SYNOPSIS
Deregulated expression of members of the Inhibitor of Apoptosis (IAP) family has been found in a wide variety of neoplastic cells, and synthetic IAP antagonists represent a promising novel class of chemotherapeutic agents. Early work focused on the ability of these compounds to block the caspase inhibitory function of XIAP. However, recent studies have shown that IAP antagonists, although primarily designed to target XIAP, trigger a ubiquitin-mediated degradation of two related proteins, c-IAP1 and c-IAP2, and through this process potentiate the death of tumor cells via autocrine cellular signaling pathways. In this context, the relative contribution of XIAP as a target of this class of compounds is unclear. Here we examine the involvement of XIAP using a recently described synthetic IAP antagonist, AEG40730, and through the comparison of a human tumor cell line targeted for XIAP with its isogenic, wild type control line. Treatment with nanomolar concentrations of AEG40730 resulted in the loss of both XIAP and c-IAP1 proteins, albeit with different kinetics. While XIAP-deficient HCT116 cells retained some sensitivity to AEG40730 to external apoptotic stimuli, the data suggest that IAP antagonists such as AEG40730 exert their apoptotic enhancing effects through XIAP in addition to the c-IAPs. These data indicate that IAP antagonists can target multiple IAPs to augment distinct pro-apoptotic signaling pathways, thereby revealing the potential for these compounds in cancer therapy and underscoring the promise of IAP-targeted therapies.
Keywords: IAP, apoptosis, caspases, SMAC, TRAIL
INTRODUCTION
Homeostatic regulation of metazoan cell number is dependent on a tightly regulated balance between the proliferation and death of cells [1, 2]. Deregulation of this balance is a hallmark of many disease states, and the activities of key components of both the proliferative and apoptotic (programmed cell death) machinery are altered in a wide variety of disorders including neurodegenerative, autoimmune and neoplastic diseases [3–5], and factors that influence this balance are of great significance in treating a host of diseases.
One important group of factors that regulate apoptosis is the TNF receptor superfamily which, following engagement with its cognate ligands, can elicit pro-survival and/or pro-apoptotic responses [6]. Several TNF receptor family members have attracted much attention as therapeutic targets for the treatment of cancers and immunological disorders. For example, cells from a range of malignancies, including cancers of prostate, colon and hepatic origin, appear highly sensitive to the pro-apoptotic ligand, TRAIL [7–9], and this has led to the development of TRAIL ligands and agonists as potential therapeutic tools for cancer treatment.
The central effectors of apoptosis are caspases, a family of intracellular cysteine proteases with specificity for aspartate-containing residues [10, 11]. Caspases function in a hierarchical manner; they are initially synthesized as inactive zymogens and, following activation, upstream or initiator caspases such as Caspases-8 and -9 can become activated by oligomerization, through a process referred to as the induced proximity model [12], which subsequently leads to the cleavage and activation of effector caspases, such as Caspases-3 and -7.
IAP (inhibitor of apoptosis) proteins are a group of intracellular proteins, several of which have been shown to directly inhibit caspases [13]. IAPs are characterized by the presence of one or more Baculoviral IAP repeat (BIR) domains, which bind directly to caspases [14]. The most intensively studied IAP protein is X-linked IAP (XIAP), which contains three BIRs. The most carboxy terminal BIR (BIR3) is necessary and sufficient for binding and inhibition of Caspase-9 by XIAP, while BIR2, together with a short proximal amino terminal domain, is involved in binding and inhibition of Caspases-3 and -7 [15, 16]. Interestingly, XIAP has been shown to be elevated in several malignancies [17–19], and so has become a promising target in anti-cancer therapies.
The caspase-inhibitory property of XIAP can be neutralized by Smac/DIABLO, a nuclear-encoded, mitochondrial protein that is released into the cytosol following mitochondrial permeabilization [20, 21]. Binding of Smac to XIAP can displace the XIAP:caspase interaction, releasing the caspase and lowering the cellular apoptotic threshold. Many seminal studies have revealed the nature of the Smac:XIAP interaction [20–23], and a number of synthetic compounds have been developed that resemble the XIAP-interacting interface in Smac. Interestingly, several of these compounds have also been found to interact with at least two other IAPs, c-IAP1 and c-IAP2, even though the experimental strategies leading to their discovery were based on the XIAP-Smac/DIABLO interaction paradigm. The data suggest that targeting c-IAP1 and c-IAP2 with Smac mimetics results in a lowering of the apoptotic threshold indirectly, by affecting the activation of the NF-κB transcriptional network, rather than directly relieving the constraints on caspases [24–26]. Much less clear, however, is the relative contribution of XIAP to the pro-apoptotic effects of these compounds, and how suitable XIAP may be as a future molecular target for drug design.
In this study we examine the role of XIAP in TRAIL-induced apoptosis, by comparing apoptotic signaling in an XIAP-expressing cell line with a derivative line in which XIAP was ablated by homologous recombination. Using this approach, we compared the effects of combined treatment of TRAIL or TNF with a recently identified IAP inhibitor, AEG40730. We find that AEG40730 can induce the degradation of the c-IAPs, and this is likely to be of central importance when compounds such as AEG40730 are used to sensitize cells to the cytotoxic effects of TNF. Interestingly, in addition to targeting c-IAPs, AEG40730 also triggered the degradation of XIAP, and this combined effect was found to significantly augment cell killing when utilized in combination with TRAIL or TNF. These findings reveal that Smac antagonists such as AEG40730 target multiple cellular IAPs, including XIAP, to potentiate apoptosis, and underscore the therapeutic potential of these compounds in combination with different primary apoptotic inducers.
EXPERIMENTAL
Cell culture, infections, plasmids
Wild type, XIAP-deficient and BAX-deficient HCT116 cells [27], kindly provided by Dr. Bert Vogelstein, Johns Hopkins University, were cultured in McCoy’s 5A media containing 10% FBS supplemented with 2 mM glutamax at 37 °C, 5% CO2.
The lentiviral expression vector FG9 was constructed by the modification of the shRNA vector FG12 [28] to include the EF-1a promoter and a multiple cloning site from the expression plasmid pEBB [29]. FG9/GFP-hygro was constructed by replacing the GFP cassette in FG9 with a GFP:hygromycin resistance fusion. Further details of the construction of these plasmids are available upon request.
The coding sequence of native XIAP, XIAPD148A, XIAPE219RH223V, XIAPW310A, XIAPD148AW310A, XIAPH467A, and Bcl-xL were subcloned from pEBB into the BamHI and NotI sites of FG9EF-1a. For stable cell line preparation, the FG9EF-1a empty vector (control) or XIAP and XIAP mutants in the FG9EF-1a vector were co-transfected with pHCMV-G, pRRE, and pRSVrev [28], which direct expression of lentiviral structural proteins, into HEK293 cells using a standard calcium phosphate transfection and incubated at 37 °C, 7% CO2. Forty hours post-transfection the virus-containing media on the HEK293 cells was collected, polybrene (Sigma) was added to a final concentration of 25 mM and the media was filtered through a 0.45 mm Millex HV PVDF filter unit (Millipore) onto HCT116 cells. The virus was incubated with the HCT116 cells for four hours followed by addition of fresh McCoy’s and incubated for an additional forty-eight hours at 37 °C, 7% CO2. Stable cells were selected by the addition of hygromycin B (Invitrogen; 200 mg/mL).
Viability experiments
HCT116 were seeded in 6 well dishes (5–6 × 105 cells/well), and stimulated with the indicated doses of recombinant TRAIL (Alexis Biochemicals) for 2 h unless stated otherwise. For experiments with TNF (Roche Diagnostics) a dose of 200 U/mL was used for 5 or 18 h. After treatment with TRAIL or TNF cells were washed with PBS and recovered in fresh media as indicated in figure legends (24 h, 48 h). For experiments with the IAP antagonist AEG40730, cells were pretreated for 24 h with a final concentration of 10 or 100 nM AEG40730 or DMSO as vehicle control. At indicated time points cells were collected by trypsinization, subsequent centrifugation and resuspension in PBS plus 1% bovine serum albumin and 2 µg/mL of propidium iodide (PI). Cell viability of PI-stained cells was analyzed by flow cytometry using a Coulter EPICS model XL-MCL flow cytometer.
Protein extraction and immunoblotting
Whole cell lysates were prepared using RIPA buffer (1% NP-40, 0.5 % sodium deoxycholate, 0.1% SDS, 1 mM DTT, 1 mM PMSF) supplemented with protease inhibitors. Samples were resolved on 4–12% gradient SDS-PAGE gels (Invitrogen), transferred onto nitrocellulose (Invitrogen) and blocked in 5% milk Tris-buffered saline containing 0.1 % Tween. Membranes were incubated at room temperature for 1 h with the following antibodies: XIAP (BD Pharmingen), β-actin (Sigma), Bcl-xL (BD Pharmingen), BAX (Santa Cruz) or c-IAP1 [24]. Secondary horseradish peroxidase-conjugated anti -mouse, anti-rabbit or anti-rat (GE Healthcare) were used for 1 h at RT. Enhanced chemiluminescence (GE Healthcare) and Kodak XAR film was used for visualization purposes.
RESULTS
XIAP modulates the sensitivity to TRAIL-mediated apoptosis
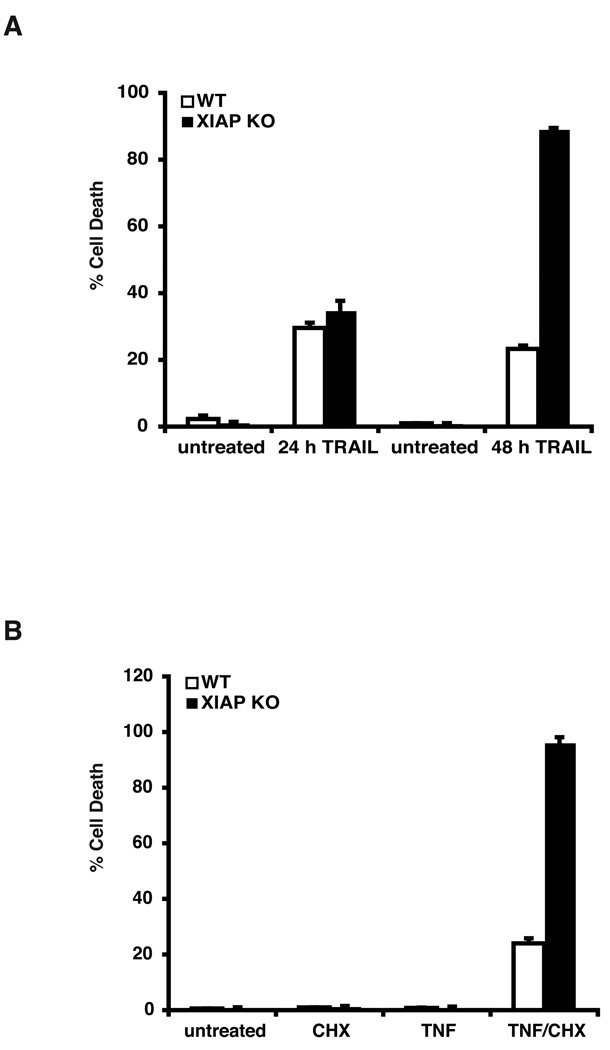
Multiple IAP targeting properties of Smac-like IAP antagonists have been recently described [24–26]. Less clear, however, are the relative contributions of specific IAPs to the apoptosis-promoting effects of these compounds, and whether these contributions differ depending on the type of apoptotic stimulus. To explore this question, we utilized the HCT116 human colon carcinoma cell line and a derivative line in which the XIAP gene had been disrupted by homologous recombination [27]. These cell lines were first examined for their responsiveness to two different apoptotic stimuli, TRAIL (TNF-related apoptosis inducing ligand) and TNF (tumor necrosis factor). XIAP-null cells were found to be highly susceptible to TRAIL-induced apoptosis after prolonged stimulation (Figure 1A; compare WT and KO at 48 h), consistent with a previous report [27]. Neither cell line appeared susceptible to treatment with TNF alone, but the XIAP-deficient cell line was found to be highly sensitive to TNF when co-incubated with the protein synthesis inhibitor, cycloheximide, compared to the parental cell line (Figure 1B). These observations defined a set of experimental conditions under which XIAP plays a clear anti-apoptotic role, and provided a framework in which the effects of IAP antagonists could be examined.
Figure 1.
XIAP-dependent differences in sensitivity to TRAIL and TNF. (A) Parental and XIAP-deficient HCT116 cells were pulsed as indicated with TRAIL (75 ng/mL) for 2 h, and subsequently maintained in fresh media for 24 or 48 h. Cell death was assessed by PI staining and subsequent flow cytometry. Data are representative of at least three independent experiments. (B) Parental and XIAP-deficient HCT116 cells were treated with TNF (200 U/mL) for 18 h, cycloheximide (5 ug/mL), or TNF plus cycloheximide, as indicated. Cells were then washed with PBS and maintained in fresh media for 48 h, before staining with PI and analysis by flow cytometry. The data depicted are representative of three independent experiments performed in triplicate.
Potentiation of TRAIL-induced apoptosis by a synthetic IAP antagonist
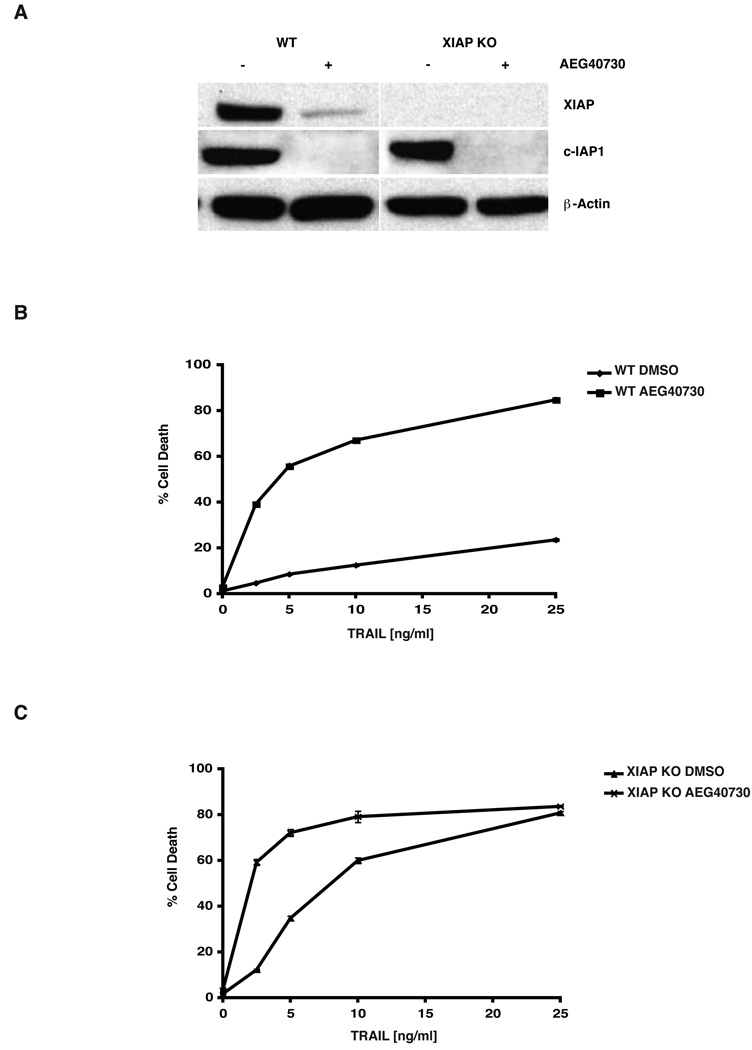
The IAP antagonist AEG40730 is a cell-permeable, synthetic molecule with nanomolar affinities not only for XIAP, but for c-IAP1 and c-IAP2 [30]. To examine its effects on IAP levels in the parental and XIAP-deficient HCT116 lines, cells were treated with AEG40730, and lysates prepared from these cells were examined by immunoblotting with antibodies to both XIAP and c-IAP1. In the parental line, XIAP protein levels were drastically diminished even at low concentrations of the drug (10 nM) after a 24 hour incubation (Figure 2A), suggesting an induced degradation of XIAP protein by the drug. Importantly, c-IAP1 protein levels were also reduced under the same conditions (Figure 2A), and since this reduction occurred in both the parental and XIAP-deficient HCT116 cells, the targeting of c-IAP1 by AEG40730 appears to occur independently of XIAP.
Figure 2.
AEG40730 triggers the degradation of IAPs. (A) Parental and XIAP-deficient HCT116 cells were treated with AEG40730 (10 nM) for 24 h. Whole cell lysates (15 µg) were resolved by SDS-PAGE, and immunoblotted with XIAP, c-IAP1 or β-actin antibodies as indicated. A representative immunoblot is shown. (B) Parental HCT116 cells were treated with vehicle control (DMSO) or AEG40730 (10 nM) for 24 h, whereupon they were either left untreated or treated with a range of concentrations of TRAIL (2.5, 5, 10, 25 ng/mL, 2 h). Following 48 h recovery in fresh media, PI-stained cells were analyzed by flow cytometry. The data shown are representative of three independent experiments, each performed in triplicate. (C) XIAP-deficient HCT116 cells were incubated with a vehicle control (DMSO) or 10 nM AEG40730. After 24 h, recombinant TRAIL (2.5, 5, 10, 25 ng/mL) was added to the treatment group for 2 h. Cells were then washed with PBS and media was replaced. Cell death was analyzed by PI exclusion and flow cytometry 48 h later. Each experiment was performed in triplicate, and data are representative of at least three independent experiments.
In pilot studies, we found that HCT116 cells could tolerate a wide range of AEG40730 concentrations when delivered as a single agent, without considerable loss of viability (data not shown). To determine whether AEG40730 could potentiate apoptosis to a second signal in our defined system, HCT116 parental cells were pre-incubated with the drug, pulsed with TRAIL and subsequently examined for viability. The combination of TRAIL and AEG40730 induced a significant level of death, even at the lowest concentrations of TRAIL (Figure 2B), presumably as a consequence of the drug targeting one or more IAPs for degradation. The involvement of XIAP was therefore examined using identical experimental conditions, but titrating TRAIL into the XIAP-deficient HCT116 line. Interestingly, XIAP-null cells, while being more sensitive to TRAIL alone, were also further sensitized by the drug AEG40730 at low concentrations of TRAIL (Figure 2C). However, this significant sensitization to TRAIL by AEG40730 in XIAP-deficient cells was no longer apparent when TRAIL concentrations were increased. Taken together, these data suggest that AEG40730 potentiates cell death, especially at lower concentrations of TRAIL, by degrading several IAPs.
XIAP reconstitution restores TRAIL resistance in XIAP-deficient cells
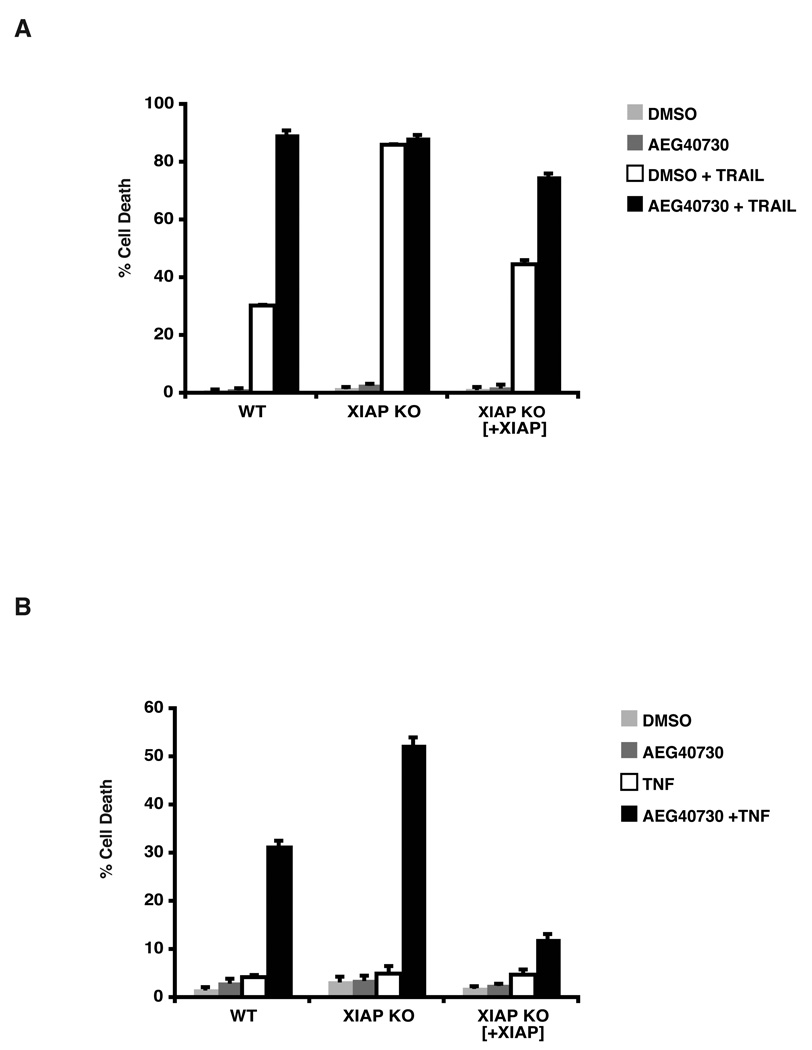
To establish definitively whether XIAP is required for resistance to TRAIL-induced apoptosis, we reconstituted XIAP-null HCT116 cells with wild type XIAP. Cells with reconstituted XIAP were found to be protected against TRAIL-induced cell death, when compared to XIAP-deficient cells (Figure 3A), and cell death was potentiated when such cells were co-treated with AEG40730, to a similar degree to that observed in AEG40730-treated parental cells. These findings are strongly indicative of a role for XIAP in resistance to TRAIL-induced death, which can be neutralized by exposure to this IAP antagonist.
Figure 3.
Reconstituted XIAP null cells protect from TRAIL mediated death. (A) Parental, XIAP-deficient and XIAP-deficient cells reconstituted with XIAP were pre-treated with DMSO or 10 nM AEG40730 for 24 h. Subsequently, cells were either left untreated or treated for 2 h with 75 ng/mL TRAIL. Thereafter cells were washed with PBS and placed in fresh media for 48 h. Cell death was measured by PI staining and flow cytometry. Each experiment was performed in triplicate, and data are representative of at least three independent experiments. (B) The indicated cells were pre-treated with vehicle control (DMSO) or AEG40730 (10 nM) for 24 h. Cells were then left untreated or treated with TNF (200 U/mL) for 5 h, washed in PBS and recovered in fresh media for 48 h. Apoptosis was measured by staining cells with PI and flow cytometry. Each experiment was performed in triplicate, and data are representative of at least three independent experiments.
Recent reports have demonstrated an important role of c-IAP1 and c-IAP2 in inhibiting TNF-mediated cell death [24–26]. We utilized the parental and XIAP-null HCT116 cells to evaluate the death response to TNF in the presence of AEG40730. Cells were pretreated with AEG40730 and subsequently pulsed with TNF, without cycloheximide. Parental HCT116 cells were not responsive to TNF (Figure 1B), however when treated with AEG40730, the parental line became sensitized to TNF-induced apoptosis (Figure 3B). Interestingly, XIAP-deficient HCT116 cells did not die following incubation with TNF alone (Figure 1B), but were also further sensitized to TNF-induced killing by pre-treatment with AEG40730 (Figure 3B), similar to the effects observed with combined treatment of AEG40730 and TRAIL. In both cases overexpression of XIAP appeared to confer a significant degree of protection against cell death by these ligands. Taken together, our data suggest that under these conditions, TNF-induced death is potentiated by degradation of c-IAPs and XIAP following treatment with AEG40730. While XIAP was found to contribute to this potentiation, XIAP deficiency alone was not sufficient to result in cell death by TNF (Figure 3B and Figure 1B).
Domains within XIAP required for downregulation by AEG40730
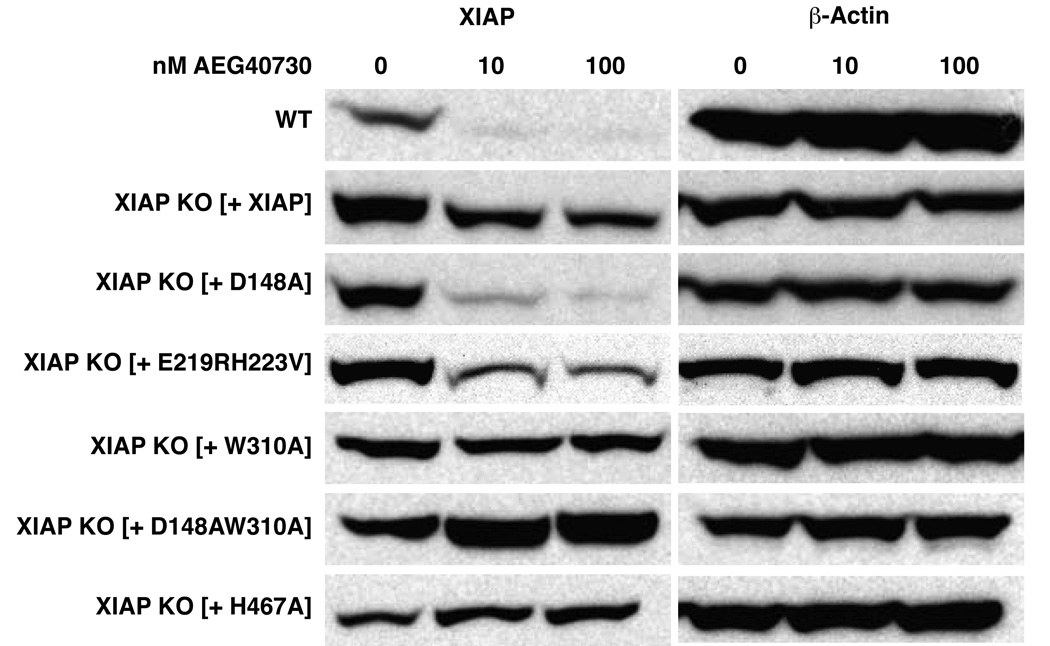
Most synthetic IAP antagonists are modeled after the N-terminal tetrapeptide of the IAP binding motif in Smac/DIABLO, which binds to the BIR3 domains of all the IAPs and several of these have recently been shown to trigger the degradation of c-IAP1 and c-IAP2 [24–26]. To further explore the mechanism of XIAP inhibition by AEG40730, we reconstituted XIAP-deficient cells with wild type XIAP or with XIAP derivatives bearing point mutations in the domains previously shown to be necessary for caspase inhibition and Smac binding. Cells were pre-incubated with AEG40730 at a range of concentrations and evaluated for changes in XIAP protein levels by immunoblotting. XIAP was degraded as a consequence of AEG40730 treatment in both the parental cells and the XIAP-deficient line in which XIAP had been reconstituted (Figure 4), although the reduction appeared less pronounced in the latter, likely due to the relative high expression of the XIAP protein in these cells. As shown in Figure 4, AEG40730 treatment exerted a similar effect in cells reconstituted with an XIAP mutant encoding a protein incapable of inhibiting caspases-3 and -7 (D148A) or (E219RH223V), but interestingly, were unable to induce the degradation of a version of XIAP (W310A) that is incapable of binding Caspase-9, which presumably interferes with the binding site in BIR3 to which AEG40730 also binds. Under the same conditions (Figure 4), drug treatment of cells reconstituted with a doubly deficient XIAP derivative (D148AW310A) also did not trigger degradation. Interestingly, AEG40730 was unable to induce degradation of XIAP in cells reconstituted with a mutant version of XIAP lacking its E3 ubiquitin ligase activity (H467A). Taken together, these data suggest that XIAP is degraded by AEG40730 upon binding to BIR3 and that this degradation is likely mediated by a process involving autoubiquitination.
Figure 4.
AEG40730 induces RING-dependent degradation upon BIR3 binding. HCT116 wt and XIAP-null cells reconstituted with wild type XIAP or the indicated XIAP derivatives were treated with vehicle control (DMSO), 10 or 100 nM AEG40730 for 24 h. Lysates of all cell lines were resolved by SDS-PAGE and immunoblotted with an antibody against XIAP or β-actin, as indicated. A representative western blot of at least three independent experiments is shown.
AEG40730 overcomes TRAIL resistance in cells overexpressing Bcl-xL or lacking BAX
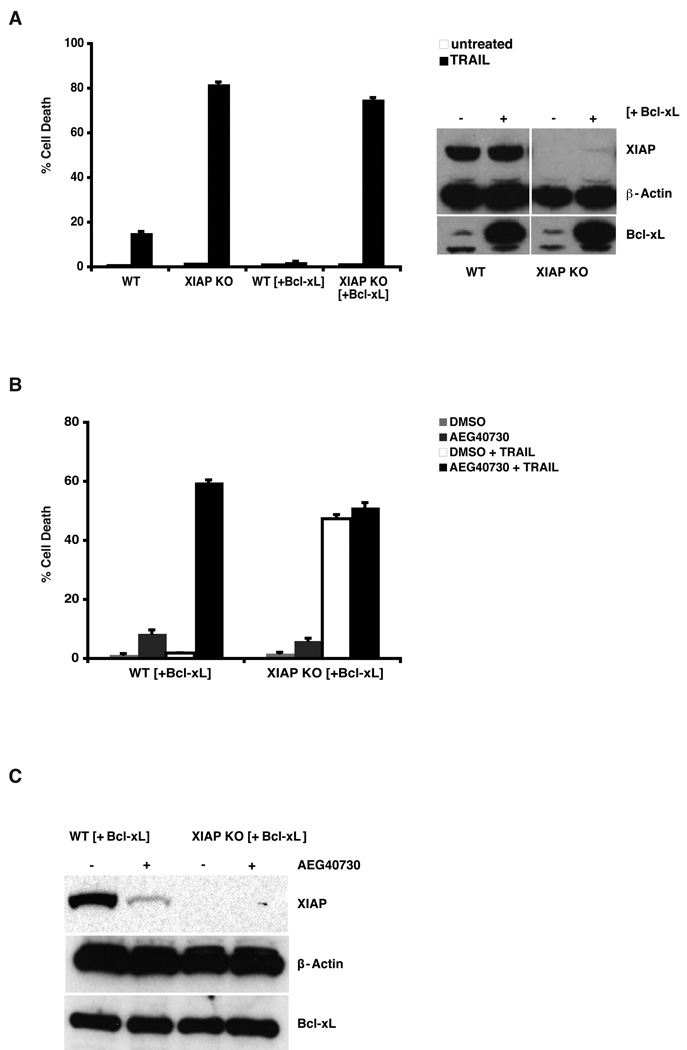
The pathological elevation of the BCL2 gene family in many cancers has been shown to cause a loss in chemosensitivity [31], predominantly through interference with BH3-mediated activation of mitochondrial death pathways, involving Caspase-9. To examine whether AEG40730 can restore sensitivity to TRAIL under conditions of elevated Bcl-xL, we introduced Bcl-xL by lentiviral infection into parental and XIAP-deficient cells, and examined sensitivity to TRAIL. Consistent with previous reports, parental cells overexpressing Bcl-xL were greatly protected against TRAIL-induced death. However, when Bcl-xL was stably expressed in XIAP-deficient cells, TRAIL was fully active in inducing cell death, and the resultant cell death was similar to that observed in XIAP-deficient cells without Bcl-xL overexpression (Figure 5A). To examine whether AEG40730 could also reverse the observed TRAIL resistance of Bcl-xL overexpressing cells, we co-treated such cells with AEG40730 and TRAIL. Combined treatment of AEG40730 and TRAIL fully restored sensitivity to TRAIL in Bcl-xL-overexpressing cells, as depicted in Figure 5B. Notably, pretreatment with AEG40730 did not further sensitize XIAP-deficient cells overexpressing Bcl-xL to TRAIL. As shown in Figure 5C, western blotting of XIAP confirmed that AEG40730 also reduced XIAP protein levels in cells with heightened Bcl-xL expression. These data point to roles not only for the c-IAPs, but also for XIAP, in blocking TRAIL-induced apoptosis, in wild type and in Bcl-xL overexpressing cells.
Figure 5.
AEG40730 sensitizes Bcl-xL overexpressing parental cells to TRAIL. (A) Effects of TRAIL stimulation on cells stably overexpressing Bcl-xL. The indicated cell lines were stimulated with TRAIL as described in the legend to Figure 3, and viability was evaluated by propidium iodide exclusion. Right panel: Cell lysates were evaluated by immunoblotting with XIAP, Bcl-xL and β-actin antibodies, as indicated. (B) A combined treatment of AEG40730 and TRAIL on stably overexpressing Bcl-xL wt HCT116 and XIAP null HCT116 cells was performed by pre-incubating the cells for 24 h with AEG40730 (10 nM) followed by a 2 h stimulation of TRAIL (75 ng/mL). After a 24 h recovery period, cell death was assessed as described above. (C) The indicated cell lines were either left untreated or incubated with 10 nM AEG40730. Whole cell lysates (50 µg) were resolved by SDS-PAGE and immunoblotted using antibodies to XIAP, Bcl-xL and β-actin, as indicated.
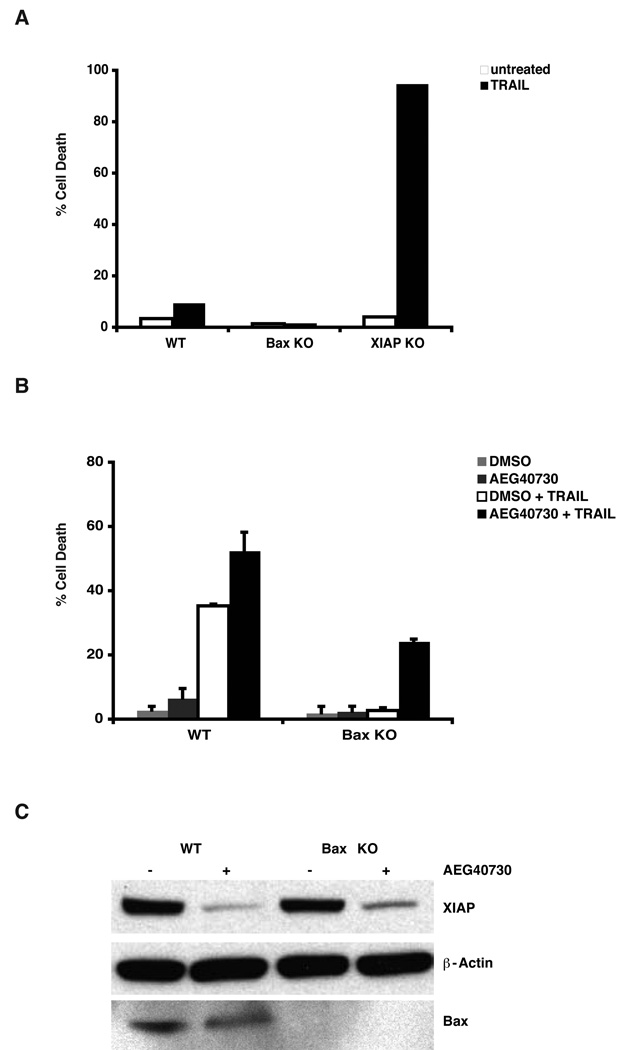
Ectopic expression of mature Smac/DIABLO and treatment with Smac antagonists have been shown to restore TRAIL sensitivity in cells that are deficient in the BAX gene [32]. To explore whether AEG40730 might be a useful means of restoring TRAIL responsiveness, in this common resistance phenotype, HCT116 cells deficient for BAX were tested for sensitivity to AEG40730, either alone or in combination with TRAIL. AEG40730 alone, did not induce cell death in BAX-deficient cells (Figure 6B) and BAX-deficient cells were fully resistant to TRAIL-induced death (Figure 6A). However, treatment of BAX-deficient cells with AEG40730 resulted in the reversal of resistance to TRAIL (Figure 6B), likely by attenuating IAP protein levels in these cells (Figure 6C). AEG40730 alone, although causing XIAP protein reduction, did not induce cell death in BAX-deficient cells (Figure 6B). Taken together, these data suggest that AEG40730 can override the cytoprotective effects of elevated expression levels of Bcl-xL, or conversely reduced levels of BAX.
Figure 6.
AEG40730 sensitizes BAX-deficient HCT116 cells to TRAIL. (A) Parental, BAX-deficient and XIAP-null cells were stimulated for 2 h with TRAIL (75 ng/mL). Cells were maintained in fresh media for a further 48 h, and viability examined as described above. Each experiment was performed in triplicate, and data are representative of at least three independent experiments. (B) Parental and BAX-deficient HCT116 cells were pre-incubated for 24 h with AEG40730 (10 nM) and subsequently stimulated with TRAIL (75 ng/mL, 2 h). (C) Parental and BAX-deficient HCT116 cells were left untreated or treated with 10 nM AEG40730 (10 nM) for 24 h. Whole cell lysates (15 µg) were resolved by SDS-PAGE and immunoblotted with XIAP, BAX and β-actin antibodies, as indicated.
DISCUSSION
Acquired resistance to apoptotic stimuli is a hallmark of many neoplastic cells [33], and a major goal of chemotherapeutic strategies is to target and reactivate this pathway. Gaining a better understanding of how cancer cells evade apoptosis and become resistant to chemotherapy is essential for drug design and the development of novel treatment approaches. XIAP has been found to be overexpressed in many cancers, including colon cancer [17–19]. Antagonists or inhibitors of the IAP related proteins may serve as new tools, or indeed exploratory therapeutics [34], and several XIAP antagonists and SMAC mimetics [34, 35] have been described and are advancing into clinical development. One remarkable effect of this class of molecules is their dramatic sensitization of cancer cells to death receptor ligands, such as TNF. The IAP antagonist AEG40730 differs in several structural aspects from the published cases, it binds with low nanomolar affinity to the BIR3 domains of c-IAP1 and 2, as well as XIAP. Treatment of cells with AEG40730 also triggers the profound degradation of c-IAP proteins (Figure 2 and data not shown), as well as XIAP.
Here we investigated the pro-apoptotic effects of the IAP antagonist AEG40730 in HCT116 cells when co-treated with TRAIL. The data presented here indicate that AEG40730 greatly potentiates TRAIL-mediated apoptosis in this system (Figure 2B and 3A). Incubation with AEG40730 result in a marked reduction in c-IAP1 protein levels (Figure 2A), but since c-IAP1 levels were equally decreased in XIAP knockout cells, we interpret these data to indicate that potentiation of TRAIL-mediated cell death by AEG40730 is also dependent on its ability to neutralize XIAP. Recent reports have demonstrated the ability of IAP antagonists to induce apoptosis in a subset of sensitive cancer cells, without the need of costimulators such as TRAIL, TNF or other chemotherapeutic agents [24–26]. It was shown that this occurs through a TNF-mediated autocrine secretion mechanism, acting upon TNF receptors, which required the initial degradation of c-IAP1 and c-IAP2 by IAP-inhibiting molecules to induce apoptotic cell death mediated by Caspase-8. Here we compared the contribution of this process between TNF- and TRAIL-mediated killing, with a particular focus on the role played by XIAP. Our data suggest that the pro-apoptotic effect of AEG40730 in TRAIL-mediated death was dependent on the targeting not only of the c-IAPs, but also of XIAP. HCT116 cells were not responsive to TNF alone, but pretreatment with AEG40730 resulted in dramatic sensitization to TNF-mediated death (Figure 3B). XIAP-deficient cells were also further sensitized to TNF by AEG40730, indicating that the sensitization by the drug to this stimulus is likely to be more dependent on the elimination of other IAPs, such as the c-IAPs. The overexpression of XIAP in XIAP-null cells treated with AEG40730 partially rescued cells from TNF-mediated death (Figure 3B), consistent with its role as a potent inhibitor of Caspase-3.
The observed involvement of XIAP in the pro-apoptotic function of AEG40730 in TRAIL-mediated death was further underscored by our finding that HCT116 wt cells with defects in the mitochondrial apoptotic pathway (BAX-null or Bcl-xL overexpression) were sensitized to TRAIL when co-treated with AEG40730 (Figure 5B and 6B). Cell death in these cells was comparable to that observed in XIAP-deficient HCT116 cells or the same cells in which Bcl-xL was ectopically expressed, treated with TRAIL alone or in combination with AEG40730 (Figure 5A and 5B). This confirms that XIAP contributed to TRAIL resistance in cells with impairment in activation of the intrinsic, mitochondrial apoptotic pathway. Taken together, these data suggest that AEG40730 can override the cytoprotective effects of heightened levels of Bcl-2 and Bcl-xL, or conversely, reduced levels of pro-apoptotic members of the Bcl-2 family, such as BAX, that are found in many neoplastic diseases. Since the neutralization of XIAP to circumvent intrinsic mitochondrial death signaling defects has been shown to restore TRAIL sensitivity and to bypass the ability of the tumor cell to evade immunotherapy and immune surveillance [31], the combination of cancer-selective drugs, such as TRAIL and IAP antagonists, represents a promising approach for cancer therapy.
ACKNOWLEDGEMENTS
We are grateful to Dr. D. Baltimore for plasmids, Dr. B. Vogelstein for cell lines, Dr. J. Silke for providing the antibody to c-IAP1, and to members of the Duckett Laboratory for helpful discussions and critical reading of the manuscript. K.A.O. is a recipient of a Pre-doctoral Award funded through the Breast Cancer Research Program of the Department of Defense [W81XWH-06-1-0429] and S.G. is a recipient of a Post-doctoral Award of the Lung Immunopathology Training Grant T32 from the National Institutes of Health. This work was supported by NIH award [R01GM067827] and the Sandler Foundation Award to C.S.D. C.S.D. is a consultant for Aegera Therapeutics Inc.
The abbreviations used are
- IAP
Inhibitor of Apoptosis
- BIR
baculoviral IAP repeat
- XIAP
X-linked IAP
- c-IAP
cellular IAP
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis inducing ligand
- HCT116
colorectal carcinoma cell line
REFERENCES
- 1.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 2.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109 Suppl:S97–S107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 3.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaux DL, Haecker G, Strasser A. An evolutionary perspective on apoptosis. Cell. 1994;76:777–779. doi: 10.1016/0092-8674(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S. Apoptosis: telling cells their time is up. Curr. Biol. 1996;6:1241–1243. doi: 10.1016/s0960-9822(02)70706-9. [DOI] [PubMed] [Google Scholar]
- 6.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 7.Bucur O, Ray S, Bucur MC, Almasan A. APO2 ligand/tumor necrosis factor-related apoptosis-inducing ligand in prostate cancer therapy. Front Biosci. 2006;11:1549–1568. doi: 10.2741/1903. [DOI] [PubMed] [Google Scholar]
- 8.Hall MA, Cleveland JL. Clearing the TRAIL for cancer therapy. Cancer Cell. 2007;12:4–6. doi: 10.1016/j.ccr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Herr I, Schemmer P, Buchler MW. On the TRAIL to therapeutic intervention in liver disease. Hepatology. 2007;46:266–274. doi: 10.1002/hep.21740. [DOI] [PubMed] [Google Scholar]
- 10.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 11.Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 12.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasula SM, Ashwell JD. IAPs: What’s in a Name? Molecular Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaux DL, Silke J. IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol. 2005;6:287–297. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- 15.Deveraux QL, Stennicke HR, Salvesen GS, Reed JC. Endogenous inhibitors of caspases. J Clin Immunol. 1999;19:388–398. doi: 10.1023/a:1020502800208. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC. A single BIR domain of XIAP sufficient for inhibiting caspases. J. Biol. Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 17.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, Andreeff M, Reed JC. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 18.Parton M, Krajewski S, Smith I, Krajewska M, Archer C, Naito M, Ahern R, Reed J, Dowsett M. Coordinate expression of apoptosis-associated proteins in human breast cancer before and during chemotherapy. Clin Cancer Res. 2002;8:2100–2108. [PubMed] [Google Scholar]
- 19.Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63:6815–6824. [PubMed] [Google Scholar]
- 20.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 21.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 22.Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang Z, Alnemri ES. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J Biol Chem. 2000;275:36152–36157. doi: 10.1074/jbc.C000533200. [DOI] [PubMed] [Google Scholar]
- 24.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004;64:3006–3008. doi: 10.1158/0008-5472.can-04-0046. [DOI] [PubMed] [Google Scholar]
- 28.Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci U S A. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF- and CD40L-mediated NF-κB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 Facilitate Cancer Cell Survival by Functioning as E3 Ligases that Promote RIP1 Ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Ravi R, Fuchs EJ, Jain A, Pham V, Yoshimura K, Prouser T, Jalla S, Zhou X, Garrett-Mayer E, Kaufmann SH, Schulick RD, Pardoll DM, Bedi A. Resistance of cancers to immunologic cytotoxicity and adoptive immunotherapy via X-linked inhibitor of apoptosis protein expression and coexisting defects in mitochondrial death signaling. Cancer Res. 2006;66:1730–1739. doi: 10.1158/0008-5472.CAN-05-3377. [DOI] [PubMed] [Google Scholar]
- 32.Kandasamy K, Srinivasula SM, Alnemri ES, Thompson CB, Korsmeyer SJ, Bryant JL, Srivastava RK. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 2003;63:1712–1721. [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 34.Vucic D, Fairbrother WJ. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res. 2007;13:5995–6000. doi: 10.1158/1078-0432.CCR-07-0729. [DOI] [PubMed] [Google Scholar]
- 35.Fischer U, Schulze-Osthoff K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005;12 Suppl 1:942–961. doi: 10.1038/sj.cdd.4401556. [DOI] [PubMed] [Google Scholar]