Abstract
Background and Purpose
Cognitive dysfunction occurs in 9% to 23% of patients during the first month after carotid endarterectomy (CEA). A 4-basepair (AAAT) tandem repeat polymorphism (either 3 or 4 repeats) has been described in the promoter region of inducible nitric oxide synthase (iNOS), a gene with complex roles in ischemic injury and preconditioning against ischemic injury. We investigated whether the 4-repeat variant (iNOS+) affects the incidence of cognitive dysfunction after CEA.
Methods
One-hundred eight-five CEA and 60 spine surgery (control) subjects were included in this nested cohort analysis. Subjects underwent a battery of 7 neuropsychometric tests before and 1 day and 1 month after surgery. Multivariate logistic regression analyses were performed to determine if the iNOS promoter variant was independently associated with the incidence of cognitive dysfunction at 1 day and 1 month. Further, all right-hand-dominant CEA subjects were grouped by operative side and performance on each test was compared between iNOS+ and iNOS− groups.
Results
Forty-four of 185 CEA subjects had at least 1 iNOS promoter allele containing 4 copies of the tandem repeat (iNOS+). iNOS+ status was significantly protective against moderate/severe cognitive dysfunction 1 month after CEA. Right-hand-dominant iNOS+ CEA subjects undergoing left-side CEA performed significantly better than iNOS− subjects on a verbal learning test and those undergoing right-side CEA performed significantly better on a test of visuospatial function.
Conclusion
We demonstrate an iNOS promoter polymorphism variant provides protection against moderate/severe cognitive dysfunction 1 month after CEA. Further, this protection appears to involve cognitive domains localized ipsilateral to the operative carotid artery.
Keywords: carotid endarterectomy, carotid stenosis, cognitive impairment, nitric oxide
Cognitive deterioration occurs in 9% to 23% of people within 1 month of carotid endarterectomy (CEA).1,2 Although the mechanism of this cognitive dysfunction is likely multifaceted, cerebral ischemia or ischemia-reperfusion injury likely plays a role because the operative carotid artery is cross-clamped for a period of ≈30 to 60 minutes during the procedure.
The presence of at least 1 apolipoprotein E (ApoE)-ε4 allele has been linked to increased risk for cognitive dysfunction after CEA.3 However, no associations have been reported between cognitive performance after CEA and genetic factors that likely play a direct role in ischemic injury or protection against ischemic injury. One such genetic factor may be a previously described polymorphism in the promoter region of the inducible nitric oxide synthase (iNOS) gene (NOS2A),4 a gene with complex roles in ischemic injury5 and ischemic and volatile anesthetic preconditioning against ischemic injury.6,7 In the population studied, this polymorphism occurred as either 3 or 4 copies of a 4-basepair (AAAT) tandem repeat located 0.7 kb 5′ to coding region, and the 4-copy variant (iNOS+) was demonstrated to induce a 25-fold gain-of-function in transfected human embryonic kidney cells.8
We present a study demonstrating that the 4-copy variant of this iNOS promoter polymorphism (iNOS+) affords protection against moderate/severe cognitive dysfunction 1 month after CEA. If this variant is indeed a gain-of-function, we propose that increased iNOS expression may be affording iNOS+ subjects enhanced cerebral ischemic tolerance via mechanisms consistent with volatile anesthetic preconditioning, an iNOS upregulation-dependent process.7
Materials and Methods
Subjects
This is a Columbia University Medical Center Institutional Review Board-approved, nested cohort analysis of 185 CEA and 60 lumbar spine surgery (laminectomy or microdiscectomy; control group) subjects who gave informed consent for genetic testing. These subjects represent a subgroup of an ongoing study of cognitive change after CEA.1–3 Only patients who were able to complete neuropsychometric (NP) tests in English and who had no history of axis I psychiatric disorders were enrolled. Because the majority of CEA patients are elderly, only spine surgery control subjects at least 60 years old were included.
Surgery and Anesthesia
All subjects received general anesthesia with routine monitoring as per normal procedure and as described previously.1 For the CEA subjects, this involved maintenance of anesthesia with a potent inhalational agent (isoflurane, end-tidal 0.6±0.1%; or sevoflurane, end-tidal 1.0±0.5%; with 70% nitrous oxide for ≈45 minutes before carotid artery cross-clamping). Intraoperative electroencephalography was used to monitor for significant cerebral ischemia during CEA procedures. A shunt was placed electively after carotid artery cross-clamping only if there was electroencephalography evidence of cerebral ischemia.
iNOS Promoter Polymorphism and ApoE Genotyping
DNA was extracted from buffy coat samples. The number of 4-basepair (AAAT) tandem repeats in the iNOS promoter region was determined by polymerase chain reaction amplification and sequencing, as described elsewhere.9 Subjects homozygous or heterozygous for the 4-copy variant were considered as 1 group (iNOS+), and subjects without a 4-copy variant allele were considered as another (iNOS−). ApoE genotypes were determined by polymerase chain reaction amplification and restriction fragment length polymorphism analysis, as described elsewhere,10 and subjects were grouped by the presences or absence of at least 1 ApoE-ε4 allele.
NP Evaluation
Subjects completed a battery of 7 NP tests; Controlled Oral Word Association, a test of verbal fluency; Boston Naming, a test of verbal function and language skills; Copy Portion of Rey Complex Figure, a test of visuospatial organization and construction; Immediate Recall Portion of Rey Complex Figure, a test of visuospatial memory; Trails Making Part A, a test of attention, visual scanning, and motor function; Trails Making Part B, a test of complex conceptual switching; and Hopkins Total Recall or Buschke Selective Reminding Test Sum Total Recall, both tests of verbal learning and memory,11 before and 1 day and 1 month after surgery. Subjects completed either Hopkins Total Recall or Buschke Selective Reminding Test Sum Total Recall, but not both, depending on the year of enrollment (both are tests of verbal learning and memory; 68% of subjects completed Buschke Selective Reminding Test). This battery offers a broad assessment of higher cortical functioning.
Statistical Analysis
As described previously,1,3 each NP test was scored individually for CEA and control (spine surgery) subjects. For each test and patient at 1 day and 1 month, a change score was calculated by subtracting the preoperative score from the 1 day and 1 month score. Each change score was converted to a z-score as follows:
Negative z-scores indicate poorer performance relative to the control mean. Z-scores were then converted to “deficit points” for each test as follows: z-score >−0.05=0; z-score between −0.5 and −1.0=1; z-score between −1.0 and −.5=2; z-score between −1.5 and −2.0=3; z-score between −2.0 and −2.5=4; z-score between −2.5 and −3.0=5; and z-score <−3.0=6 deficit points. Deficit points were then averaged across all 7 NP tests for each patient to generate an “average deficit score” at 1 day and 1 month (higher scores indicate poorer cognitive performance). By averaging deficit points across NP tests, we adjusted for patients who refused or were unable to complete 1 or 2 tests (only patients completing at least 5 tests were included in this analysis to insure average deficit scores represented broad assessments of cognitive function). Subjects were categorized as having “severe cognitive dysfunction” at 1 day or 1 month if their average deficit score was at least 2 standard deviations higher than the mean of the spine surgery control group and as having “moderate/severe cognitive dysfunction” if their average deficit score was at least 1.5 standard deviations higher than the mean of the control.
Key demographic and perioperative variables and baseline NP test scores were compared between the iNOS− and iNOS+ CEA groups using parametric (Student t tests for continuous and χ2 tests for categorical variables) or nonparametric tests (Mann–Whitney U test for continuous and Fisher exact test for categorical variables) as appropriate (Table 1). The iNOS promoter polymorphism genotypes were assessed for Hardy-Weinberg equilibrium by χ2 analysis.
Table 1.
Key Demographic and Perioperative Variables
| iNOS− |
iNOS+ |
||||
|---|---|---|---|---|---|
| Mean or Fraction | SE or % | Mean or Fraction | SE or % | P | |
| N at 1 day | 141 | 44 | |||
| Age | 69.56 | 0.75 | 72.52 | 1.13 | 0.14 |
| Male | 97/141 | 69.8% | 33/44 | 75.0% | 0.43 |
| White | 131/141 | 92.9% | 41/44 | 93.2% | 1.00 |
| Obese | 26/141 | 18.4% | 14/44 | 31.8% | 0.06 |
| Diabetes mellitus | 31/141 | 21.9% | 12/44 | 27.8% | 0.47 |
| Hypertension | 89/141 | 63.1% | 27/44 | 61.4% | 0.83 |
| Hypercholesterolemia | 85/141 | 60.3% | 32/44 | 72.7% | 0.14 |
| Previous CVA | 60/141 | 42.6% | 18/44 | 40.9% | 0.85 |
| Previous MI | 33/141 | 23.4% | 7/44 | 15.9% | 0.29 |
| Previous contralateral CEA | 15/141 | 10.6% | 3/44 | 6.8% | 0.57 |
| Years of education | 14.61 | 0.26 | 14.86 | 0.46 | 0.38 |
| Right-hand-dominant | 133/141 | 94.3% | 40/44 | 90.9% | 0.48 |
| Right-side CEA | 75/141 | 53.2% | 21/44 | 47.7% | 0.53 |
| Surgery duration, min | 156.14 | 3.31 | 154.18 | 5.12 | 0.90 |
| Clamp duration, min | 44.09 | 1.52 | 44.32 | 2.30 | 0.56 |
| Fentanyl, mcg/kg | 2.27 | 0.11 | 2.45 | 0.25 | 0.84 |
| Midazolam, mg/kg | 0.034 | 0.001 | 0.032 | 0.002 | 0.90 |
| Intraoperative EEG change | 7/143 | 4.90% | 1/44 | 4.28% | 0.68 |
| Shunt placed | 5/143 | 3.50% | 1/44 | 2.27% | 1.00 |
P are from Mann–Whitney U test for continuous and Fisher exact test for categorical variables.
CVA indicates cerebrovascular accident; EEG, electroencephalogram; MI, myocardial infarction; SE, standard error of the mean.
Average deficit scores and incidence of moderate and severe cognitive dysfunction at 1 day and 1 month were compared between the iNOS− and iNOS+ CEA groups using Mann–Whitney U test and Fisher exact tests as appropriate.
As the primary analysis, multivariate logistic regression analyses were performed to determine if iNOS promoter polymorphism variant influenced the incidence of severe (Table 2) and moderate/ severe (Table 3) cognitive dysfunction 1 day and 1 month after surgery while controlling for variables previously reported to affect cognitive performance in CEA and other surgical populations (age,2,3,12 years of education,12 obesity,3 presence of ApoE-ε4 allele,3 diabetes mellitus,2,3 and history of a cerebrovascular accident12). All variables were initially included in the model and stepwise reverse elimination was subsequently performed until only those variables achieving P<0.10 in the multivariate model remained.
Table 2.
Multivariate Logistic Regression Models of Severe Cognitive Dysfunction After Reverse Elimination
| 1 Day |
1 Month |
|||
|---|---|---|---|---|
| OR (CI) | P | OR (CI) | P | |
| Age | 1.12 (1.05–1.19) | <0.001* | … | … |
| Years of education | … | … | … | … |
| Previous CVA | … | … | 3.53 (CI) | 0.02* |
| Diabetes mellitus | … | … | 2.62 (0.83–8.26) | 0.099 |
| Obesity | … | … | … | … |
| ApoE-ε4 | 2.31 (0.91–5.90) | 0.08 | … | … |
| iNOS+ | … | … | … | … |
| Whole final model | … | 0.0002* | … | 0.01* |
All independent variables listed were included in model before reverse elimination.
Covariate P are from Wald tests and whole model P are from likelihood ratio tests.
Only those variables with OR and P listed remained after reverse elimination.
OR for age are presented as per year.
Statistically significant.
ApoE-ε4 indicates presence of at least 1 ApoE-ε4 allele; iNOS+, presence of at least 1 iNOS promoter insertion allele.
Table 3.
Multivariate Logistic Regression Models of Moderate/ Severe Cognitive Dysfunction After Reverse Elimination
| 1 Day |
1 Month |
|||
|---|---|---|---|---|
| OR (CI) | P | OR (CI) | P | |
| Age | 1.10 (1.04–1.16) | 0.001* | … | … |
| Years of education | … | … | … | … |
| Previous CVA | 1.93 (0.90–4.17) | 0.09 | … | … |
| Diabetes mellitus | … | … | … | … |
| Obesity | … | … | … | … |
| ApoE-ε4 | … | … | … | … |
| iNOS+ | … | … | 0.11 (0.01–0.83) | 0.03* |
| Whole final model | … | 0.0002* | … | 0.003* |
All independent variables listed were included in model before reverse elimination.
Covariate P are from Wald tests and whole model P are from likelihood ratio tests.
Only those variables with OR and P listed remained after reverse elimination.
OR for age are presented as per year.
Statistically significant.
To determine if hemisphere-specific cognitive effects of iNOS promoter variant exist, all right-hand-dominant CEA subjects were grouped by operative side and z-scores for each test at 1 day and 1 month were compared between iNOS+ and iNOS− groups using Mann–Whitney U tests (Table 4).
Table 4.
Mean (SE) 1 Day and 1 Month Z-Scores for All Right-Hand-Dominant CEA Patients by Operative Side and iNOS Promoter Variant
| Left-Side CEA |
Right-Side CEA |
|||||
|---|---|---|---|---|---|---|
| 1 Day Test | iNOS− | iNOS+ | P | iNOS− | iNOS+ | P |
| COWA | −0.06 (0.13) | −0.11 (0.28) | 0.82 | 0.06 (0.12) | 0.15 (0.23) | 0.92 |
| Boston Naming | −0.07 (0.08) | −0.20 (0.11) | 0.39 | −0.12 (0.12) | −0.18 (0.23) | 0.95 |
| Rey, Copy | −0.30 (0.13) | −0.43 (0.27) | 0.55 | −0.58 (0.19) | 0.22 (0.21) | 0.04 |
| Rey, Recall | −0.22 (0.14) | 0.23 (0.26) | 0.17 | −0.03 (0.23) | 0.01 (0.37) | 0.77 |
| Trails A | −0.42 (0.28) | −0.08 (0.32) | 0.52 | −0.17 (0.22) | −0.09 (0.41) | 0.85 |
| Trails B | −0.16 (0.17) | −0.15 (0.20) | 0.48 | −0.33 (0.18) | −0.50 (0.32) | 0.36 |
| Buschke SRT | −0.26 (0.14) | 0.54 (0.10) | 0.01 | 0.18 (0.12) | 0.27 (0.40) | 0.94 |
| Hopkins TR | −0.60 (0.45) | −0.52 (0.41) | 0.87 | −0.34 (0.33) | −0.72 (0.63) | 0.60 |
| Left-Side CEA |
Right-Side CEA |
|||||
| 1 Month Test | iNOS− | iNOS+ | P | iNOS− | iNOS+ | P |
|
| ||||||
| COWA | −0.23 (0.18) | 0.02 (0.31) | 0.43 | −0.09 (0.16) | 0.24 (0.24) | 0.25 |
| Boston Naming | −0.05 (0.11) | −0.36 (0.21) | 0.14 | 0.16 (0.13) | −0.28 (0.16) | 0.12 |
| Rey, Copy | 0.06 (0.13) | 0.66 (0.23) | 0.04 | −0.38 (0.12) | 0.44 (0.22) | 0.01 |
| Rey, Recall | −0.03 (0.14) | 0.44 (0.26) | 0.16 | 0.08 (0.17) | 0.29 (0.29) | 0.61 |
| Trails A | 0.34 (0.22) | 1.13 (0.40) | 0.14 | 0.66 (0.18) | 0.36 (0.32) | 0.13 |
| Trails B | −0.04 (0.18) | 0.18 (0.13) | 0.67 | −0.13 (0.15) | −0.35 (0.21) | 0.37 |
| Buschke SRT | −0.08 (0.17) | 0.95 (0.22) | 0.01 | 0.35 (0.18) | 0.38 (0.25) | 1.00 |
| Hopkins TR | −1.15 (0.49) | −1.08 (0.63) | 0.81 | −0.15 (0.37) | 0.60 (0.51) | 0.16 |
Boldface type indicates significance by Mann–Whitney U test.
Buschke LTR indicates Buschke Long-Term Retrieval; COWA, Controlled Oral Word Association Test; Hopkins TR, Hopkins Total Recall.
Results
All study subjects possessed only alleles with 3 or 4 copies of the AAAT tandem repeat in the iNOS promoter. Forty-four of 185 CEA subjects (23.8%) were found to be iNOS+ (42 heterozygous, 2 homozygous for 4 copies). The 4-copy allele frequency (0.12) was similar to other published reports in Australian (0.14),13 British (0.15),4 and French (0.15)14 populations, and the iNOS promoter genotypes were in Hardy-Weinberg equilibrium (P=0.99). Baseline performance on each NP test did not significantly differ between iNOS− and iNOS+ groups (data not presented), and follow-up rates at 1 month were similar for both groups (79.4% for iNOS−, 84.1% for iNOS+; P=0.50 by ε2 test).
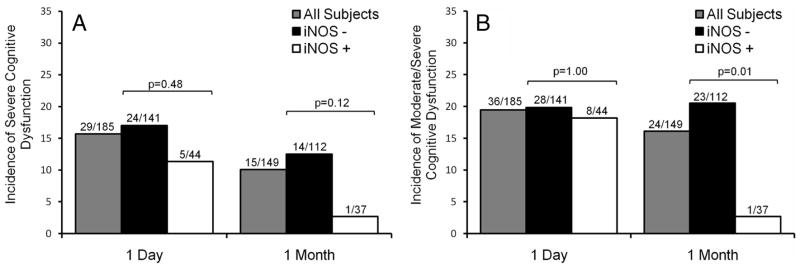
iNOS+ subjects had a significantly lower average deficit score at 1 month (mean±standard error, 0.362±0.053 vs 0.611±0.051; P=0.01), but not at 1 day (0.714±0.087 vs 0.775±0.064; P=0.86). At 1 day and 1 month, 16.2% and 10.1% of all CEA subjects met criteria for severe cognitive dysfunction, and 19.5% and 16.1% met criteria for moderate/severe cognitive dysfunction, respectively (Figure; in the control group, 4 [6.7%] and 1 [2.4%] subjects had severe cognitive dysfunction, and 5[8.3%] and 4 [9.5%] subjects had moderate dysfunction at 1 day and 1 month, respectively). By univariate analysis, there was no significant difference in incidence of severe cognitive dysfunction between iNOS+ and iNOS− CEA groups at 1 day or 1 month (Figure, A). However, significantly fewer iNOS+ CEA subjects had moderate/severe cognitive dysfunction at 1 month (Figure, B).
Figure.
Incidence of severe (A) and moderate/severe (B) cognitive dysfunction for all, iNOS−, and iNOS+ carotid endarterectomy patients 1 day and 1 month postoperatively. The bars indicate the incidences of dysfunction as percentages of corresponding groups. There was no significant difference in the incidence of cognitive dysfunction between iNOS+ and iNOS− subjects at 1 day or 1 month (A). However, significantly fewer iNOS+ subjects had moderate/severe cognitive dysfunction at 1 month by Fisher exact test (B). Bar graph shows incidence of severe and moderate cognitive dysfunction for all, iNOS−, and iNOS+ carotid endarterectomy patients 1 day and 1 month postoperatively.
The primary analysis, multivariate logistic regression analyses of cognitive dysfunction, demonstrates that iNOS+ status was not significantly associated with presence of severe cognitive dysfunction 1 day or 1 month after CEA (Table 2). However, iNOS+ was significantly protective against moderate/severe cognitive dysfunction at 1 month (P=0.03; Table 3).
Test-by-test z-score analysis of all right-hand-dominant CEA subjects was performed. Right-hand-dominant iNOS+ subjects undergoing right-side CEA performed significantly better than iNOS− subjects on the Copy Portion of Rey Complex Figure Test at 1 day and 1 month. Right-hand-dominant iNOS+ subjects undergoing left-side CEA perform significantly better than iNOS− subjects on Buschke Selective Reminding Test at 1 day and 1 month. At 1 month, right-hand-dominant iNOS+ subjects undergoing left-side CEA subjects also performed better than iNOS− subjects on the Copy Portion of Rey Complex Figure Test (Table 4). By definition, the mean z-scores of the control group were zero for each test.
Discussion
Multivariate analyses demonstrate that a polymorphism (4 copies of a tandem repeat [AAAT]) in the promoter region of iNOS+ provides protection against moderate/severe cognitive dysfunction 1 month after CEA endarterectomy independent of previously reported potential risk factors for poorer postoperative cognitive function. Furthermore, test-by-test analysis suggests that this protection is “hemisphere-specific,” because it protects visuospatial function (Copy Portion of Rey Complex Figure) in right-side-dominant CEA subjects and language function/verbal learning and memory (Buschke Selective Reminding Test) in left-side CEA subjects. This “hemisphere-specific” effect supports the possibility, given the known role of iNOS in ischemia and cerebral preconditioning, that the protection afforded by the iNOS+ allele relates to cerebral ischemia. The hemisphere-specific effect may also explain why the overall protection afforded by iNOS+ status was only significantly protective against moderate/severe cognitive dysfunction, not severe cognitive dysfunction, because severe dysfunction potentially requires injury to more diffuse neuroanatomical and corresponding cognitive domains.
iNOS+ left-side CEA subjects also performed better on the Copy Portion of Rey Complex Figure at 1 month, which would not be predicted when considering lateralization of cognitive function. However, both iNOS+ and iNOS− groups performed insignificantly better than controls (P=0.07 and P=0.78, respectively) on this test at 1 month (both groups had positive mean z-scores). This evidence of more “widespread” cognitive protection or improvement at 1 month may explain why iNOS+ status was only shown to be a significantly protective against cognitive dysfunction at 1 month, not 1 day, in multivariate analyses. Because left-side CEA patients performed better on Buschke Selective Reminding Test at 1 day and 1 month, one might also expect them to perform significantly better on Hopkins Total Recall, a similar test. However, fewer subjects completed the Hopkins Total Recall than Buschke Selective Reminding, diminishing the ability to detect a statistical difference. Further, Buschke Selective Reminding Test, an intensive test involving 12 trials of verbal reminding/learning, is a more sensitive measure than Hopkins Total Recall (however, patients find it more difficult and therefore it is inconvenient to administer and complete; thus, we switched tests in our battery during recruitment of subjects for this study).
Transient transfection experiments in human embryonic kidney 293 cells have demonstrated that the iNOS+ allele is associated with a 25-fold gain-of-function.8 If the polymorphism is indeed a gain-of-function in iNOS− expressing cells in the brain, the mechanism of protection seen in data presented here may be consistent with known mechanisms of “delayed-phase” ischemic and volatile anesthetic preconditioning. Briefly, repeated transient ischemic exposures and volatile anesthetics have been shown to protect the brain,6,7,15–17 heart,18–20 kidney,21,22 and retina23 from injury resulting from subsequent significant ischemic insults. Furthermore, animal model studies have shown that this protection is dependent on an increase in iNOS expression7,17,19 and that specific iNOS inhibitors6,7,16–18, 20 or iNOS gene knockouts6,16,24,25 completely abolish the protective effect. In addition, there is evidence that increased iNOS expression is sufficient for preconditioning (ie, without previous transient ischemic or volatile anesthetic exposures), at least with regard to the myocardium, as iNOS overexpression by gene therapy before left anterior descending artery occlusion decreases infarct size in rodent hearts (similar to preconditioned animals).26 To the extent they are understood, the mechanisms of volatile anesthetic preconditioning appear to be similar in the brain and heart.
The downstream mechanisms by which increased iNOS expression and the resultant increased nitric oxide production protect against ischemic injury are still unclear. However, there is evidence suggesting that preconditioning protects mitochondrial integrity during ischemic stress.6,27–31 Further, increased neurogenesis in the adult rodent denote gyrus after ischemic injury has been shown to be iNOS expression-dependent.32
Thus, if the iNOS promoter polymorphism in question has a gain-of-function effect, we could hypothesize that the iNOS+ subjects may have better cognitive outcomes because they are “preconditioned at baseline” secondary to increased basal iNOS expression. Although iNOS is commonly thought to be expressed when “induced,” the human embryonic kidney cell experiments referenced suggest the iNOS+ allele may lead to higher basal expression.8 Alternatively, it is possible that iNOS expression is “more inducible” in iNOS+ subjects, and hence they are afforded preconditioning protection even though they only receive volatile anesthetics for ≈45 minutes before cross-clamp (near the lower limit of the time necessary to produce preconditioning protection before ischemic insult in animal models15) at ≈0.6% (for patient receiving isoflurane; lower concentration than tested in animal models15).
It is interesting to note that there is clinical evidence suggesting that the iNOS+ genotype may be associated with a “high-iNOS” state, because the polymorphism has been linked to conditions with key elements of inflammatory pathophysiology: giant cell arteritis,33 increased severity of diabetic nephropathy,8 and increased severity of coronary artery disease.34
Further studies are needed to investigate the potential link between the iNOS+ genotype and preconditioning-like mechanisms of cerebral ischemic protection. Notably, the polymorphism has only been described as a gain-of-function in transient transfection assays in human embryonic kidney cells, in which the promoter variants were driving reporter-gene expression.8 Thus, the phenotype of this promoter variant is unknown in the cell types that likely express the iNOS that is thought to contribute to cerebral preconditioning (cerebrovascular endothelial cells6). Further, even if iNOS transcription is increased in the appropriate cells in iNOS+ subjects, it is not known if this increased expression leads to increased amounts of functional protein or activity.
A limitation of this, and all other, studies of postoperative cognitive change is that terms such as “cognitive dysfunction” are somewhat subjective and are not universally defined.35 Two points pertaining to this issue need to be addressed. First, we previously published a report (which included a minority of the subjects included in this analysis) that the presence of at least 1 ApoE-ε4 allele leads to an increased likelihood of cognitive dysfunction after CEA, a finding that is not confirmed by multivariate analysis here. This previous study included a battery of 5 NP tests that form a subset of the 7 tests presented here, so the extent of ApoE-ε4-positive subjects’ “dysfunction” may have been diluted by the inclusion of 2 more tests (these 2 tests, Immediate Recall Portion of Rey Complex Figure and Hopkins Total Recall/Buschke Long-Term Retrieval, were not included in the previous study because they were not administered to a significant number of the participating subjects). Further, the inclusion of another variable, iNOS promoter variant, in the multivariate models of cognition after CEA may have caused ApoE-ε4 status to lose significance at 1 month. Consistent with these notions, the presence of at least 1 ApoE-ε4 allele has been shown to both affect36 and not affect37 postoperative cognition in studies of different surgical populations completed by independent groups. Second, to address the issue of subjectivity and variability in the categorization of postoperative cognitive outcomes, we present analysis of both “moderate/severe” and “severe” cognitive dysfunction.
Although cerebral precondition against ischemic damage has long been discussed and carotid endarterectomy has been noted as a potential area of its application,15 this may be the first demonstration to our knowledge of the cerebroprotective effects of “preconditioning-like” mechanisms in humans.
Acknowledgments
The authors thank H.T. Lee, MD, PhD, Department of Anesthesiology, Columbia University, for his kind advice and assistance with genotyping.
Sources of Funding
G.T.Y. was supported in part by a grant from Doris Duke Charitable Foundation, New York, NY; E.J.H. was supported in part by a grant from the NIA (RO1 AG17604) and the Department of Anesthesiology, Columbia University; New York, NY.
Footnotes
Disclosures
None.
References
- 1.Heyer EJ, Sharma R, Rampersad A, Winfree CJ, Mack WJ, Solomon RA, Todd GJ, McCormick PC, McMurtry JG, Quest DO, Stern Y, Lazar RM, Connolly ES. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mocco J, Wilson DA, Komotar RJ, Zurica J, Mack WJ, Halazun HJ, Hatami R, Sciacca RR, Connolly ES, Jr, Heyer EJ. Predictors of neurocognitive decline after carotid endarterectomy. Neurosurgery. 2006;58:844–850. doi: 10.1227/01.NEU.0000209638.62401.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heyer EJ, Wilson DA, Sahlein DH, Mocco J, Williams SC, Sciacca R, Rampersad A, Komotar RJ, Zurica J, Benvenisty A, Quest DO, Todd G, Solomon RA, Connolly ES., Jr Apoe-epsilon4 predisposes to cognitive dysfunction following uncomplicated carotid endarterectomy. Neurology. 2005;65:1759–1763. doi: 10.1212/01.wnl.0000184579.23624.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy R, Hill AV. A biallelic tetranucleotide repeat in the promoter of the human inducible nitric oxide synthase gene. Clin Genet. 1997;52:192–193. doi: 10.1111/j.1399-0004.1997.tb02544.x. [DOI] [PubMed] [Google Scholar]
- 5.Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 6.Cho S, Park EM, Zhou P, Frys K, Ross ME, Iadecola C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:493–501. doi: 10.1038/sj.jcbfm.9600058. [DOI] [PubMed] [Google Scholar]
- 7.Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible no synthase dependent. Stroke. 2002;33:1889–1898. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- 8.Morris BJ, Markus A, Glenn CL, Adams DJ, Colagiuri S, Wang L. Association of a functional inducible nitric oxide synthase promoter variant with complications in type 2 diabetes. J Mol Med. 2002;80:96–104. doi: 10.1007/s00109-001-0287-1. [DOI] [PubMed] [Google Scholar]
- 9.Morris BJ, Glenn CL, Wilcken DE, Wang XL. Influence of an inducible nitric oxide synthase promoter variant on clinical variables in patients with coronary artery disease. Clin Sci (Lond) 2001;100:551–556. [PubMed] [Google Scholar]
- 10.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein e by gene amplification and cleavage with hhai. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 11.Lezak MD. Neuropsychological assessment. Oxford; New York: Oxford University Press; 2004. [Google Scholar]
- 12.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 13.Glenn CL, Wang WY, Morris BJ. Different frequencies of inducible nitric oxide synthase genotypes in older hypertensives. Hypertension. 1999;33:927–932. doi: 10.1161/01.hyp.33.4.927. [DOI] [PubMed] [Google Scholar]
- 14.Cambien F, Poirier O, Nicaud V, Herrmann SM, Mallet C, Ricard S, Behague I, Hallet V, Blanc H, Loukaci V, Thillet J, Evans A, Ruidavets JB, Arveiler D, Luc G, Tiret L. Sequence diversity in 36 candidate genes for cardiovascular disorders. Am J Hum Genet. 1999;65:183–191. doi: 10.1086/302448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Traystman RJ, Murphy SJ. Inhalational anesthetics as preconditioning agents in ischemic brain. Curr Opin Pharmacol. 2007 doi: 10.1016/j.coph.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano T, Kunz A, Abe T, Girouard H, Anrather J, Zhou P, Iadecola C. Inos-derived no and nox2-derived superoxide confer tolerance to excitotoxic brain injury through peroxynitrite. J Cereb Blood Flow Metab. 2007;27:1453–1462. doi: 10.1038/sj.jcbfm.9600449. [DOI] [PubMed] [Google Scholar]
- 17.Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in neonatal rats. Anesthesiology. 2004;101:695–703. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Takano H, Manchikalapudi S, Tang XL, Qiu Y, Rizvi A, Jadoon AK, Zhang Q, Bolli R. Nitric oxide synthase is the mediator of late preconditioning against myocardial infarction in conscious rabbits. Circulation. 1998;98:441–449. doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- 19.Wakeno-Takahashi M, Otani H, Nakao S, Imamura H, Shingu K. Isoflurane induces second window of preconditioning through upregulation of inducible nitric oxide synthase in rat heart. Am J Physiol Heart Circ Physiol. 2005;289:H2585–H2591. doi: 10.1152/ajpheart.00400.2005. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Chuang JH, Liu K, Chan JY. Nitric oxide triggers delayed anesthetic preconditioning-induced cardiac protection via activation of nuclear factor-kappaB and upregulation of inducible nitric oxide synthase. Shock. 2008;30:241–249. doi: 10.1097/SHK.0b013e318162ad19. [DOI] [PubMed] [Google Scholar]
- 21.Joo JD, Kim M, D’Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006;17:3115–3123. doi: 10.1681/ASN.2006050424. [DOI] [PubMed] [Google Scholar]
- 22.Park KM, Byun JY, Kramers C, Kim JI, Huang PL, Bonventre JV. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem. 2003;278:27256–27266. doi: 10.1074/jbc.M301778200. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto K, Yonoki Y, Kubota Y, Kuwagata M, Saito M, Nakahara T, Ishii K. Inducible nitric oxide synthase inhibitors abolished histological protection by late ischemic preconditioning in rat retina. Exp Eye Res. 2006;82:512–518. doi: 10.1016/j.exer.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Zhao T, Xi L, Chelliah J, Levasseur JE, Kukreja RC. Inducible nitric oxide synthase mediates delayed myocardial protection induced by activation of adenosine a(1) receptors: Evidence from gene-knockout mice. Circulation. 2000;102:902–907. doi: 10.1161/01.cir.102.8.902. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible no synthase gene. Proc Natl Acad Sci U S A. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Guo Y, Xuan YT, Lowenstein CJ, Stevenson SC, Prabhu SD, Wu WJ, Zhu Y, Bolli R. Gene therapy with inducible nitric oxide synthase protects against myocardial infarction via a cyclooxygenase-2-dependent mechanism. Circ Res. 2003;92:741–748. doi: 10.1161/01.RES.0000065441.72685.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxiareoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–C1590. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 28.Dave KR, Saul I, Busto R, Ginsberg MD, Sick TJ, Perez-Pinzon MA. Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus. J Cereb Blood Flow Metab. 2001;21:1401–1410. doi: 10.1097/00004647-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Bejui F, Robert D, Ovize M. Preconditioning delays ca2+-induced mitochondrial permeability transition. Cardiovasc Res. 2004;61:115–122. doi: 10.1016/j.cardiores.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhan RZ, Fujihara H, Baba H, Yamakura T, Shimoji K. Ischemic preconditioning is capable of inducing mitochondrial tolerance in the rat brain. Anesthesiology. 2002;97:896–901. doi: 10.1097/00000542-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Zhang HX, Du GH, Zhang JT. Ischemic pre-conditioning preserves brain mitochondrial functions during the middle cerebral artery occlusion in rat. Neurol Res. 2003;25:471–476. doi: 10.1179/016164103101201878. [DOI] [PubMed] [Google Scholar]
- 32.Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–229. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Gay MA, Oliver J, Sanchez E, Garcia-Porrua C, Paco L, Lopez-Nevot MA, Ollier WE, Martin J. Association of a functional inducible nitric oxide synthase promoter variant with susceptibility to biopsy-proven giant cell arteritis. J Rheumatol. 2005;32:2178–2182. [PubMed] [Google Scholar]
- 34.Kunnas TA, Mikkelsson J, Ilveskoski E, Tanner MM, Laippala P, Penttila A, Perola M, Nikkari ST, Karhunen PJ. A functional variant of the inos gene flanking region is associated with lad coronary artery disease: An autopsy study. Eur J Clin Invest. 2003;33:1032–1037. doi: 10.1111/j.1365-2362.2003.01271.x. [DOI] [PubMed] [Google Scholar]
- 35.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: A systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Lelis RG, Krieger JE, Pereira AC, Schmidt AP, Carmona MJ, Oliveira SA, Auler JO., Jr Apolipoprotein e4 genotype increases the risk of postoperative cognitive dysfunction in patients undergoing coronary artery bypass graft surgery. J Cardiovasc Surg (Torino) 2006;47:451–456. [PubMed] [Google Scholar]
- 37.Abildstrom H, Christiansen M, Siersma VD, Rasmussen LS. Apolipoprotein e genotype and cognitive dysfunction after noncardiac surgery. Anesthesiology. 2004;101:855–861. doi: 10.1097/00000542-200410000-00009. [DOI] [PubMed] [Google Scholar]