Abstract
Background and purpose
Over the past five years, experimental data has emerged that ischemia-induced cell death pathways may differ in males and females. Cell death in males is triggered by Poly(ADP-ribose) polymerase (PARP) activation and nuclear translocation of apoptosis-inducing factor (AIF). We have previously shown that interference with this pathway benefits males but not females after an experimental stroke. In contrast caspase activation may be the major pathway activated after ischemic injury in females. The aim of this study is to examine whether sex differences exist in caspase activation in adult mice after stroke and to determine if interference with stroke-induced caspase activation preferentially protects females.
Methods
Focal stroke was induced by reversible middle cerebral artery occlusion (MCAO; 90 minutes) in young and aging C57BL6 mice of both sexes. The pan-caspase inhibitor, quinoline-Val-Asp(Ome)-CH2-O-phenoxy (Q-VD-OPh) was administered at reperfusion. Histological outcomes was assessed 48 hours after MCAO. Separate cohorts were utilized for protein analysis of key cell death proteins including caspase-3, caspase-8, cytochrome C and Apoptosis Inducing Factor (AIF).
Results
Drug-treated female mice had significantly decreased infarct volumes and improved neurological deficits after stroke compared to vehicle-treated mice. Q-VD-OPh administration had no effect in male mice. The expression of cytochrome C and nuclear caspase-8 levels were increased in females after stroke.
Conclusions
Female mice had an early release of cytochrome C and enhanced caspase activation after MCAO. Caspase inhibition benefited females but not males. Sex differences exist in both the response to ischemic injury and the efficacy of neuroprotective agents.
Keywords: MCAO, cytochrome C, caspase, sex differences, stroke
Text
We have known for some time that the epidemiology of human stroke is sexually dimorphic until late in life, well beyond the years of reproductive senescence and menopause. The principal mammalian estrogen (17-β estradiol or E2) is neuroprotective in many types of brain injury and has been the major focus of investigation over the past several decades1-4. However, despite preclinical and observational evidence of a protective role for estrogen, recent randomized clinical trials such as the Women's Health Initiative (WHI) have failed to translate the beneficial effects of estrogen into a viable therapy for stroke prevention in post-menopausal women, as treatment with estrogen led to an unexpected increase in stroke rates5. In addition, women continue to have a decreased incidence of stroke compared to men well beyond (>20 years) the menopause, suggesting that not all the observed “female protection” is mediated by gonadal steroids6-7. Ischemic sexual dimorphism also exists in the outcome from brain injury in subjects before puberty, when estrogen is less likely to be involved8.
Over the past five years, data is emerging that suggests that experimental stroke outcomes are influenced by biological sex as well as hormone exposure and that parallel, yet divergent cell death pathways are activated after an ischemic injury. One well investigated cell death pathway is mediated by neuronal Nitric Oxide (NO). Rising levels of NO and peroxynitrite (ONOO) leads to DNA damage and the subsequent activation of the DNA repair enzyme poly ADP ribose polymerase-1 (PARP-1). Over activation of PARP consumes NAD+, leads to energy failure, PAR polymer formation, and the subsequent release of apoptosis inducing factor (AIF) from the mitochondria9-12. AIF translocates to the nucleus, and leads to caspase-independent apoptosis11 Key evidence in establishing NO toxicity/PARP-1 activation as a major cytotoxic mechanism has accumulated from exclusively male animals or mixed sex primary neuronal cell cultures12-14. It has been recently shown that this ischemic death pathway shows dramatic sexual dimorphism. Males benefit from inhibition of nNOS or PARP-1, and knockout mice are protected from ischemic injury. In contrast, interference with this pathway leads to a striking exacerbation of injury in female animals15.
Similar sex-specificity can be modeled in cell culture when background sex steroids are removed from the media. After cytotoxic challenge, programmed cell death in cortical neurons proceeded predominately via an AIF-dependent pathway in male (XY) neurons, versus a cytochrome c-dependent pathway in female (XX) neurons16. Large scale DNA fragments (50-kbp) (the biochemical hallmark of AIF-mediated cell death17 were increased in XY neurons after ONOO- exposure. In contrast, mitochondrial release of cytochrome c, caspase-3 proteolysis, and oligonucleosomal DNA fragmentation (the phenotypic hallmarks of caspase-mediated programmed cell death16 were more prominent in female neurons.
Recent work in neonatal animals has shown that females are exquisitely sensitive to caspase-mediated cell death, as caspase inhibition dramatically reduced injury after a hypoxic-ischemic insult18. Acknowledging that rates of apoptosis differ among developmental ages, it is not known whether similar sex differences in caspase-mediated cell death exist in adult animals19.
Q-VD-OPh is a novel, cell-permeable broad-spectrum caspase inhibitor which has a potent effect on suppressing caspase-induced apoptosis20. In this study we administered Q-VD-OPh to test the hypothesis that caspase-dependent cell death pathways are preferentially activated in the female brain after experimental stroke.
Materials and Methods
Animals
C57BL/6 mice were purchased from Charles River Laboratories. All experiments were performed according to National Institutes of Health guidelines for the care and use of animals in research and under protocols approved by the University of Connecticut Animal Care and Use Committee. Both young mice (9-12 weeks; 21-25 gms) and aging mice (16 months, males 35-50gms, females 26-38gms) of both sexes were utilized.
Ovariectomy and E2 treatment
In ovariectomized (Ovx) females the ovaries were surgically removed 10 days prior to MCAO as described previously 15. In E2 treated mice 17β-estradiol was delivered by subcutaneous SILASTIC capsule (0.062 inch inner diameter; 0.125 inch outer diameter) filled with 0.035 ml of 17β-estradiol (180μg/ml; Sigma) 15 in sesame oil implanted at the time of ovariectomy. Control mice were implanted with oil containing capsules filled. Serum 17β-estradiol was measured using ELISA kit (IBL HAMBURG, Hamburg, Germany).
Focal Cerebral Ischemic Model
Focal transient cerebral ischemia was induced by MCAO (0.21mm suture) for 90 minutes followed by reperfusion as described previously 15. In aging mice a larger 0.23mm silicone coated suture was utilized to achieve occlusion. Cortical perfusion was measured by Laser Doppler flowmetry (LDF, Moor Instruments Ltd, England) . Only the mice whose cerebral blood flow (CBF) dropped to less than 85% of control were included.
Behavioral Scoring
Neurological deficits were scored at 1.5h, 6h, or 48h post-stroke. The scoring system was as follows: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; and 4, no spontaneous locomotor activity or barrel rolling.
Drug administration
Q-VD-OPh (MP Biomedicals, Aurora, Ohio, USA) was dissolved in DMSO and further diluted with sterile PBS. OPh (5-20 mg/kg ) was injected intraperitoneally at reperfusion (90 minutes post-occlusion). Control mice were injected with vehicle alone.
Histological Assessment
48 hours after stroke the mice were euthanized and the brains were removed and cut into 5 2-mm slices. The slices were stained with 1.5% 2,3,5-triphenyltetrazolium solution at 37°C for 30 minutes. The stained slices were fixed with 4% formalin, images were digitalized, and the infarct volumes (corrected for edema) were analyzed using computer software (Sigmascan Pro5) as previously described 15.
Sub-cellular Fractionation
Brain samples were obtained by rapid removal of the brain, followed by the immediate removal of the cerebellum and occipital pole and olfactory/frontal pole 21. Samples were gently homogenized in Dounce homogenizers with cold lysis solution (10mM Tris-HCl pH 7.5, 5mM MgCl2, 0.1mM EDTA, 1.5mM CaCl2, 0.25mM sucrose, 1M DDT, 10% Triton X-100, 1:50 protease inhibitor cocktail). The homogenates were centrifuged at 800g for 10 min at 4°C, and the pellet (P1) contained the nuclear fraction; the supernatant was further centrifuged at 14000g for 30min and served as the cytosolic fraction. P1 was resuspended in lysis buffer and was run through a sucrose gradient composed of 1.8M and 2.3M sucrose with ultra-centrifugation at 30,000g for 45 min. The pellets (P2) were collected with Nuclei pure storage buffer (Sigma-Aldrich, St. Louis, MO), and centrifuged at 2300rpm for 10min. The pellets were resolved with Extraction Buffer (Sigma-Aldrich, St. Louis, MO) as nuclear fraction, sonicated 10 sec three times, and stored at -80°C. All samples represent pooled samples (5 stroke; 2 sham/gp). Due to the nuclear extraction process, such a small amount of nuclear protein is obtained from a single mouse, that pooling samples from each group was required
Western Blots
The fractionated protein concentration was determined by BCA™ Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL) and the protein was subjected to Western Blotting as previously described 21. Briefly sample proteins were resolved on 4% to 20% sodium dodecyl sulfate - polyacrylamide electrophoresis gels and transferred to a polyvinylidene difluoride membrane. Cytochrome C, AIF, caspase-3, -8, and -9 were detected using corresponding antibodies from Cell Signaling (1:1000). β-actin (1:1000; Sigma), β-tubulin (1:1000; Santa Cruz), and Histone H3 (1:4000; Sigma, for nuclear fraction) were used as loading controls. All blots were incubated overnight in primary antibodies at 4°C in Tris-buffered saline buffer containing 4% bovine serum albumin and 0.1% Tween 20. The secondary antibodies (goat anti-rabbit IgG 1:5000, goat anti-mouse IgG 1:2000, donkey anti-goat IgG 1:1000; Santa Cruz) were diluted and ECL detection kit (Amersham Biosciences) was used for signal detection. The densitometry of Western Blotting images was performed with computer software (Scion Image). Each sample was run in triplicate.
Statistics
Data from individual experiments were presented as Mean±SEM. One-way analysis of variance (with Turkey post-hoc correction, when appropriate) was used for the comparison of the means between the experimental groups. P<0.05 was considered statistical significant. Induction of ischemia, behavioral and histological assessments were done by an investigator blinded to drug treatment.
Results
Sex Differences in Caspase Activation
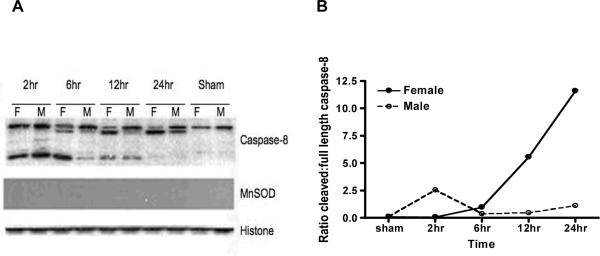
To determine if differences in caspase activation were present between male and female mice after MCAO we began by assessing levels of caspase 8 to evaluate the extrinsic pathway, and caspase 9 to assess a component of the apoptosome/intrinsic pathway22. No differences were seen in caspase 9 levels or cleavage products between male and gonadally intact female wildtype mice (data not shown) at any time point in the nuclear, cytoplasmic, or mitochondrial fractions. However, nuclear caspase-8 levels varied dramatically by gender (Figure 1). Cleaved caspase 8 (full length-57 kDa; cleaved forms- 43, 18, 10 kDa) steadily increased in the nuclear fraction in both males and females after stroke. However the cleaved form rapidly peaked in males, whereas female mice had a sustained increase in nuclear cleavage products.
Figure 1.
Caspase-8 protein levels in the nuclear fraction in young mice after stroke. A, Mice brains were subjected to fractionation at different time points after stroke, and nuclear proteins were Western-blotted for caspase-8 expression. Full length caspase-8 (57kDa), and cleaved forms (43, 18, and 10kDa) were analyzed. MnSOD was used to confirm the lack of mitochondrial contamination and Histone was used as a loading control. B. The optic density of Western Blots were measured and ratios were calculated by cleaved forms of caspase-8 vs. full length of caspase-8.
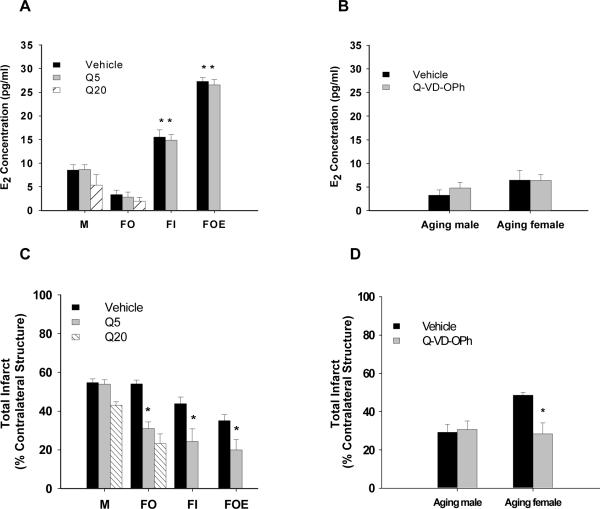
E2 levels were not significantly different between drug- and vehicle-treated mice
In adult animals, the neuroprotective effects of estrogen have been well described 1,2, To ensure that the confounding effect of estrogen was removed in our studies we subsequently examined intact, ovariectomized and E2-replaced mice in our Q-VD-OPh studies. Serum 17β-estrodial levels were equivalent within the drug- and vehicle-treated male (M), Ovx female (FO), intact female (FI), and Ovx plus E2 treated female (FOE) group. Levels in the E2-replaced FOE mice were significantly higher than those in any other group. As expected, the estrogen levels in IF were significantly higher than those in either the M or FO group (Figure 2A). E2 levels were also measured in our male and female aging mice (Figure 2B). There was no significant difference in estrogen levels, confirming loss of estrogen in aging females.
Figure 2.
Serum levels of 17β-estrodial and infarct volumes after stroke. A. E2 levels in young mice. B. There were no significant differences in E2 levels in aging mice. C. Total infarct volumes in young mice. There were no significant differences between vehicle- and drug-treated mice in the male group. There was a significant reduction in infarct in the drug-treated FO, FI, and FOE groups. D. Aging mice were treated with 10mg/kg Q-VD-OPh. There was no significant difference between vehicle- and drug-treated mice in aging male group. Drug-treated aging females had significantly decreased infarct volume than vehicle-treated females. P*<0.05, one-way analysis of variance. Q5, Q-VD-OPh 5mg/kg; Q20, Q-VD-OPh 20mg/kg.
Stroke Outcomes in Young and Aging Mice
We first administered 5mg/kg of Q-VD-OPh intraperitoneally to young (9-12 weeks old) male (M), Ovx female (FO), intact female (FI), or Ovx plus E2 treated female mice (FOE). The infarct volumes were measured after 48 hours of stroke. Drug-treated female mice had significantly decreased infarct volumes compared to vehicle-treated mice regardless of their hormone status: FO (total: vehicle 53.9±2.1%, n=6 versus drug 31.0±3.3%, n=6, P<0.05), FI (total: vehicle 43.8±3.4%, n=9 versus drug 24.4±6.4%, n=6, P<0.05), and FOE (total: vehicle 35.0±3.1%, n=7 versus drug 20.0±5.4%, n=7, P<0.05) groups. As expected, the E2-replaced and intact female mice had smaller strokes than either males or ovariectomized females (P<0.05) consistent with estrogen's known neuroprotective effects. No neuroprotective effect of QVD was seen in male animals (total: vehicle 53.9±2.2%, n=6 versus drug 54.6±2.0%, n=6, P>0.05) (Figure 2C). When comparing males to females, we found that each drug-treated female group had significantly smaller infarct volumes than drug-treated males (P<0.05, Fig 2C).
To determine whether administration of a higher dose of Q-VD-OPh could protect males and lead to a more robust decrease of infarct volumes in females, we injected a high dose (20mg/kg; ip.) of Q-VD-OPh to male and Ovx female mice. Q-VD-OPh- treated Ovx females had significantly smaller infarct volumes than vehicle treated Ovx females or males. There were no differences in infarct volumes between the low dose and the high dose Q-VD-OPh administration in males (Figure 2C) or females.
As caspase-induced cell death may be developmentally regulated, and the vast majority of clinical strokes occur in the aged, we also administered Q-VD-OPh to aging mice to determine if the sex-dependent effect of Q-VD-OPh exists in aging animals. Male and female aging mice were treated with 10mg/kg Q-VD-OPh and stroke outcome assessed at 48h. There was no significant difference in infarct volume between vehicle- and drug-treated male aging mice; however Q-VD-OPh continued to have a significant neuroprotective effect in females (total: vehicle 48.5±1.6%, n=6 versus drug 28.4±5.7%, n=6,P<0.05) (Figure 2D). Aging male mice had smaller infarcts than young male mice, yet had higher mortality and neurological deficits (Table 1).
Table 1.
Behavioral scores after stroke in low (a) and high (b) dose Q-VD-Oph treated mice
| a | 5mg/kg |
|||||||
|---|---|---|---|---|---|---|---|---|
| v-male | q-male | vfO-ovx | qfO-ovx | vf-intact | qf-intact | vfE-ovx | qfE-ovx | |
| 1.5h | 2.5±0.2 | 2.7±0.2 | 2.6±0.4 | 2.8±0.4* | 2.7±0.2 2 | 2.7±0.3* | 2.1±0.1 | 2.3±0.2* |
| 6h | 2.1±0.2 | 2.3±0.2 | 2.2±0.5 | 1.3±0.2 | 2.3±0.2 | 1.3±0.2 | 2.0±0.0 | 1.1±0.1 |
| 48h | 1.3±0.2 | 1.5±0.2 | 1.4±0.2 | 0.8±0.2 | 1.2±0.1 | 0.7±0.2 | 1.0±0.0 | 0.7±0.2 |
| b | 20mg/kg |
Aging mice (10mg/kg) |
||||
|---|---|---|---|---|---|---|
| q-male | qf-ovx | va-male | qa-male | va-female | qa-female | |
| 1.5h | 2.6±0.3 | 2.5±0.3* | 4.0±0.0 | 3.8±0.2 | 3.2±0.2 | 3.3±0.2* |
| 6h | 2.4±0.2 | 1.2±0.2 | 3.8±0.2 | 3.8±0.2 | 3.3±0.2 | 2.2±0.2 |
| 48h | 1.0±0.2 | 0.8±0.2 | 3.8±0.2 | 3.5±0.2 | 2.8±0.2 | 2.0±0.3 |
Young mice were administered either 5 mg/kg or 20 mg/kg Q-VD-OPh, and aging mcie 10 mg/kg. There were no significant differences between 1.5h and 6h post-stroke behavior in young and aging males or in young and aging vehicle-treated females. There was a significant difference between 1.5h and 6h or 48h post-stroke behavioral scores in young and aging female drug-treated mice.
P<0.05
one-way ANOVA: v, vehicle; q, Q-VD-OPh; f, female; O, oil; E, 17β-estrodial; a, aging.
Mice with LDF values of more than 15% of pre-occlusion LDF values or subarachnoid hemorrhage were excluded (15 young and 7 aging mice). Mortality within 48 hours was 6% for young and 27% for aging mice. Neurological deficits were significantly improved in drugtreated female young and aging mice (Table 1).
Cytosolic Cytochrome C Increases Early in Female Mice After Stroke
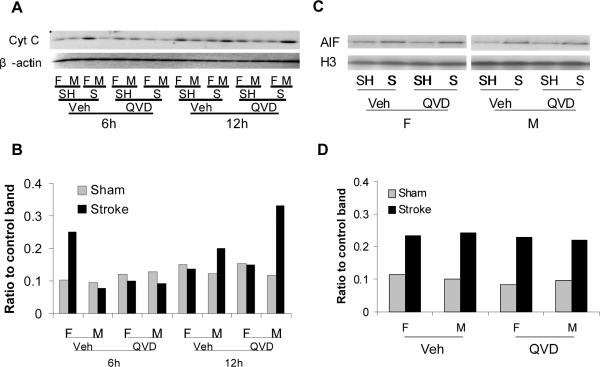
We then examined the expression of Cytochrome C in the cytosol and AIF translocation to the nucleus after stroke. The expression of cytochrome C dramatically increased in vehicle-treated female mice compared to males at 6h of stroke, and Q-VD-OPh treatment attenuated this increase. Males had increased levels of cytochrome C 12h after stroke compared to females, and the levels were higher with Q-VD-OPh treatment (Figure 3A & B). Both male and female mice had increased nuclear translocation of AIF after stroke. Treatment with Q-VD-OPh treatment had no effect on AIF translocation (Figure 3C & D).
Figure 3.
Cytochrome C and AIF expression after stroke. A & B. The expression of cytochrome C in cytosol was dramatically increased in vehicle-treated female mice compared to males at 6h of stroke, and Q-VD-OPh treatment attenuated this increase. Males had increased levels of cytochrome C at 12h of stroke compared to females, and the levels were higher with Q-VD-OPh treatment. C & D. Both male and female mice had increased expression of AIF in nuclei at 6h after stroke, which was unaffected by Q-VD-OPh administration. The optical density was expressed as the ratio of cytochrome C or AIF protein bands to control bands. F,female; M, male; SH,sham; S, stroke; Veh, vehicle; QVD, Q-VD-OPh; H3, Histone.
Q-VD-OPh Inhibited Caspase-3 Activation in Both Female and Male Mice
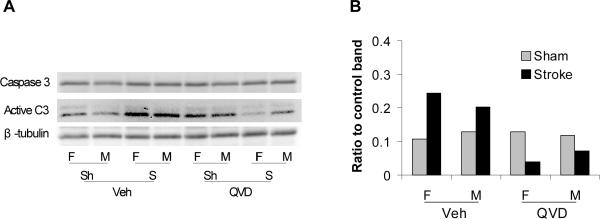
To assess the effect of Cytochrome C activation on caspase activation, we detected the expression of caspase-3, -8 and -9 with Western Blots. Expression of Active (cleaved) caspase-3 was up-regulated in the cytosol after stroke in both male and female mice. Q-VD-OPh inhibited caspase-3 cleavage in both female and male mice after stroke (Figure 4 A&B). There were no differences in caspase-8 and -9 expression in the cytosol after stroke (data not shown)
Figure 4.
Caspase-3 protein levels in cytosol at 6h of stroke. Active (cleaved) caspase-3 increased in cytosol in both male and female vehicle-treated mice after stroke, but decreased in Q-VD-OPh treated mice.
Discussion
The present study demonstrates several important findings. Firstly, mitochondrial cytochrome C release, a major triggering event for subsequent caspase activation, is higher in female mice shortly after an induced stroke. Secondly, there are sex differences in the timing and duration of caspase activation after ischemia. Cleaved caspase-8 levels increase in the nucleus in both sexes after MCAO, but this predominates in females. Caspase-3 cleavage occurs in both sexes after MCAO, although this is more pronounced in the female brain. To evaluate the functional significance of the enhanced cytochrome C-caspase activation in females, we administered a pan-caspase inhibitor to mice of both sexes. We found that Q-VD-OPh was neuroprotective in females, and had no effect in males. The activation of caspase-3 was reduced in both sexes after Q-VD-OPh treatment; this effect was more robust in females. These findings are consistent with the hypothesis that female cell death after MCAO appears to be mediated primarily by caspase activation. Thirdly, treatment with Q-VD-OPh treatment had no effect on ischemia-induced nuclear AIF translocation in either sex, demonstrating the relative specificity of this agent for inhibiting caspase-mediated cell death. Fourthly, the sexually dimorphic protective effect of Q-VD-OPh was seen in intact females, ovariectomized females and estrogen-replaced females, suggesting that its neuroprotective effect is independent of ovarian hormone availability. Finally, as caspase-mediated cell death may play a more important role in developmental cell death (ie. in neonatal and young mice), and to better model clinical stroke, we administered Q-VD-OPh to aged mice of both sexes. Aging females continued to have a dramatic neuroprotective response to caspase inhibition. Similar to our results in young males, we found no protective response in aging male mice.
Over the past several years, data has been emerging which suggests that cell death mechanisms are sexually dimorphic both in vivo and in vitro (see23 for review). In a study using cytotoxic agents to induce cell death, female neurons demonstrated greater resistance to nitrosative stress than male neurons16 yet had higher levels of cytosolic cytochrome C, an initiating event in the intrinsic caspase cascade. Additionally, male and female neurons responded differently to drugs targeting specific proteins and pathways. In cultured neurons, female derived neurons were differentially protected by z-VAD.fmk, a pan-caspase inhibitor19. In sex-selected hippocampal slices exposed to oxygen-glucose deprivation (OGD) male-derived slices were protected with nNOS inhibitors, female slices were not24. This suggests that female neurons may be differentially sensitive to caspase-induced cell death, and male neurons, nitrosative cell death.
These sex differences also have been demonstrated in vivo. Caspase-3 activation, with enhanced protein, cleavage products, and caspase activity has been seen in neonatal (P9) females compared to males after a hypoxic-ischemic (HI) episode19. In the immature brain, male neurons displayed a more pronounced translocation of AIF than females after HI. Surprisingly we saw no sex differences in nuclear AIF translocation in this study (Figure 3C&D), this may be due to differences in developmental age or the ischemic model used. Q-VD-OPh had no effect on AIF translocation in either sex. It appears that the caspase-independent AIF-mediated cell death pathway11 may not be a major contributor to cell death in females as the continued translocation of AIF (Figure 3C) did not ameliorate the protective effect of Q-VD-OPh. In contrast males showed no neuroprotective effect of Q-VD-OPh treatment, consistent with the hypothesis that the cytochrome C-caspase pathway does not play a major role in cell death in males.
Caspases play an essential role during apoptotic cell death and are synthesized as inactive zymogens that are proteolytically processed to produce mature, active proteases (for review 22,25,26). The involvement of caspases in ischemia-induced cell death has been inferred from studies demonstrating that the broad-spectrum caspase inhibitors such as zVAD.fmk are neuroprotective in animal models of ischemia 27-29. Genetic deletion of caspase-3 leads to protection after focal stroke; however, the gender of the animals used was not reported30. The literature examining the neuroprotective effects of caspase inhibition is quite mixed, with some showing protection, and others not 31. Often, the gender of the animals is not mentioned which could, as noted by our results, lead to significant variability.
The question remains as to whether these differences are secondary to enhanced caspase activation in females, or an intrinsic female sensitivity to caspase-induced cell death. Recently, it was shown that P7 female rats were dramatically protected when given the pan-caspase inhibitor Q-VD-OPh at reperfusion after a 50 min focal injury, while males showed no protection from the treatment18. Neonatal males had a large increase in cytosolic cytochrome C levels (implying its release from the mitochondria) 6-12 hrs. after reperfusion, whereas females had a gradual appearance of cytosolic cytochrome C which peaked at 12 hrs. In adult mice, a different pattern of cytochrome C release was seen. Females had an early, robust release of cytochrome C at 6 hours (Fig 3A/band 3), which was attenuated with Q-VD-OPh treatment. In contrast males had the most robust increase at 12h after stroke (Figure 2A/band 16), which was not reduced by caspase inhibition. At this relatively late time point after stroke, it is likely that other parallel cell death pathways are activated, which could be detrimental in males. These differences in the pattern of Cytochrome C release may reflect the different developmental age (neonates vs. adults) or models (global/HI vs. reperfusion/focal).
We examined Caspase-3 in our studies as it is a major downstream executioner caspase and mediates apoptosis in both the extrinsic and intrinsic apoptotic pathways 22,32. Our results revealed that the expression of cleaved caspase-3 increased 6h after stroke which was attenuated by Q-VD-OPh treatment in both male and female mice with a more robust effect in females (Figure 3A & B). The result is consistent with the early release of cytochrome C in females (Fig 1A at 6 hours). Caspase-3 is cleaved and activated by initiator caspases such as caspase-8 and -9 which are activated by the assembly of a multimetric complex (dubbed the apoptosome) involving Apaf-1 and cytochrome C 25. Surprisingly, in the present study we found no differences in caspase-8 or -9 cleavage or activation in the cytosol after stroke. However, striking sex differences were present in the nuclear activation of caspase-8, with females having a dramatic increase in cleavage products compared to males at 6h, 12h and 24h after stroke (Figure 1). Caspase-8 is thought to be the primary activator of the extrinsic, death receptor pathways of apoptosi35,35. However, caspase-8 can also induce apoptosis through the intrinsic mitochondrial pathway by cleaving the cytoplasmic factor Bid, a proapoptotic member of the Bcl-2 family34. Nuclear translocation of caspase-8 occurs after stroke in male animals, where it is able to cleave and inactivate PARP-2 35. Deletion of PARP-2-/- is protective in male mice 36, therefore the reduction in PARP-2 activity by caspase-8 could be beneficial in stroke. However, we have previously shown that deletion of the major isoform of PARP, PARP-1 is detrimental in female animals15. The role of PARP-2 in female ischemic cell death has not yet been investigated, but as nuclear levels of active caspase-8 are so much higher in females after stroke, this could lead to a differential reduction in PARP-2 activation, which analogous to the loss of PARP-1, could exacerbate injury in females. Interactions between PARP and caspase-8 is an area of current investigation.
It is widely accepted that estrogen is neuroprotective after induced stroke 1,2. As expected, FI and FOE mice had smaller histological damage than FO mice in our vehicle-treated groups (Figure 2C). Q-VD-OPh maintained its protective effect in all female groups, including aging mice. As the majority of stroke deaths in the United State now occur in women who are post-menopausal 37, this is an important point if these agents are to be developed for clinical use. As has been documented by others, aging female mice had larger infarct volumes than young female mice after stroke38 likely secondary to the loss of estrogen with aging (Table 1, Figure 2D). Aging males had significant smaller strokes compared to young males (total infarct: aging 30.7±10.8%, n=6 vs young 54.6±2.0%, n=6, P<0.01; Figure 2D), consistent with previously published studies 39,40.
In conclusion, we have shown sex differences in stroke outcomes after administration of the broad-spectrum caspase inhibitor Q-VD-OPh: Female mice have improved neurological deficits and decreased infarct volumes, whereas males do not. Females have early release of cytochrome C and caspase activation after stroke. Q-VD-OPh treatment attenuates the increase in cleaved caspase-3 in both male and female animals. These data provide evidence for parallel sex-dependent cell death pathways in the ischemic brain. These sex differences need to be further explored if we are to develop efficacious neuroprotective agents for use in stroke patients.
Acknowledgements
LDM is supported by NIH RO1 NS050505 and NS055215
Sources of Funding This work was supported by The American Heart Association (L.D.M.) and the National Institute of Neurological Disorders and Stroke (LDM NS055215).
Footnotes
Disclosures None.
References
- 1.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: An integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 2.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bushnell CD. Stroke and the female brain. Nat Clin Pract Neurol. 2008;4:22–33. doi: 10.1038/ncpneuro0686. [DOI] [PubMed] [Google Scholar]
- 4.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Genderlinked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The women's health initiative randomized controlled trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 6.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 7.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and hispanic residents of an urban community: The northern manhattan stroke study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 8.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- 9.Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N. Apoptosis-inducing factor triggered by poly(adp-ribose) polymerase and bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moroni F. Poly(adp-ribose)polymerase 1 (parp-1) and postischemic brain damage. Curr Opin Pharmacol. 2008;8:96–103. doi: 10.1016/j.coph.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(adp-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 12.Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(adp-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 14.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (adp-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 16.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 17.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 18.Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C. Specific caspase inhibitor q-vd-oph prevents neonatal stroke in p7 rat: A role for gender. J Neurochem. 2007;100:1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Sugama S, Mischak RP, Kiaei M, Bizat N, Brouillet E, Joh TH, Beal MF. A novel systemically active caspase inhibitor attenuates the toxicities of mptp, malonate, and 3np in vivo. Neurobiol Dis. 2004;17:250–259. doi: 10.1016/j.nbd.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 21.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of amp-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: Life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- 23.Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: Are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann Neurol. 2005;58:317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- 25.Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem Res. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- 26.Stefanis L. Caspase-dependent and -independent neuronal death: Two distinct pathways to neuronal injury. Neuroscientist. 2005;11:50–62. doi: 10.1177/1073858404271087. [DOI] [PubMed] [Google Scholar]
- 27.Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T, Hyman BT, Moskowitz MA. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Mouw G, Zechel JL, Zhou Y, Lust WD, Selman WR, Ratcheson RA. Caspase-9 inhibition after focal cerebral ischemia improves outcome following reversible focal ischemia. Metab Brain Dis. 2002;17:143–151. doi: 10.1023/a:1019921904378. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Colbourne F, Sun P, Zhao Z, Buchan AM, Iadecola C. Caspase inhibitors reduce neuronal injury after focal but not global cerebral ischemia in rats. Stroke. 2000;31:176–182. doi: 10.1161/01.str.31.1.176. [DOI] [PubMed] [Google Scholar]
- 30.Le DA, Wu Y, Huang Z, Matsushita K, Plesnila N, Augustinack JC, Hyman BT, Yuan J, Kuida K, Flavell RA, Moskowitz MA. Caspase activation and neuroprotection in caspase-3- deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci U S A. 2002;99:15188–15193. doi: 10.1073/pnas.232473399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loetscher H, Niederhauser O, Kemp J, Gill R. Is caspase-3 inhibition a valid therapeutic strategy in cerebral ischemia? Drug Discov Today. 2001;6:671–680. doi: 10.1016/s1359-6446(01)01826-8. [DOI] [PubMed] [Google Scholar]
- 32.Troy CM, Salvesen GS. Caspases on the brain. J Neurosci Res. 2002;69:145–150. doi: 10.1002/jnr.10294. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez I, Xu CJ, Juo P, Kakizaka A, Blenis J, Yuan J. Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron. 1999;22:623–633. doi: 10.1016/s0896-6273(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of bid by caspase 8 mediates the mitochondrial damage in the fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 35.Benchoua A, Couriaud C, Guegan C, Tartier L, Couvert P, Friocourt G, Chelly J, Menissier-de Murcia J, Onteniente B. Active caspase-8 translocates into the nucleus of apoptotic cells to inactivate poly(adp-ribose) polymerase-2. J Biol Chem. 2002;277:34217–34222. doi: 10.1074/jbc.M203941200. [DOI] [PubMed] [Google Scholar]
- 36.Kofler J, Otsuka T, Zhang Z, Noppens R, Grafe MR, Koh DW, Dawson VL, de Murcia JM, Hurn PD, Traystman RJ. Differential effect of parp-2 deletion on brain injury after focal and global cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:135–141. doi: 10.1038/sj.jcbfm.9600173. [DOI] [PubMed] [Google Scholar]
- 37.Website. AHA Statistics for 2005
- 38.DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL. Early disruptions of the bloodbrain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging. 2008;29:753–764. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petcu EB, Sfredel V, Platt D, Herndon JG, Kessler C, Popa-Wagner A. Cellular and molecular events underlying the dysregulated response of the aged brain to stroke: A mini-review. Gerontology. 2008;54:6–17. doi: 10.1159/000112845. [DOI] [PubMed] [Google Scholar]
- 40.Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925:148–158. doi: 10.1016/s0006-8993(01)03270-x. [DOI] [PubMed] [Google Scholar]