Abstract
Placental hypoxia as a result of impaired trophoblast invasion is suggested to be involved in the pathophysiology of preeclampsia. Hypoxia is a potent stimulus for the release of adenosine, and the actions of adenosine are mediated through four adenosine receptors, A1, A2A, A2B and A3. We investigated the presence, distribution and expression of adenosine receptor subtypes in the human placenta, the expression of the adenosine receptors in placentas from pregnancies complicated by preeclampsia, small for gestational age (SGA) infants and uncomplicated pregnancies, and the effect of hypoxia on placental adenosine receptor expression. Immunofluorescent microscopy localized A1, A2A, A2B and A3 adenosine receptors to the syncytiotrophoblast, endothelial cells and myo-/fibroblasts within the human placenta. Adenosine receptor protein and message expression levels were significantly higher in placentas from preeclamptic pregnancies with or without SGA infants, but not different in pregnancies with SGA infants alone. In vitro exposure of placental villous explants to hypoxia (2% oxygen) increased the expression of A2A adenosine receptor 50%. These data indicate that all four known adenosine receptors are expressed in the human placenta and adenosine receptor expression is significantly higher in pregnancies complicated by preeclampsia. These data are consistent with the hypothesis that differences in placental adenosine receptors may contribute to alterations in placental function in preeclampsia.
Introduction
Preeclampsia, a multi-systemic syndrome of pregnancy, affects 3–5% of all pregnancies and is a leading cause of fetal and maternal morbidity, iatrogenic prematurity and intrauterine growth restriction [1, 2]. Several changes in placental morphology and function have been described in pregnancies complicated by preeclampsia and fetal growth restriction in the absence of preeclampsia [3, 4]. The mechanisms associated with these alterations are not well understood, however placental hypoxia as a result of impaired trophoblast invasion is implicated in both conditions [5]. Studies indicate that several signals including adenosine are produced in response to hypoxia. Adenosine concentrations are higher in women with preeclampsia and in women with growth-restricted infants in the third trimester of pregnancy [6, 7].
Adenosine, a metabolite of adenine nucleotides, is produced in several tissues, including placenta, in response to hypoxia, ischemia and inflammation [8, 9]. Functional attributes of adenosine include regulation of vascular tone, [10] promotion of angiogenesis, [11] proliferation, [12] inflammation [13] and protection against oxidative stress [9, 14]. The physiological effects of adenosine are mediated via specific adenosine receptors [15]. The adenosine receptor family belongs to the category or purinergic P1 receptors and includes four gene products, A1, A2A, A2B and A3, identified by pharmacological, biochemical, and molecular biological studies [16, 17].
Pharmacological studies have demonstrated adenosine receptors in human placenta. In these studies A2 receptors were present in human placenta and chorionic vessels [10, 18]. A recent report that studied adenosine transport in uncomplicated and preeclamptic pregnancies identified and described functional A2A and A2B receptors in placental microvascular endothelium by Western blot and PCR [19]. However, to date complete descriptions of the presence and distribution of all four known adenosine receptor subtypes in the human placenta is lacking. Moreover, little is known about the expression of these receptor subtypes in uncomplicated pregnancies versus pregnancies complicated with placental hypoxic pathologies, such as preeclampsia and SGA.
The objectives of the current study were first to demonstrate the presence and distribution of the A1, A2A, A2B and A3 adenosine receptors in term human placenta using western blot analysis, real time RT-PCR, and immunofluorescent microscopy and second to compare the expression of these receptors in placentas of uncomplicated pregnancies and pregnancies complicated by preeclampsia or small for gestational age infants. Finally, we addressed the affect of hypoxia on adenosine receptor expression, using an in vitro placental villous explants model.
Materials and Methods
Placenta collection and processing
Placentas from uncomplicated or complicated pregnancies delivered by vaginal or cesarean section were obtained within 10 min of delivery. Biopsies were collected from the maternal side of the placenta, after removal of the decidua, from a central part of cotyledons between the umbilical cord insertion site and the peripheral edge of the placenta that was free of infarcts. The University of Pittsburgh Institutional Review Board approved the study and informed written consent was obtained from each patient. For studies involving analysis of placental proteins, biopsies were flash frozen in liquid nitrogen and stored at −80°C until use. For the preparation of placental villous explants, placental villous tissue was excised and transported in sterile PBS to the laboratory at room temperature. Tissue for immunohistochemistry was fixed in OCT and stored at −80°C.
Preeclampsia (PE) was diagnosed by the presence of gestational hypertension and proteinuria beginning after the 20th week of pregnancy with resolution of clinical symptoms postpartum. Gestational hypertension was defined as an absolute blood pressure ≥ 140mmHg systolic and/or ≥90mmHg diastolic after 20 weeks of gestation. Proteinuria was defined as ≥ 300 mg per 24-hour urine collection, ≥ 2+ protein on voided urine sample, ≥ 1+ protein on catheterized urine specimen, or a protein-creatinine ratio of ≥ 0.3. Small for gestational age (SGA) infants were defined by infant birth weight ≤ 10th centile for gestational age, after adjusting for race and gender, in an otherwise uncomplicated, normotensive pregnancy based on data from over 10,000 deliveries at Magee-Womens Hospital, Pittsburgh, PA USA. SGA infants with clinical or pathological evidence of chronic intrauterine infection or chromosomal abnormalities were excluded from the study. The clinical and demographic data for the uncomplicated and complicated pregnant subjects in this study are presented in the Table. Maternal age, maternal pre-pregnancy body-mass index, maternal race and parity were not statistically different between the groups of pregnant women (Table). Importantly, the number of placentas obtained by cesarean section, the number of women that were induced (p=0.45) and the number of women in labor (p=0.49) were not different between groups. By definition, women with PE had significantly higher systolic and diastolic blood pressures at delivery compared to the other pregnant study groups. As is typical, the average gestational age at delivery was significantly earlier in the women with PE & SGA compared to the other study groups. Infant birth weights and birth weight percentiles were lower in the PE & SGA group and the SGA group without preeclampsia compared to the infants of NP women or women with PE only.
Table.
Patient demographics and pregnancy information.
| Uncomplicated pregnancies (N=6) | Preeclampsia (N=6) | Preeclampsia with SGA (N=6) | Small for gestational age only (N=6) | |
|---|---|---|---|---|
| Maternal Age (years) | 22.3 ± 2.2 | 22.5 ± 7.2 | 24.7 ± 5.8 | 25.8 ± 5.6 |
| Maternal BMI (kg/m2) | 24.1 ± 4.5 | 25.7 ± 4.3 | 25.2 ± 3.3 | 22.6 ± 2.8 |
| Maternal Race (B and other/W) | 2/4 | 1/5 | 2/4 | 1/5 |
| Nulliparous/Total | 6/6 | 6/6 | 6/6 | 5/6 |
| Gestational weeks at delivery | 39.5 ± 1.3 | 38.0 ± 3.7 | 32.4 ± 3.4#** | 36.6 ± 3.4 |
| Blood pressure at delivery | 126.2 ± 9.0/74.0 ± 5.0 | 147.3 ± 9.2/90.5 ± 6.1# | 156.2 ±11.2 98.7 ± 13.5# | 108.6 ± 11.4/64.8 ± 12.0* ** |
| Cesarean section/Total | 2/6 | 5/6 | 5/6 | 3/6 |
| Labor/Total | 5/6 | 5/6 | 4/6 | 6/6 |
| Birth weight (g) | 3341.3 ± 293.5 | 3032.3 ± 1242.7 | 1378.7 ± 546.5#** | 1824.0 ± 645.4# |
| Birth weight percentile | 57.1 ± 29.7 | 41.5 ± 42.7 | 7.2 ± 1.4# | 1.9 ± 1.3# |
Data are mean ± SD,
p<0.05 vs. NP;
p<0.05 vs. PE, and
p<0.05 vs. PE with SGA.
Immunohistochemistry
The distribution of adenosine receptors was determined using immunofluorescent staining (A1:A-268, A2A: A-269 A3: A4229, all rabbit, Sigma, St. Louis, MI; A2B: goat, sc-7505, Santa Cruz, CA). Transverse sections, 7μm thick, were cut at −20°C with a microtome. Before staining, sections were fixed by incubation in −20°C acetone for 10 min. The sections were then carefully rinsed with phosphate buffered saline (PBS) and incubated with Super Block solution (ScyTek, UT, USA) for 5 min. The sections were incubated for 1 h at room temperature with the primary antibody specific to adenosine receptor A1 and A2A, whereas sections with A2B and A3 antibody were incubated overnight at 37°C. Dilution used for primary antibodies was 1:50. Immunoreactivity was visualized with Alexa 568-conjugated secondary antibody (Invitrogen, 11036, goat, 1:100 dilution). The sections were mounted in mounting medium containing DAPI (Vector, CA, USA). Specificity of the antibody staining was determined with non-immune sera and secondary antibody coupled directly to fluorescein complex. Immunoreactive cells were examined and photographed under fluorescent illumination in a photomicroscope (Zeiss, Axioscop 40). Co-localization of A1, A2A, A2B and A3 receptor immunoreactivity with trophoblast (cytokeratin 18, DAKO, M7010, dilution 1:50), endothelium (CD31, BD Bioscience, 550389, dilution 1:100), and fibroblast cells (α-smooth muscle actin, Abcam ab7817, dilution 1:100) were also determined. Immunoreactivity was visualized with a FITC-conjugated secondary antibody (Millipore, AP124F, 1:200 dilution).
Western blot analysis
Western blot analysis was performed according to published protocols on total protein extracts of placental biopsies and villous explants [20]. Total protein was extracted by sonication (Ultrasonic Processor, Tekmar, OH; microprobe setting 70 for 30 seconds) of 10 mg of tissue in 8 volumes of 1X Laemmli buffer (50 mM Tris HCl, pH 6.8, 2% SDS, 10% Glycerol) containing 5 mM DTT, 0.5 mM phenylmethyl sulfonyl fluoride (PMSF), 1mM sodium vanadate and 1 μl per ml of protease inhibitors cocktail (Calbiochem, San Diego, CA). Thirty to fifty micrograms of proteins were separated on a SDS containing 10% polyacrylamide gel. A human striatum sample was loaded as positive control for all adenosine receptors on each gel. Incubation with specific primary antibodies (A1:A-268, A2A: A-269 A3: A4229, all rabbit, Sigma, St. Louis, MI, A2B: goat, sc-7505, Santa Cruz, CA, HIF-1α: Transduction Laboratories, Lexington, KY) to the proteins of interest were performed for one hour (A1, A2A, HIF-1α) at room temperature or overnight (A2B, A3) at 4°C. For peptide neutralization (A1, A2A, A2B and A3) 2 μg of antibody was mixed with 5 μg of corresponding blocking peptide antigen in 2 ml of blocking buffer for 4h. After incubation with primary antibodies, membranes were washed 3x in TBS-0.05%Tween20 buffer for 10 min each and incubated with a species specific secondary antibody (Promega, Madison, WI) for 30 min. Chemiluminescent detection was carried out using the CDP Star detection system as per the manufacturer's protocol (Roche, Indianapolis, IN). Membranes were exposed for different times to Kodak Bio-Max–AR film. Films were scanned and density of the proteins of interest were estimated using the software, Unscanit (Silk Scientific, Orem, UT). For the analysis of β-actin, the membranes were stripped and reprobed with anti β-actin antibody to account for protein loading variations.
RNA isolation and Real-Time RT-PCR
Total RNA was extracted from placental biopsies or placental villous explants using Trizol reagent (Invitrogen, Carlsbad, CA). In all cases, the amount of RNA was estimated spectrophotometrically at 260/280 nm and aliquots (2μg) of this RNA was fractionated on agarose/ethidium bromide gels to check RNA integrity. Using two microgram of total RNA, cDNA synthesis was carried out using a commercially available kit as per manufacturer’s instructions (Retroscript Kit, Ambion, TX).
Quantitative real-time RT-PCR assays of A1, A2A, A2B and A3 cDNA were carried out using gene-specific TaqMan probes (Applied Biosystems, Foster City, CA) in an ABI Prism 7900 Sequence Detection System (Applied Biosystems) in accordance with the manufacturer’s recommendations. Identical PCR conditions were performed using 1μL of cDNA, and normalization was achieved in all cases by comparing the gene of interest against the housekeeping genes coding for human 18S and TBP (TATA box binding protein) [21]. A sample without cDNA was subjected to this protocol as a negative control. The following TaqMan Gene expression assays (Applied Biosystems) were used for real-time RT-PCR: A1 Hs00181231_m1, A2A Hs00169123_m1, A2B Hs00386497, A3 Hs00181232_m1, 18S Hs99999901_s1 and TBP Hs00427620_m1. The PCR thermal profiles were 2 min at 50°C and for 40 cycles 10 min at 95°C, 15 sec at 95°C, 60 sec at 60°C. Real-time RT-PCR data were analyzed as percent expression relative to a striatum sample, designated the calibrator, which was included in each real-time RT-PCR run to serve as an internal standard [22]. Striatum is known to express all four adenosine receptors [23]. These data were then compared between the groups and expressed as median fold changes compared to uncomplicated pregnancies (NP; group comparisons) or compared to 21% oxygen of NP or PE pregnancies (hypoxia experiments).
Villous explants culture
Placental villous explants (1–2 mg each in size) were dissected and used for hypoxia experiments. Fifty mg of finely dissected villous tissue was placed into each well of a 24-well plate (Becton Dickinson, Franklin Lakes, NJ, USA) containing 1.0 ml of Medium 199 (Mediatech, Cellgro, Herndon, VA) supplemented with 10% Fetal Bovine Serum (FBS, Summit Technology, Ft. Collins, CO), gentamicin (0.1%, Sigma, St. Louis, MI) and penicillin-streptomycin (100 IU/ml, Sigma, St. Louis, MI). The pH was reported at the end of the incubation. Villous explants were incubated for an 18–24 h preincubation period at 37°C on an orbital shaker (60 rpm, Belly Dancer, Stovall Life Science Inc., Greensboro, NC) under standard tissue culture conditions of 5% CO2-balance room air (nonhypoxic condition, pO2 140 mmHg or 20.94% O2) in a cell culture incubator (Forma Scientific, Marietta, OH). After a medium change the plates were placed on an orbital shaker for 24 h at 37°C under either normoxic (21% O2) or reduced O2 conditions (hypoxia, pO2 20 mmHg or 2 % O2 5 % CO2-balance nitrogen, Coy hypoxic glove box, Grass Lake, MI). Villous tissue was flash frozen in liquid nitrogen and stored at −80°C until further use.
Data Quantification and Statistical Analysis
Demographic data are presented as means and standard deviations. Significant differences between groups for continuous variables were evaluated by analysis of variance. If overall significance was observed, then individual group means were compared by Fisher’s PLSD post hoc testing to adjust for multiple comparisons. Statistical analyses of categorical variables was performed using Fishers exact test. Western and PCR data are presented as median fold changes (interquartile ranges, as appropriate) compared to the group of uncomplicated pregnancies (group comparisons) or fold changes compared to control samples exposed to 21% oxygen (NP or PE pregnancies). These data were analyzed using the nonparametric Wilcoxon-signed rank test. Differences were considered significant at P<0.05.
Results
Human placenta expresses all known adenosine receptors, A1, A2A, A2B and A3
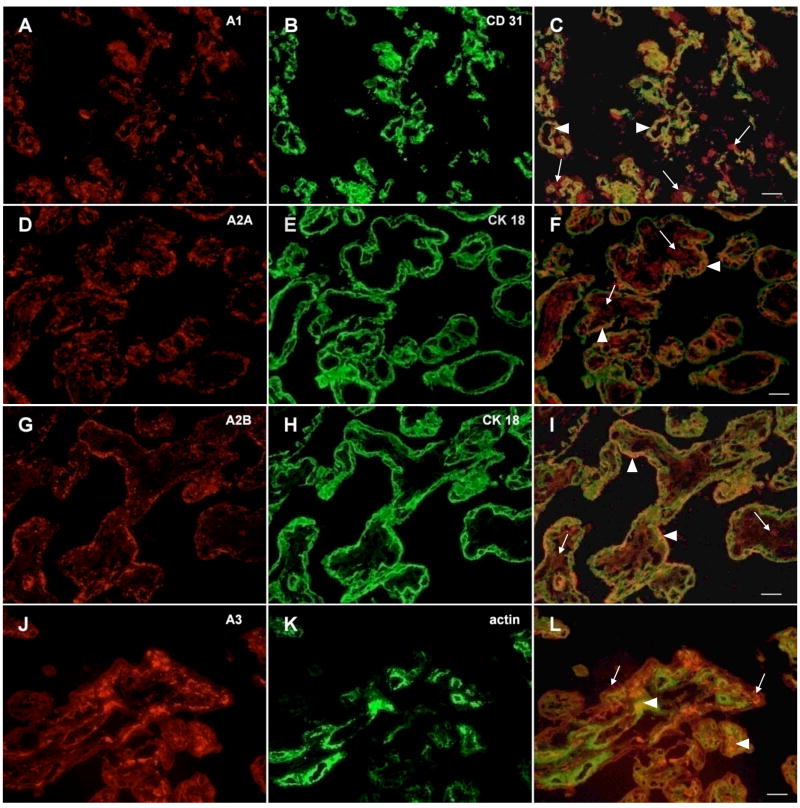
The pattern of adenosine receptor distribution was examined by immunocytochemistry of placentas from uncomplicated pregnancies. Representative immunohistochemical photographs for each receptor with a selected marker protein are shown in Figure 1. Immunostaining for the adenosine A1, A2A, A2B and A3 receptors was observed in trophoblast cells (localized by cytokeratin 18 staining), and on endothelial cells (localized by CD31 staining, Figure 1). The A1, A2A and A3 receptors were also co-localized with fibroblasts and myofibroblast surrounding the blood vessels and filling the interstitial space of the placental villous tree (localized by a-actin staining, Figure 1). However, the A2B receptor was not detected in fibroblasts or myofibroblasts.
Figure 1.
Localization of A1, A2A, A2B and A3 receptors in human placenta. Immunohistochemistry of frozen sections was performed as described in Materials and Methods. A, D, G, J) Positive fluorescent staining with an A1, A2A, A2B and A3(A-268, A-269, sc7505, A4229) adenosine receptor antibody. B) Fluorescent staining of endothelial cells with an CD31 antibody (BD 50389), E, H) of syncytiotrophoblast with cytokeratin 18 antibody (M7010) and K) of fibroblasts and myofibroblasts with an α-smooth muscle actin antibody (ab7817). Co-localization of adenosine receptors on endothelial (C), syncytiotrophoblast (F, I) or fibroblast cells (L), merged photographs. Arrows represent adenosine receptors only, arrowheads show co-localization areas. Scale bars: 20μm.
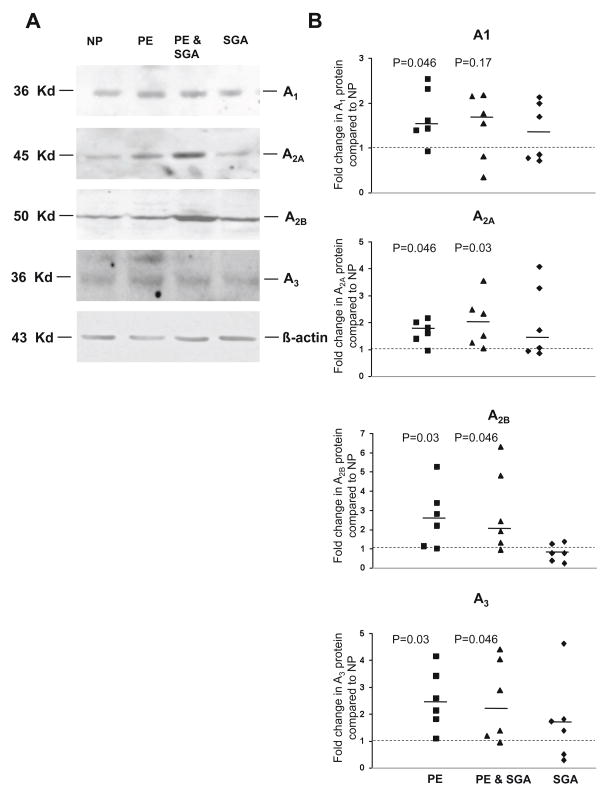
Adenosine receptor A1, A2A, A2B and A3 protein expression is higher in preeclampsia
Adenosine receptor protein was analyzed by western blot analysis on placental biopsies. The A1, A2A, A2B and A3 adenosine receptors were present in placental homogenate as verified by immunoreactive protein bands at molecular weights of 36, 45, 52 and 36 kDa, respectively (Figure 2) [24, 25]. All four adenosine receptor subtypes were significantly higher in the placentas of PE and PE & SGA when compared to uncomplicated pregnancies (NP) (Figure 2). The receptor protein concentration of A1, A2A, A2B and A3 were 1.52 fold (1.39–2.42, P=0.046), 1.71 fold (1.4–2.02, P=0.046), 2.52 fold (1.15–3.41, P=0.03) and 2.36 fold (1.81–3.42, P=0.03) higher in PE compared to NP, respectively. Subjects with PE & SGA had a 1.65 fold (0.8–2.15, P=0.17), 1.92 (1.24–2.49, P=0.03), 2.16 fold (1.32–4.8, P=0.046) and 2.13 fold (1.19–4.04, P=0.046) higher concentration of the A1, A2A, A2B and A3 adenosine receptors compared to NP, respectively. The amount of the adenosine receptors was not significantly higher in placentas of women with SGA infants without preeclampsia compared to uncomplicated pregnancies (P=0.35; 0.25; 0.42; 0.25).
Figure 2.
Adenosine receptor A1, A2A, A2B and A3 protein expression in placental biopsies of women with uncomplicated (NP), preeclamptic (PE), preeclamptic with SGA (PE & SGA) and SGA pregnancies (N=6 for each group). A) Representative western Blot of A1, A2A, A2B and A3 receptors and β-actin as loading control. B) Placentas of women with PE and PE & SGA have a higher protein expression of adenosine receptors A1, A2A, A2B and A3. Scatter plots represent fold changes compared to uncomplicated pregnancies (dashed line) after normalization to β-actin. A dashed line indicates a ratio of 1.0 and a solid line indicates the median fold change of each group. All P values refer to comparisons of data from uncomplicated pregnancies.
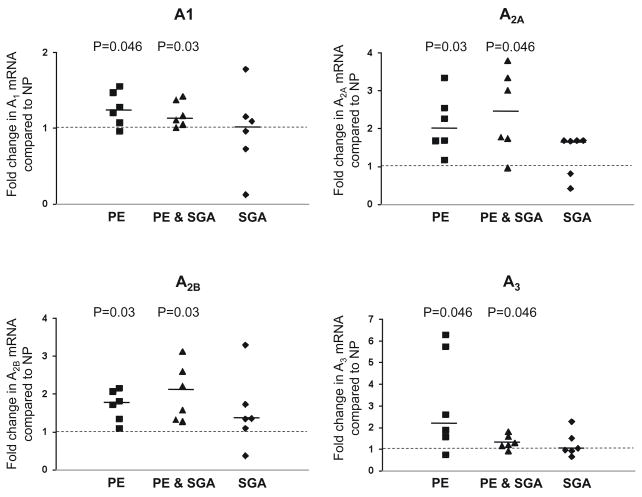
Adenosine receptor A1, A2A, A2B and A3 mRNA expression is higher in preeclampsia
To determine whether the increases in A1, A2A, A2B and A3 receptor protein were associated with an increase in mRNA encoding the A1, A2A, A2B and A3 receptor, mRNA content was determined with quantitative real-time RT-PCR of mRNA from placental biopsies (Figure 3) The expression of the adenosine receptors was normalized to the expression of two endogenous references (18S, TBP) in each sample. Results were similar whether 18S or TBP was used as endogenous control. In placentas of preeclamptic women, the expression of the A1, A2A, A2B and A3 receptors was elevated 1.25 fold (1.08–1.47, P=0.046), 1.98 fold (1.68–2.55, P=0.03), 1.77 fold (1.34–2.07, P=0.03) and 2.24 fold (1.57–5.74, P=0.046) compared to uncomplicated pregnancies, respectively. In preeclampsia with SGA, A1, A2A, A2B and A3 receptor expression was elevated 1.14 fold (1.05–1.37, P=0.03), 2.38 fold (1.73–3.33, P=0.046), 1.89 fold (1.32–2.59, P=0.03) and 1.23 fold (1.15–1.58, P=0.046). By contrast, in placentas of women with SGA infants without preeclampsia receptor expression was not significantly different from uncomplicated pregnancies (P=0.91; 0.12; 0.17; 0.75).
Figure 3.
Adenosine receptor A1, A2A, A2B and A3 mRNA expression in placentas of women with preeclamptic (PE), preeclamptic with SGA (PE & SGA) and SGA pregnancies compared to women with uncomplicated pregnancies (NP), (dashed line, N=6 for each group). Placentas of women with PE and PE & SGA have a higher mRNA expression of adenosine receptors A1, A2A, A2B and A3 compared to uncomplicated pregnancies. SGA pregnancies are not different from uncomplicated pregnancies. Scatter plots represent fold changes compared to uncomplicated pregnancies (dashed line). A dashed line indicates a ratio of 1.0 and a solid line indicates the median fold change for each group. All P values refer to comparisons of data from uncomplicated pregnancies.
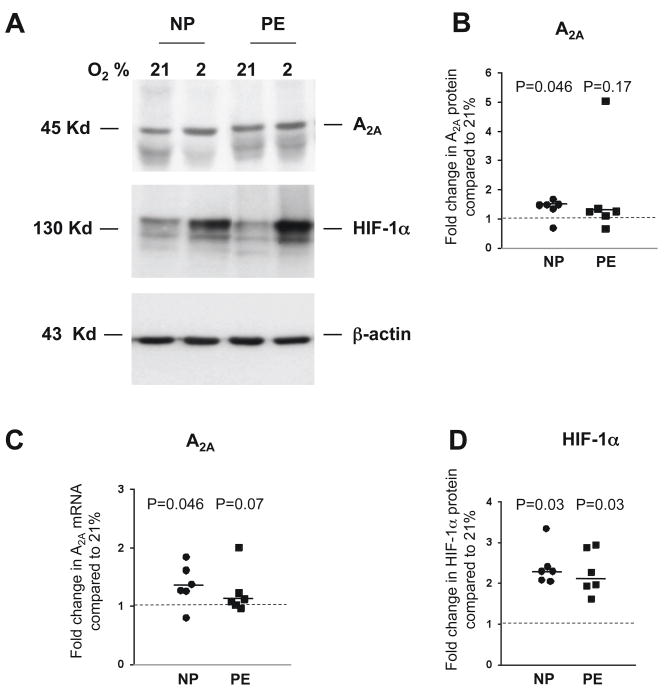
Hypoxia increases placental expression of the A2A receptor
To determine whether adenosine receptor protein and mRNA expression are influenced by hypoxia, we incubated placental villous explants for 24 h at 21% or 2% oxygen. In vitro hypoxia increased the protein amount of the A2A receptor 1.46 fold (1.33–1.48, P=0.046) and mRNA expression 1.32 fold (1.25–1.61, P=0.046) in explants from uncomplicated pregnancies compared to paired samples exposed to 21% oxygen (Figure 4A-C). Elevations in HIF-1α were measured to confirm the effect of hypoxia on placental explants. HIF-1α protein increased 2.3 fold in villous explants from uncomplicated pregnancies exposed to 2% oxygen compared to paired samples exposed to 21% oxygen (N=6, Figure 4D). Protein and mRNA for the A2A receptor were 1.25 fold (1.11–1.35, P=0.17) and 1.1 fold (1.02–1.32, P=0.07) higher in preeclamptic placentas, after exposure to 2% oxygen compared to paired explant samples exposed to 21% oxygen (Figure 4A-C). The expression of the A1, A2B and A3 adenosine receptors was not significantly affected by exposure to hypoxia (data not shown).
Figure 4.
Adenosine receptor A2A and HIF-1α expression in placental villous explants of women with uncomplicated (NP) and preeclamptic (PE) pregnancies after incubation for 24 h at 21% and 2% oxygen (N=6 for each group). A) Representative western blot of adenosine receptor A2A, HIF-1α and β-actin. B) Adenosine receptor A2A protein expression is significantly higher after exposure to hypoxia in placental villous explants of women with uncomplicated pregnancies. Values were normalized to β-actin. C) Real time RT-PCR revealed that adenosine receptor A2A mRNA expression is significantly higher after exposure to hypoxia in placental villous explants of women with uncomplicated pregnancies. Scatter plots represent fold changes compared to 21% oxygen of NP or PE pregnancies. D) Hypoxia induced HIF-1α proteins in placental villous explants from uncomplicated and preeclamptic pregnancies as assessed by western analyses. A dashed line indicates a ratio of and a solid line the median fold change of each group. All P values refer to comparisons of data from normoxia.
Discussion
In this study we provide evidence for the presence and demonstrate the cellular localization of the A1, A2A, A2B and A3 adenosine receptors in human term placenta. We also report that adenosine receptor protein concentration and mRNA expression of all four receptors are higher in placentas of pregnancies complicated by PE, but not SGA alone compared to uncomplicated pregnancies. Lastly, our results indicate that hypoxia increases placental adenosine receptor A2A expression in an in vitro placental villous explant model.
Immunohistochemistry indicates the presence of receptor subtypes in different placental cell types. All four receptors showed prominent immunoreactivity in syncytiotrophoblast cells. Co-localization studies with the endothelial cell marker CD31 demonstrated immunoreactivity for all four receptors on endothelial cells as well. The A1, A2A and A3, adenosine receptors but not A2B were also localized on fibroblasts and myofibroblast within the placental villous stroma. These findings are in agreement with previous studies of adenosine receptors in human placenta. Competitive binding studies confirmed the presence of adenosine receptors and suggested that these receptors include both high- and low-affinity subtypes consistent with subtypes of A2 receptors [26, 27]. Recent studies indicate that adenosine, a potent vasoactive nucleoside, regulates vascular tone in the feto-placental circulation via activation of A2A and A3 receptors [10, 18]. In contrast to the present study, none of these studies documented the receptor protein or mRNA but rather used pharmacological or biochemical tools [26, 28, 29]. Only one other study identified the A2A and A2B adenosine receptor by protein and mRNA expression in placental cells, specifically microvascular endothelial cells [19].
In this study we also evaluated the impact of preeclampsia with and without SGA infants and SGA pregnancies alone on placental adenosine receptor concentration. Adenosine receptor concentration and expression was significantly higher in placentas of PE women with and without SGA infants compared to uncomplicated pregnancies. Higher maternal and fetal plasma adenosine concentrations have been reported in PE, and maternal adenosine concentration correlates with the severity of the syndrome [7]. Placental adenosine receptor protein and mRNA expression was not different between uncomplicated pregnancies and pregnancies with SGA infants without PE. Both preeclampsia and fetal growth restriction are proposed to share a common pathology of deficient trophoblast invasion/spiral artery remodeling and poor placental perfusion leading to placental hypoxia. However, preeclampsia is associated with fetal growth restriction in only approximately one-third of cases. Perhaps the differences in adenosine receptor concentration in the two disorders are relevant to this difference. In contrast to our study Escudero et al. reported that mRNA and protein for the A2A receptor is reduced, whereas that for A2B is unaltered in preeclamptic placenta [19]. However, this study specifically looked at the expression in placental microvascular endothelium and not whole placental tissue which might explain these differences.
Our finding of adenosine receptors on endothelial cells, may suggest a role for adenosine in the regulation of angiogenesis and vascular tone in the placenta. In other tissues, evidence suggests that adenosine, a well-characterized pro-angiogenic nucleoside, may increase tissue oxygenation by stimulating the growth of blood vessels. The four adenosine receptors are reported to be involved in the angiogenic actions of adenosine on endothelial cells, smooth muscle, fibroblasts, monocytes, macrophages and mast cells [30, 31]. The impaired invasion and failed vascular remodeling of the extravillous trophoblast cells in preeclampsia is thought to result in reduced, and possibly oscillating, blood flow to the developing placenta, contributing to intermittent hypoxia, oxidative stress and placental damage [32]. In vitro hypoxia decreases cytotrophoblast invasiveness [33, 34]. Perhaps increased adenosine and adenosine receptors are an adaptive response to attempt to overcome the resulting reduced perfusion. Interestingly our data indicate that this response, at least as it relates to adenosine receptor concentration, is not present in SGA pregnancies.
Our finding of the presence of adenosine receptors on the syncytiotrophoblast may indicate a role in the regulation of nutrient exchange between mother and fetus. The transport of nutrients across the syncytiotrophoblast cell membranes is an important step in the regulation of fetal growth Treatment of pregnant mice with an adenosine A2A receptor inhibitor in first or second trimester reduced embryonic arterial blood flow and fetal growth [35]. The activity and function of system A amino acid transporter, one major player in transport of essential amino acids to the fetus, has been shown to be reduced by hypoxia in cultured term human trophoblast [36]. However, high concentrations of adenosine returned the net transport of glutamate and aspartate toward baseline levels in a blood brain barrier model [37]. Adenosine also induced system A amino acid transport in cultured rat hepatocytes. In the kidney, extracellular adenosine is an autocrine/paracrine modulator of several physiological functions including sodium excretion [38]. It has been proposed that the adenosine A1 and A2A receptors regulate at least in part active sodium transport along the nephron by Na+/K+ ATPase [39, 40]. We propose that these mechanisms seen in other tissues may also be present in the placenta. Consistent with this, we have found that in placentas from SGA infants from mothers without PE in which adenosine receptors were not increased in our studies there was reduced uptake by system A amino acid transport in vitro [41]. However, in placentas from PE pregnancies with SGA infants in which adenosine receptors are increased, system A uptake was normal [41].
We further investigated the effect of hypoxia on placental adenosine receptor expression and found that hypoxia increased adenosine A2A receptor protein and mRNA expression levels in healthy term placenta. In contrast, hypoxia had no effect on A1, A2B and A3 receptor expression. The fact, that adenosine A2A receptor protein and mRNA were not statistically different after hypoxia exposure may possibly be attributed to the fact that in PE the compromised tissue is not able to respond to hypoxia by up-regulating hypoxia-dependent genes under in vitro conditions to the same extent as tissue from uncomplicated pregnancies [42]. However, dissection of villous explants from placental biopsies results in mainly intact syncytiotrophoblast cell interactions but in a removal of blood vessels with endothelial cells. Since adenosine receptor expression was shown on endothelial cells in several other studies, removal of these cells may change the overall ability of the tissue to adapt to hypoxia under in vitro conditions. In addition, the small sample size of the preeclampsia group (n=6) may also be a contributing factor to the lack of statistically significant differences in the adenosine A2A receptor protein and mRNA in response to hypoxia.
Hypoxia inducible transcription factors, e.g. HIF-1α, are major transducers of hypoxia mediated gene expression. Increased HIF-1α protein expression in NP and PE villous explants proved the activation of hypoxic pathways in our model [20]. Since HIF-1α protein levels in PE under normoxic conditions are higher than in NP the ratio of hypoxic/normoxic HIF-1α levels is lower in PE. In contrast to our results, a previous study found increased A3 receptor mRNA and protein expression in villous explants under hypoxic tissue culture conditions. However, in this study explants were incubated for 5 days instead of 1 day [43]. It was reported that hypoxia increased expression of A2A receptors in rat PC12 pheochromocytoma cells [44]. It should be noted, however, that hypoxia does not always up-regulate A2A receptors. Feokistov et al. observed that reduced oxygen concentrations modulate the expression of adenosine receptors in human endothelial and smooth muscle cells towards an A2B phenotype [11]. It is possible, therefore, that the effects of hypoxia on the expression of A2A adenosine receptors can be cell- and tissue- specific. While hypoxia has been shown to be highly involved in the regulation of A2A receptor expression in several tissues other factors that are involved in the pathophysiology of PE, such as inflammation might also play a role in the up-regulation of placental adenosine receptors. Therefore, we hypothesize that hypoxia might be the dominant factor for A2A receptor expression while factors such as inflammation are more important for the regulation of the A1, A2B, and A3 receptors.
The physiologic relevance of data obtained from in vitro studies should be interpreted with caution. First we used the term “normoxia” to refer to the standard experimental conditions used in vitro (21% O2). Oxygen concentrations are considerably lower in vivo, depending on the localization of cells within the placenta and the gestational age when the tissue was obtained. Normal oxygen tension in the intervillous space of human placentas at term is believed to be 45–50 mmHg (8% O2) [45]. Furthermore, our cohort is limited in sample size, and our two groups with SGA infants were compiled according to available birth weight data and no information about the Doppler status or the symmetry of growth was available. However, the severe degree of growth restriction (1.9 percentile in the SGA without preeclampsia) indicates that in this group the infants were more likely to be growth restricted. Villous explants studies are limited by the in vitro nature of the approach and may not adequately represent what occurs in an in vivo system. Lastly, while the differences in placental adenosine receptors between preeclampsia and uncomplicated pregnancies is intriguing, since these biopsies were collected during the clinical manifestations of the syndrome it is impossible to make any conclusions regarding the relevance of these differences in relation to the pathogenesis of preeclampsia.
In conclusion, we have demonstrated the presence and distribution of adenosine receptors in human term placentas and their differential expression profiles with the complication of pregnancy, PE and SGA. We further linked hypoxia to alterations in certain adenosine receptors. Our localization findings suggest possible roles for adenosine in the regulation of placental functions such as nutrient transport and placental hemodynamic control. As the placenta relies entirely upon circulating and locally produced vasoactive substances for vascular control the detection of vasoactive adenosine and its receptors within placental blood vessels is likely of physiological significance. Further functional studies are warranted to increase our understanding of the complex molecular regulation underlying the actions of adenosine in the placenta.
Acknowledgments
This project was supported by National Institutes of Health grant number P01-HD30367, Preeclampsia Foundation Vision Grant, Irene McLenahan Young Investigators Research Grant and German Research Foundation Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertension in Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 2.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, Farnot U, Bergsjo P, Bakketeig L, Lumbiganon P, Campodonico L, Al-Mazrou Y, Lindheimer M, Kramer M World Health Organization Antenatal Care Trial Research G. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? American Journal of Obstetrics & Gynecology. 2006;194:921–931. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 3.Benirschke K, Kaufmann P, Baergen R. Pathology of the Human Placenta. Springer; 2006. [Google Scholar]
- 4.Fox HSNJ. Pathology of the Placenta. Saunders Elsevier; 2007. [Google Scholar]
- 5.Kingdom JC, Kaufmann P. Oxygen and placental vascular development. Advances in Experimental Medicine & Biology. 1999;474:259–275. doi: 10.1007/978-1-4615-4711-2_20. [DOI] [PubMed] [Google Scholar]
- 6.Yoneyama Y, Wakatsuki M, Sawa R, Kamoi S, Takahashi H, Shin S, Kawamura T, Power GG, Araki T. Plasma adenosine concentration in appropriate- and small-for-gestational-age fetuses. American Journal of Obstetrics & Gynecology. 1994;170:684–688. doi: 10.1016/s0002-9378(94)70248-9. [DOI] [PubMed] [Google Scholar]
- 7.Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Increased plasma adenosine concentrations and the severity of preeclampsia. Obstetrics & Gynecology. 2002;100:1266–1270. doi: 10.1016/s0029-7844(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 8.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends in Immunology. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Saito H, Nishimura M, Shinano H, Makita H, Tsujino I, Shibuya E, Sato F, Miyamoto K, Kawakami Y. Plasma concentration of adenosine during normoxia and moderate hypoxia in humans. American Journal of Respiratory & Critical Care Medicine. 1999;159:1014–1018. doi: 10.1164/ajrccm.159.3.9803100. [DOI] [PubMed] [Google Scholar]
- 10.Sobrevia L, Yudilevich DL, Mann GE. Activation of A2-purinoceptors by adenosine stimulates L-arginine transport (system y+) and nitric oxide synthesis in human fetal endothelial cells. Journal of Physiology. 1997;499:135–140. doi: 10.1113/jphysiol.1997.sp021916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feoktistov I, Ryzhov S, Zhong H, Goldstein AE, Matafonov A, Zeng D, Biaggioni I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension. 2004;44:649–654. doi: 10.1161/01.HYP.0000144800.21037.a5. [DOI] [PubMed] [Google Scholar]
- 12.Grant MB, Davis MI, Caballero S, Feoktistov I, Biaggioni I, Belardinelli L. Proliferation, migration, and ERK activation in human retinal endothelial cells through A(2B) adenosine receptor stimulation. Investigative Ophthalmology & Visual Science. 2001;42:2068–2073. [PubMed] [Google Scholar]
- 13.Olah ME, Roudabush FL. Down-regulation of vascular endothelial growth factor expression after A(2A) adenosine receptor activation in PC12 pheochromocytoma cells. Journal of Pharmacology & Experimental Therapeutics. 2000;293:779–787. [PubMed] [Google Scholar]
- 14.Ajamieh HH, Candelario-Jalil E, Fernandez OS, Gerbes AL. Ischaemic and pharmacological preconditionings protect liver via adenosine and redox status following hepatic ischaemia/reperfusion in rats. Clinical Science. 2008;115:69–77. doi: 10.1042/CS20070415. [DOI] [PubMed] [Google Scholar]
- 15.Olah ME, Stiles GL. Adenosine receptor subtypes: characterization and therapeutic regulation. Annual Review of Pharmacology & Toxicology. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- 16.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- 17.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological Reviews. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 18.Donoso MV, Lopez R, Miranda R, Briones R, Huidobro-Toro JP. A2B adenosine receptor mediates human chorionic vasoconstriction and signals through arachidonic acid cascade. American Journal of Physiology - Heart & Circulatory Physiology. 2005;288:H2439–2449. doi: 10.1152/ajpheart.00548.2004. [DOI] [PubMed] [Google Scholar]
- 19.Escudero C, Sobrevia L. A hypothesis for preeclampsia: adenosine and inducible nitric oxide synthase in human placental microvascular endothelium. Placenta. 2008;29:469–483. doi: 10.1016/j.placenta.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta. 2003;24:199–208. doi: 10.1053/plac.2002.0893. [DOI] [PubMed] [Google Scholar]
- 21.Meller M, Vadachkoria S, Luthy DA, Williams MA. Evaluation of housekeeping genes in placental comparative expression studies. Placenta. 2005;26:601–607. doi: 10.1016/j.placenta.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Lacey HA, Nolan T, Greenwood SL, Glazier JD, Sibley CP. Gestational profile of Na+/H+ exchanger and Cl−/HCO3− anion exchanger mRNA expression in placenta using real-time QPCR. Placenta. 2005;26:93–98. doi: 10.1016/j.placenta.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Marala RB, Mustafa SJ. Immunological characterization of adenosine A2A receptors in human and porcine cardiovascular tissues. J Pharmacol Exp Ther. 1998;286:1051–1057. [PubMed] [Google Scholar]
- 24.Kobayashi R, Saitoh O, Nakata H. Identification of adenosine receptor subtypes expressed in the human endothelial-like ECV304 cells. Pharmacology. 2005;74:143–151. doi: 10.1159/000084547. [DOI] [PubMed] [Google Scholar]
- 25.Puffinbarger NK, Hansen KR, Resta R, Laurent AB, Knudsen TB, Madara JL, Thompson LF. Production and characterization of multiple antigenic peptide antibodies to the adenosine A2b receptor. Molecular Pharmacology. 1995;47:1126–1132. [PubMed] [Google Scholar]
- 26.Fox IH, Kurpis L. Binding characteristics of an adenosine receptor in human placenta. Journal of Biological Chemistry. 1983;258:6952–6955. [PubMed] [Google Scholar]
- 27.Schocken DD, Schneider MN. Use of multiple radioligands to characterize adenosine receptors in human placenta. Placenta. 1986;7:339–348. doi: 10.1016/s0143-4004(86)80152-7. [DOI] [PubMed] [Google Scholar]
- 28.Sexl V, Mancusi G, Baumgartner-Parzer S, Schutz W, Freissmuth M. Stimulation of human umbilical vein endothelial cell proliferation by A2-adenosine and beta 2-adrenoceptors. British Journal of Pharmacology. 1995;114:1577–1586. doi: 10.1111/j.1476-5381.1995.tb14942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zolnierowicz S, Work C, Hutchison K, Fox IH. Partial separation of platelet and placental adenosine receptors from adenosine A2-like binding protein. Mol Pharmacol. 1990;37:554–559. [PubMed] [Google Scholar]
- 30.Auchampach JA. Adenosine receptors and angiogenesis. [comment] Circulation Research. 2007;101:1075–1077. doi: 10.1161/CIRCRESAHA.107.165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark AN, Youkey R, Liu X, Jia L, Blatt R, Day YJ, Sullivan GW, Linden J, Tucker AL. A1 adenosine receptor activation promotes angiogenesis and release of VEGF from monocytes. Circulation Research. 2007;101:1130–1138. doi: 10.1161/CIRCRESAHA.107.150110. [DOI] [PubMed] [Google Scholar]
- 32.Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 33.Karumanchi SA, Lindheimer MD. Preeclampsia pathogenesis: "triple a rating"-autoantibodies and antiangiogenic factors. Hypertension. 2008;51:991–992. doi: 10.1161/HYPERTENSIONAHA.107.100735. [DOI] [PubMed] [Google Scholar]
- 34.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29 (Suppl A):S73–77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Momoi N, Tinney JP, Liu LJ, Elshershari H, Hoffmann PJ, Ralphe JC, Keller BB, Tobita K. Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth. American Journal of Physiology - Heart & Circulatory Physiology. 2008;294:H2248–2256. doi: 10.1152/ajpheart.91469.2007. [DOI] [PubMed] [Google Scholar]
- 36.Nelson DM, Smith SD, Furesz TC, Sadovsky Y, Ganapathy V, Parvin CA, Smith CH. Hypoxia reduces expression and function of system A amino acid transporters in cultured term human trophoblasts. American Journal of Physiology - Cell Physiology. 2003;284:C310–315. doi: 10.1152/ajpcell.00253.2002. [DOI] [PubMed] [Google Scholar]
- 37.Grant GA, Meno JR, Nguyen TS, Stanness KA, Janigro D, Winn RH. Adenosine-induced modulation of excitatory amino acid transport across isolated brain arterioles. Journal of Neurosurgery. 2003;98:554–560. doi: 10.3171/jns.2003.98.3.0554. [DOI] [PubMed] [Google Scholar]
- 38.Macala LJ, Hayslett JP. Basolateral and apical A1 adenosine receptors mediate sodium transport in cultured renal epithelial (A6) cells. American Journal of Physiology - Renal Physiology. 2002;283:F1216–1225. doi: 10.1152/ajprenal.00085.2002. [DOI] [PubMed] [Google Scholar]
- 39.Wengert M, Berto C, Jr, Kaufman J, Leao-Ferreira LR, Paes-de-Carvalho R, Lopes AG, Caruso-Neves C. Stimulation of the proximal tubule Na+-ATPase activity by adenosine A(2A) receptor. International Journal of Biochemistry & Cell Biology. 2005;37:155–165. doi: 10.1016/j.biocel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Caruso-Neves C, Francisco-Pedro LG, Souza LP, Chagas C, Lopes AG. Effect of adenosine on the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochimica et Biophysica Acta. 1997;1329:336–344. doi: 10.1016/s0005-2736(97)00121-1. [DOI] [PubMed] [Google Scholar]
- 41.Shibata E, Hubel CA, Powers RW, von Versen-Hoeynck F, Gammill H, Rajakumar A, Roberts JM. Placental system A amino acid transport is reduced in pregnancies with small for gestational age (SGA) infants but not in preeclampsia with SGA infants. Placenta. 2008;29:879–882. doi: 10.1016/j.placenta.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajakumar A, Jeyabalan A, Markovic N, Ness R, Gilmour C, Conrad KP. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2007;293:R766–774. doi: 10.1152/ajpregu.00097.2007. Epub 2007 May 2016. [DOI] [PubMed] [Google Scholar]
- 43.Kim YH, Hwang HS, Kim YT, Kim HS, Park YW. Modulation of matrix metalloproteinase secretion by adenosine A3 receptor in preeclamptic villous explants. Reproductive Sciences. 2008;15:939–949. doi: 10.1177/1933719108322431. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi S, Conforti L, Pun RY, Millhorn DE. Adenosine modulates hypoxia-induced responses in rat PC12 cells via the A2A receptor. Journal of Physiology. 1998;508:95–107. doi: 10.1111/j.1469-7793.1998.095br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, Huppertz B. Human placental explants in culture: approaches and assessments. Placenta. 2005;26:439–448. doi: 10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]