved separation of saliva supernatant from cellular elements by a simple filtration step (bypassing the need for centrifugation).
Keywords: Saliva diagnostics, Saliva stabilizer, Salivary biomarkers, Proteins, DNA, RNA
1. Introduction
Human saliva is an informative body fluid containing an array of analytes (proteins, mRNA and DNA) that can be used as biomarkers for translational and clinical applications.1,2 We have discovered seven saliva mRNA biomarkers with high sensitivity and specificity for oral cancer detection by using microarray profiling followed by quantitative PCR validation.3 Such a panel of biomarkers are currently being validated through a clinical study sponsored by the Early Disease Research Network (EDRN) of the National Cancer Institute. We have also conducted systematic analysis of human saliva proteome4–10 and identified potential protein biomarkers for oral cancer and Sjögren syndrome.11–13 These novel targets, if successfully validated on multi-center clinical trials, will allow non-invasive detection of these disease entities.
A major challenge in the utilization of biological analytes for clinical applications is the necessity to stabilize and maintain the integrity of informative biomarkers for clinical diagnostics. DNA, RNA and proteins are unstable biomolecules, which leads to difficulties in sample storage and handling. We have shown that saliva β-actin mRNA in saliva was degraded with half-life of 12 min.14 One of the values of saliva is the ease of sampling and high subject compliance for sample collection, which includes field applications as well as home collection. The ability to provide a user friendly, easy to use collector, processor and universal stabilizer for major salivary diagnostic analytes is a key enabling pre-analytic technology for saliva diagnostics.
This work is part of our saliva research efforts to develop ambient temperature stabilization protocols for salivary analytes. In our previous work, we found that RNAprotect® Saliva Reagent (RPS, QIAGEN Inc., Valencia, CA) could stabilize RNA in saliva samples at room temperature for up to 12 weeks.15 No significant loss or change in RNA integrity was observed. In this study, we have investigated the use of RPS as a global pre-analytic solution for stabilization of DNA, RNA and proteins in saliva at ambient temperature.
2. Materials and methods
2.1. Sample collection and processing
Saliva samples were obtained from five healthy adult individuals under approved institutional review board protocols and informed consents. As described previously,1 saliva collection was performed in the morning between 9 AM and 11 AM and after 2 h of fasting. Subjects were told to rinse twice with distilled water before sample collection. To prepare saliva supernatant and filtrates, saliva supernatant was collected after centrifugation at 5,000 × g for 10 min at 4 °C. Saliva filtrates were obtained with Millipore Millex-HV 0.45 μm or Millex-SV 5.0 μm sterile low protein binding PVDF Durapore membrane syringe filters. All sample processing was carried out on the ice to minimize protein degradation. Total protein assay was performed with the 2D Quant kit (Amersham). Cell viability assay in saliva samples was performed using the Vi-Cell cell viability analyzer (Beckman, Fullerton, CA) to test the presence or absence of live cells in saliva samples.
RPS-incubated saliva samples were prepared by mixing saliva (whole saliva, saliva supernatant or filtrates) with RPS at a ratio of 1:5 (as recommended in RPS user instructions). Both RPS-incubated saliva samples and corresponding saliva samples without RPS stabilization (controls) were stored at room temperature for specified incubation time and then stored in −80 °C freezer until analysis. The stabilities of salivary protein and DNA/RNA were tested for different durations. For salivary proteins, the samples were incubated for up to 6 days because proteins degraded quickly at ambient temperature and we aim to preserve most proteins with RPS during the time required for regular international shipping. For salivary DNA and RNA, they degrade slowly so we incubated the samples for up to 10 weeks to examine the long-term preservation ability of the RPS. We also prepared RPS-incubated saliva samples at saliva/RPS ratio of 1:3, 1:1 and 2:1 to examine how low this ratio can go while still keeping acceptable performance of protein stabilization.
2.2. SDS-PAGE and immunoblotting
Protein precipitation was carried out by adding cold acetone pre-chilled at −20 °C to saliva samples (sample: acetone = 1:9) and leaving the mixture at −20 °C overnight. After spinning at 14,000 × g for 20 min and washing the pellets with cold acetone, supernatant was removed to obtain pellets. Add to the pellet 3 μl of NuPAGE reducing agent (10×), 7.5 μl of NuPAGE LDS sample buffer (4×, both from Invitrogen) and 19.5 μl of water. The mixture was vortexed briefly andthenheatedat 70 °C for 10 min. Ten microlitres of the heated mixture was loaded immediatelyontoNuPAGE10% Bis–Tris gel. Five microlitres of SeeBlue Plus2 pre-stained standard was loaded as protein standards. Electrophoresis was in a MES SDS running buffer at 120 V for about 75 min. The gel was transferred to nitrocellulose membrane immediately with iBlot apparatus and minigel transfer stacks (Invitrogen, Carlsbad, CA). The membrane was saturated with 5% milk in TBST solution at room temperature for 2 h. To measure β-actin, the blots were then incubated with primary rabbit polyclonal actin antibody (Sigma, St. Louis, MO), followed by horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody (Amersham, Arlington, IL). To measure cystatin C, we used the primary rabbit polyclonal cystatin C antibody (UpState Biotechnology, Lake Placid, NY) and same secondary antibody. Primary antibodies were diluted 250 times with 5% milk/TBST and incubated at room temperature for 2 h. Secondary antibody was diluted 1000 times with 5% milk/TBST and incubated for 1 h. After washing, bands were visualized using enhanced chemiluminescence kit (Amersham).
2.3. RNA/DNA in whole saliva
Pooled whole saliva samples were incubated at room temperature for up to 10 weeks with or without the presence of RPS (saliva/RPS = 1:5). At time points 0, 2, 6 and 10 weeks, 200 μl of saliva without RPS (control) and 1.2 ml of RPS-incubated saliva (both in triplicate) were removed and stored at −80 °C. Before analysis, 1 ml of RPS was added to the control samples so that both control and RPS-incubated samples have the same amount of RPS during the DNA/RNA extraction to avoid any possible variations in RNA recovery due to RPS presence.
Total nucleic acids in whole saliva were extracted with QIAmp RNA Viral mini kit (QIAGEN Inc. Valencia, CA) according to the manufacturer's instructions with minor modifications. We used 50 μl of buffer AVE to elute total nuclei acids. For DNA analysis, 3 μl of eluate was 10-fold diluted with water and then 2 μl was used per qPCR reaction with a primer pair that targets a region of chromosome 18. The forward primer sequence is tgacaaccaaacgtgtgttctg, and the reverse primer sequence is agcagcgacttctttaccttgataa. We selected this primer set because it consistently produced amplified PCR product from different batches of saliva extracted DNA. For RNA analysis, 20 μl of eluate from total nucleic acid extraction was treated with RNase-free DNase (DNaseI-DNA-free, Ambion Inc.) to remove DNA completely, and 3 μl was used for 20 μl RT reaction. RT-qPCR for β-actin was performed as described previously.15
3. Results
3.1. Protein stabilization
To investigate if RPS can stabilize saliva proteins, whole saliva, saliva supernatant and saliva filtrate (5.0 μm) were incubated with RPS and without RPS (as control) at room temperature for 5 days. We selected β-actin (43 kDa) as a marker saliva protein to evaluate RPS using immunoblotting analysis as it is readily detectable in saliva and widely used for protein normalization purpose. In this study, frozen saliva with RPS were prepared by precipitating proteins with cold acetone immediately after addition of RPS and then storing at −80 °C. Frozen saliva without RPS were prepared the same way except that RPS was not added. Frozen saliva samples were used as references to calculate the intact protein percentages in saliva samples at room temperature with and without RPS, respectively.
Five individual samples were tested for the comparison of the residual protein levels after incubation at room temperature with and without RPS stabilization. Fig. 1A is the results of one of the five subjects showing the relative levels of actin as measured by immunoblotting. After incubation at room temperature for 5 days, β-actin in all types of tested saliva samples disappeared if RPS was not added (lane 1, Fig. 1A). With addition of RPS, however, a differential effect on β-actin stabilization was observed with respect to whole saliva versus supernatant saliva. The β-actin band disappeared in Fig. 1A (lane 3 top panel) or was very weak in other three individual samples (not shown). Its average intact protein percentage in whole saliva decreased to 24 ± 22.5% while saliva supernatant β-actin was at 91.4 ± 6.8% (Fig. 1B). This result indicates that RPS can preserve the proteins in supernatant but cannot stabilize the proteins in whole saliva. This is likely due to the presence of cellular and microbial elements in whole saliva which may release proteolytic enzymes that are beyond the proteolytic inhibitory abilities of the RPS. Cells need to be removed to stabilize saliva proteins with RPS.
Fig. 1.
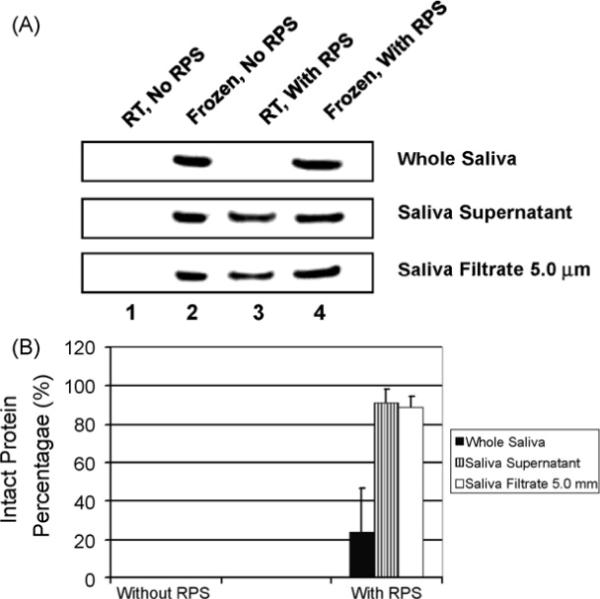
Immunoblotting assay of β-actin after RPS-incubation. (A) Saliva samples, including whole saliva, saliva supernatant and 5.0 μm saliva filtrate were mixed with RPS and incubated at room temperature (RT) for 5 days. After SDS-PAGE, actin was detected by immunoblotting. (B) The intact protein percentage (relative actin level) was calculated by dividing the band intensity of actin in saliva preserved with or without RPS by that of corresponding reference saliva frozen at −80 °C. The error bars represent S.D.
The observed ability of RPS to stabilize β-actin in saliva supernatant but not in whole saliva prompted us to seek for technologies that will allow for cellular separation in saliva without the need for centrifugation. This is of particular importance as it applies to field and epidemiological research and applications. We tested if cellular elements can be removed by filtration without significant loss of protein content comparing to centrifugation method. Two pore sizes filters: 0.45 μm and 5.0 μm were explored to examine the effect of pore size on protein loss. To determine the effectiveness of cell removal and ease of use, we examined the presence of cellular elements in whole saliva and filtered saliva. Cells were readily detectable in whole saliva but none were observed in saliva supernatant or filtrates using either the 0.45 μm or 5.0 μm filter. Cell viability assays (Beckman Vi-Cell cell viability analyzer) also confirmed a viability value of 0% for both supernatant and filtrates but 29.4% for whole saliva.
To determine if the filtration process results in more loss of salivary protein compared to centrifuged supernatant, we measured the total protein concentration for the filtered saliva and centrifuged saliva supernatant. The total protein concentration for centrifuged saliva supernatant was determined as 0.55 ± 0.08 mg/ml (n = 5), which is about half of the one for whole saliva (1.11 ± 0.11 mg/ml, n = 5). However, the total protein concentration for 5.0 (0.52 ± 0.08 mg/ml, n = 5) and 4.5 μm (0.47 ± 0.07 mg/ml, n = 5) filtered saliva are within the similar range as the one for the centrifuged saliva. Based on these findings we conclude that the 5.0-μm filter is suitable for the cellular removal from saliva. It should also be noted that the 5.0 μm filter also allowed easier processing of saliva filtration as its larger pore size permits easier flow through and less clogging.
We then proceed to test if filtered saliva incubated with RPS can also stabilize saliva β-actin protein at room temperature. The ability to do so will present a highly desirable scenario whereby saliva can be filtered, stabilized and stored at ambient temperature, all at the point of care. Three different saliva samples: whole saliva, saliva supernatant and 5.0-μm saliva filtrate, were incubated with RPS at room temperature for 5 days. As shown in Fig. 1A (lane 3), there was no significant difference in intensity for β-actin among those of frozen saliva samples without RPS and those of frozen reference saliva samples with RPS. This result indicates that filtration with 5.0 μm filters can replace centrifugation without significantly affecting protein contents. Fig. 1B shows that 89.3 ± 5.2% of actin in 5.0-μm filtrate remains intact after 5-day incubation with RPS, very similar to that of the centrifuged saliva. The difference is not significant (p > 0.05).
To further understand the degradation kinetics of saliva proteins, a time course analysis was performed for up to 6 days, where the salivary β-actin levels on each day were analyzed. Fig. 2A shows the 6-day β-actin degradation time courses in 5.0-μm saliva filtrate at room temperature. Actin without RPS stabilization (control) exhibited rapid decay kinetics from day 1. On the contrary, β-actin in RPS-incubated saliva filtrate remained similar band intensity from day 0 through day 6. After normalized to band intensity on day 0, we found that 88.2 ± 22.7% of RPS-incubated β-actin remained intact on day 6 while control β-actin without RPS decreased to 22.3 ± 16.4% on day 1 and dropped to <5% after 3 days (Fig. 2B). From the actin decay time kinetics, the half-lives of actin in saliva filtrate with and without RPS stabilization were calculated to be 12.5 ± 5.9 and 0.69 ± 0.33 days, respectively.
Fig. 2.
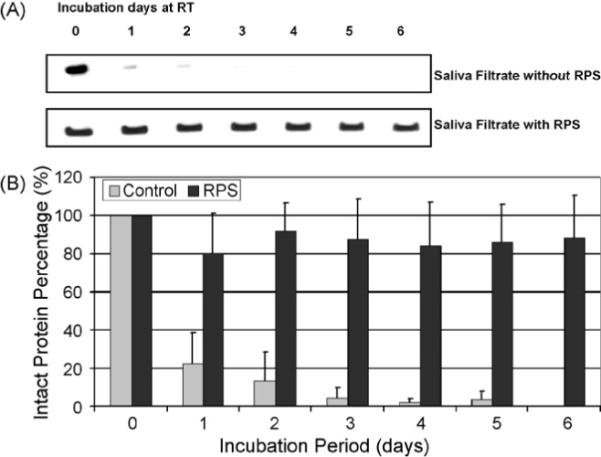
Degradation time course of β-actin in 5.0 μm saliva filtrate. (A) 5.0 μm saliva filtrate samples were incubated with or without RPS at RT for up to 6 days. Samples were taken every day. After SDS-PAGE, actin was detected by immunoblotting. (B) Actin bands were digitized and normalized to the band intensity on day 0. The error bars represent S.D.
To compare the protein stabilization performance of RPS with other protein inhibitors, we added commonly used protein inhibitor cocktails that consist of 1 μl of 10 mg/ml aprotinin, 3 μl of 400 mM sodium orthovanadate and 10 μl of 10 mg/ml phenylmethanesulfonyl fluoride per ml of saliva sample, and incubated at room temperature for 5 days. As shown in Fig. 3 lane 2, β-actin was not detectable after 5 days with the protease inhibitors in both filtered and centrifuged supernatants. These data suggest that these protein inhibitors do not work well to preserve salivary proteins at room temperature. As the references, protein inhibitors-incubated saliva samples were prepared by extracting proteins immediately after addition of inhibitors and storing pellets at −80 °C. They produced strong bands, similar to RPS-incubated saliva samples, suggesting that the degradation of proteins in protein inhibitors-incubated saliva samples is due to enzymatic digestion without any effective protein stabilization at room temperature.
Fig. 3.
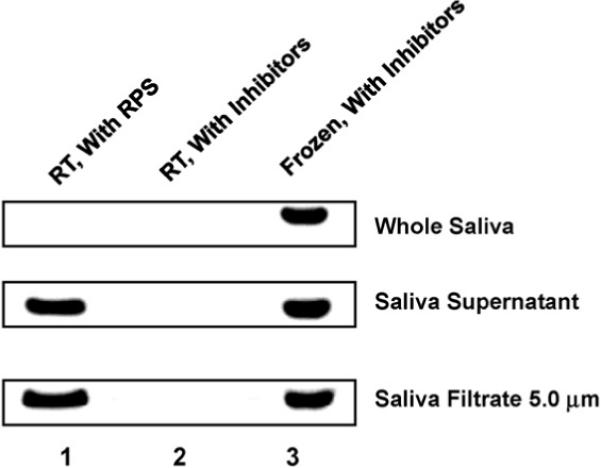
Comparison of RPS-incubated and protease inhibitors-incubated saliva samples. Saliva samples, including whole saliva, saliva supernatant and 5.0 μm saliva filtrate were mixed with RPS or protease inhibitors, and then incubated at room temperature for 5 days. After SDS-PAGE, actin was detected by immunoblotting.
We also examined the effect of RPS on stabilization of another saliva protein cystatin C (16 kDa) using immunoblotting and similar results were observed. As shown in Fig. 4, cystatin C band disappeared completely after incubation at RT for 5 days. However, in the presence of RPS, 70.3% of cystatin C remained intact.
Fig. 4.
Immunoblotting assay of cystatin C after RPS-incubation. Saliva supernatant was mixed with RPS and incubated at RT for 5 days. After SDS-PAGE, cystatin C was detected by immunoblotting.
3.2. DNA/RNA stabilization
We previously showed that RPS could stabilize RNA in saliva samples at room temperature for up to 12 weeks.14 No significant loss or change in RNA integrity was observed. Here we have evaluated the effect on RPS, in addition to preserving salivary RNA stability, it effect on stabilizing salivary genomic DNA. As genomic DNA reside primarily in the cellular phase of saliva, whole saliva was used for this assessment. Since we have already thoroughly evaluated the effect of RPS on salivary supernatant RNA stability,15 there is no need to duplicate the supernatant assessment. Instead we assess the effect of RPS on whole saliva RNA.
To evaluate the DNA/RNA stability in whole saliva with and without RPS, pooled whole saliva was assayed at room temperature up to 10 weeks. Samples at week 0, 2, 6 and 10 were tested for DNA/RNA levels. For DNA analysis, we used a primer pair that targets a region in chromosome 18, and for RNA analysis, we used a primer pair that targets β-actin mRNAs. The qPCR results of chromosome 18 DNA are shown in Fig. 5A. The cycle threshold (Ct) values for the control samples increased significantly from 26.4 at week 0 to 37.9 at week 10, while RPS-incubated saliva showed no significant (p > 0.05) Ct values differences throughout the time course. Fig. 5B shows the results of RT-qPCR for β-actin mRNA. Similarly, the control Ct value increased significantly from 30.3 at beginning to 37.2 at week 2, thereafter decreased slowly to 35.6 at week 10. RPS-stabilized saliva shows no significant (p > 0.05) increase in Ct values. These data allow us to conclude that RPS can stabilize both DNA and RNA at room temperature for up to 10 weeks without significant loss.
Fig. 5.
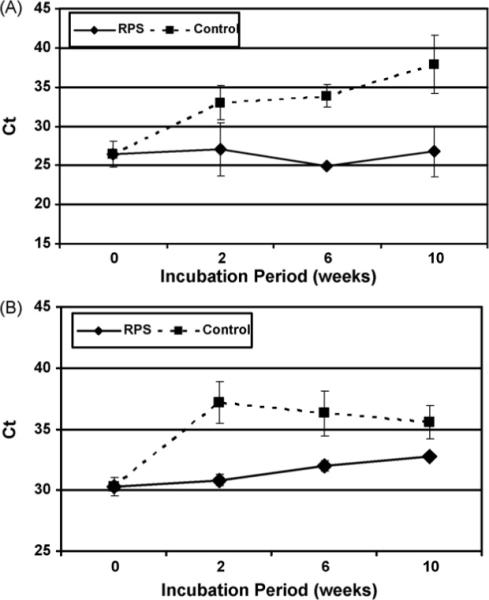
RPS stabilization for DNA/RNA in whole saliva. Whole saliva samples were incubated with or without RPS for 0, 2, 6, 10 weeks. At each time point, triplicate aliquots of 200 μl of saliva were used to extract total DNA/RNA with RNA Viral mini kit. (A) qPCR of chromosome 18 DNA. (B) RT-qPCR for β-actin mRNA. The error bars represent S.D.
4. Discussion
The goal of this work is to test the hypothesis that RPS can be used for stabilization of proteins and nucleic acids in saliva and to develop a simple saliva collection/stabilization protocol at ambient temperature. Previously we demonstrated that RPS could stabilize β-actin mRNA in saliva and RPS performed better preservation capability than SUPER-ase•In™ RNase inhibitor and RNALater®.15 In this study, we have found that RPS can stabilize both DNA and RNA in whole saliva for up to 10 week without significant changes in Ct values.
We have further tested the degradation time course of β-actin in RPS-incubated saliva supernatant. Immunoblot data show that RPS can stabilize proteins at ambient temperature if cells in saliva are removed. This can be done by centrifugation or simply by filtering. Total protein assay data show that the loss of proteins after filtration is similar to that obtained after centrifugation. No significant difference in protein loss was observed between 0.45 μm and 5.0 μm filters. However saliva is a body fluid of high viscosity. Considering the ease of sample handling and processing, 5.0 μm filter would be a good choice for sample preparation.
In conclusion, we have demonstrated that RPS not only stabilize RNA but also provides effective preservation at room temperature to other important macromolecules such as DNA and proteins. By using 5.0 μm hydrophilic membrane syringe filters to replace centrifugation, saliva sample collection becomes much simpler and specially trained personnel are no longer needed. Based on our studies, a simple “spit-and-mail” saliva collection kit can therefore be designed to allow saliva samples collected by disease patients at home. This will greatly facilitate the application of saliva as an important diagnostic biofluid for large-scale oral disease screening and monitoring.
Acknowledgements
This work was supported by R21 CA126733 U01-DE017790 (D. Wong) and R03-DE017144 (S. Hu).
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Conflict of interest statement
None.
REFERENCES
- 1.Li Y, Zhou X, St John MA, Wong DT. RNA profiling of cell-free saliva using microarray technology. J Dent Res. 2004;83:199–203. doi: 10.1177/154405910408300303. [DOI] [PubMed] [Google Scholar]
- 2.St John MA, Li Y, Zhou X, Denny P, Ho CM, Montemagno C, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:929–35. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, St John M, Zhou X, Kim Y, Sinha U, Jordan RC, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10:8442–50. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 4.Hu S, Xie Y, Ramachandran P, Ogorzalek Loo RR, Li Y, Loo JA, et al. Large-scale identification of proteins in human salivary proteome by liquid chromatography/mass spectrometry and two-dimensional gel electrophoresismass spectrometry. Proteomics. 2005;5:1714–28. doi: 10.1002/pmic.200401037. [DOI] [PubMed] [Google Scholar]
- 5.Hu S, Denny P, Denny P, Xie Y, Loo JA, Wolinsky LE, et al. Differentially expressed protein markers in human submandibular and sublingual secretions. Int J Oncol. 2004;25:1423–30. [PubMed] [Google Scholar]
- 6.Hu S, Loo J, Wong DT. Human saliva proteome analysis. Ann N Y Acad Sci. 2007;1098:323–9. doi: 10.1196/annals.1384.015. [DOI] [PubMed] [Google Scholar]
- 7.Hu S, Loo J, Wong DT. Human body fluid proteome analysis. Proteomics. 2006;6:6326–53. doi: 10.1002/pmic.200600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu S, Loo Y, Wang J, Xie Y, Tjon K, Wolinsky L, et al. Human saliva proteome and transcriptome. J Dent Res. 2006;85:1129–33. doi: 10.1177/154405910608501212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu S, Loo J, Wong DT. Human saliva proteome analysis and disease biomarker discovery. Expert Rev Proteomics. 2007;4:531–8. doi: 10.1586/14789450.4.4.531. [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Yen Y, Ann D, Wong D. Implications of salivary proteomics in drug discovery and development: a focus on cancer drug discovery. Drug Discov Today. 2007;12:911–6. doi: 10.1016/j.drudis.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Hu S, Wang J, Meijer J, Ieong S, Xie Y, Yu T, et al. Salivary proteomic and genomic biomarkers for primary Sjögren's Syndrome. Arthritis Rheum. 2007;56:3588–600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Hu S, Wong DT. Salivary biomarkers for the detection of primary Sjögren's Syndrome. Future Rheum. 2007;2:447–9. [Google Scholar]
- 13.Hu S, Yu T, Xie Y, Yang Y, Li Y, Zhou X, et al. Discovery of oral fluid biomarkers for human oral cancer by mass spectrometry. Cancer Genomics Proteomics. 2007;4:55–64. [PubMed] [Google Scholar]
- 14.Park NJ, Li Y, Yu T, Brinkman BM, Wong DT. Characterization of RNA in saliva. Clin Chem. 2006;52:988–94. doi: 10.1373/clinchem.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park NJ, Yu T, Nabili V, Brinkman BM, Henry S, Wang J, et al. RNAprotect saliva: an optimal room-temperature stabilization reagent for the salivary transcriptome. Clin Chem. 2006;52:2303–4. doi: 10.1373/clinchem.2006.075598. [DOI] [PubMed] [Google Scholar]


