Abstract
Introduction
Activator protein (AP)-1 family members play important roles in the development and maintenance of the adult skeleton. Transgenic mice that overexpress the naturally occurring ΔFosB splice variant of FosB develop severe osteosclerosis. Translation of Δfosb mRNA produces both ΔFosB and a further truncated isoform (Δ2ΔFosB) that lacks known transactivation domains but, like ΔFosB, induces increased expression of osteoblast marker genes.
Materials and Methods
To test Δ2ΔFosB’s ability to induce bone formation in vivo, we generated transgenic mice that overexpress only Δ2ΔFosB using the enolase 2 (ENO2) promoter-driven bitransgenic Tet-Off system.
Results
Despite Δ2ΔFosB’s failure to induce transcription of an AP-1 reporter gene, the transgenic mice exhibited both the bone and the fat phenotypes seen in the ENO2-ΔFosB mice. Both ΔFosB and Δ2ΔFosB activated the BMP-responsive Xvent-luc reporter gene and increased Smad1 expression. Δ2ΔFosB enhanced BMP-induced Smad1 phosphorylation and the translocation of phospho-Smad1 (pSmad1) to the nucleus more efficiently than ΔFosB and showed a reduced induction of inhibitory Smad6 expression.
Conclusions
ΔFosB’s AP-1 transactivating function is not needed to induce increased bone formation, and Δ2ΔFosB may act, at least in part, by increasing Smad1 expression, phosphorylation, and translocation to the nucleus.
Key words: bone formation, osteoblasts, activator protein-1, Smad, ΔFosB
INTRODUCTION
Development and proper maintenance of the skeleton depends on the synchronized actions of bone-forming osteoblasts and bone-resorbing osteoclasts.(1,2) Several in vivo studies using knockout and/or transgenic approaches have shown the important role of activator protein (AP)-1 family members in the development and maintenance of the adult skeleton as regulators of osteoblast and/or osteoclast differentiation and function.(3,4) The AP-1 transcription factors, which bind TPA response elements (TREs) in gene promoters, are formed by the heterodimerization of Jun (JunD, c-Jun, and JunB) and Fos (c-Fos, FosB, Fra-1, Fra-2) proteins in interfamilial combinations to form a number of transcriptionally active regulatory complexes that differ in their stability, DNA binding affinity, and transactivation potential.(5–7) JunB plays a regulatory role in both osteoblast and osteoclast differentiation,(8) and all of the Fos proteins contribute to the regulation of bone cell differentiation and growth. Mice lacking c-Fos are osteopetrotic, because of deficient osteoclast differentiation,(9,10) whereas c-Fos overexpression in mice results in osteogenic osteosarcomas.(11) Mice that overexpress Fra-1 have an osteosclerotic phenotype,(12) as do mice that overexpress Fra-2,(13) and deletion of the fra-1 or the fra-2 genes leads to osteopenia.(13)
Interestingly, whereas the overexpression or deletion of FosB has not been reported to induce a skeletal phenotype,(11,14,15) we have shown that transgenic mice that overexpress the naturally occurring ΔFosB variant of FosB (FosB 1–237, generated by alternative splicing of the fosb gene transcript)(16) under the control of the enolase 2 (ENO2; previously neuron-specific enolase/NSE) promoter or the osteoblast-specific osteocalcin (OG2) promoter develop a severe and progressive osteosclerosis,(17–19) much like that seen in the Fra-1-overexpressing mice. The induction of the osteosclerotic phenotype by overexpressing ΔFosB specifically in osteoblasts using the OG2 promoter,(19) as well as in vitro studies,(17) showed that ΔFosB increases osteoblast function at least in part by a cell autonomous mechanism that remains to be elucidated. Interestingly, the relative levels of full-length FosB, ΔFosB, and Δ2ΔFosB (FosB 79–237), a further truncated isoform produced from the Δfosb mRNA by the use of an alternative translation initiation codon, vary in response to osteogenic stimuli,(17,20,21) suggesting that one or both truncated FosB isoforms are physiologically important regulators of osteoblast differentiation.
ΔFosB lacks the 101 C-terminal residues of FosB, which includes a potent transactivation domain,(22–24) but retains the N-terminal Fos homology domain (FHD),(16,25–27) which contributes to the transcriptional activity of FosB-Jun complexes.(28) Δ2ΔFosB lacks the FHD but retains the basic DNA-binding and leucine zipper dimerization motifs (Fig. 1)(29) and, as shown here, the ability to form DNA-binding heterodimers with Jun family members. Interestingly, the doubly truncated isoform induces at least some of the same changes in the expression of osteoblast marker genes (runx2, collagen I, osteocalcin) as the FHD-containing ΔFosB when expressed in primary calvarial cells or mesenchymal cell lines,(17) despite the absence of both the C-terminal transactivation domain and the FHD. Because runx2, collagen type I, and osteocalcin are all bone morphogenetic protein (BMP)-responsive genes,(30,31) we hypothesized that this further truncated FosB isoform might be sufficient to induce high bone mass, and that this may occur, at least in part, by modulating the BMP/Smad signaling pathway.
FIG. 1.
FosB isoforms. ΔFosB arises as a consequence of alternative splicing of the fosb mRNA and lacks FosB’s 101 C-terminal amino acids, which contain a potent proline-rich transactivation domain (PPP). The further truncated Δ2ΔFosB isoform arises as a consequence of initiation of translation from Met79 of the ΔFosb mRNA and lacks both the C-terminal transactivation domain that is missing in ΔFosB and the N-terminal Fos homology domain (FH). All three isoforms retain the basic DNA-binding domain (B) and the leucine zipper (LZ), which mediates the binding to Jun proteins to form AP-1 complexes.
To test this hypothesis and determine whether the AP-1 transcriptional activity of ΔFosB was required to induce bone formation, we generated transgenic mice that overexpress only the Δ2ΔFosB isoform using the ENO2 promoter-driven bitransgenic Tet-Off system. Overall examination and histomorphometric analysis of these mice showed that the Δ2ΔFosB isoform, which lacks any known transactivation domain and which we show here does not activate transcription from AP-1 sites, is able to induce both the bone and the fat phenotypes seen in the ENO2-ΔFosB mice. Analysis of the effects of ΔFosB and Δ2ΔFosB overexpression on the BMP-2/Smad signaling pathway showed that Δ2ΔFosB increased Smad1 expression and enhanced BMP-induced Smad1 phosphorylation and the translocation of phospho-Smad1 (pSmad1) to the nucleus more efficiently than ΔFosB while reducing BMP-2–induced expression of inhibitory Smad6. Thus, our data indicate that the AP-1 transactivating activity of overexpressed ΔFosB is not needed to induce an increase in bone formation and suggest that this effect on bone formation is mediated, at least in part, by increasing Smad1 levels downstream of activated BMP receptors and by promoting Smad1 phosphorylation and translocation to the nucleus, with the consequent induction of osteoblast target gene expression.
MATERIALS AND METHODS
Construction of plasmids
ΔFosB, ΔFosB2i3i, and Δ2ΔFosB constructs in pcDNA3.1 have been described elsewhere.(17,19) GFP-fusion constructs were generated by amplifying the coding sequences of the corresponding pcDNA3.1 constructs by PCR and ligating the products into E-GFP (Clontech, Mountain View, CA, USA). C-terminally FLAG-tagged Smad1 (Smad1-FLAG), N-terminally Xpress-tagged Smad4 (Xpress-Smad4), Smad6, and hBMP-2 plasmids were kindly provided by Dr G Rawadi (Prostrakan Pharmaceuticals, Romainville, France) and were used both for transfections and as cDNA probes in Northern blot analysis.
The pTetOp-ΔFosB vector(32) containing ΔFosB cDNA under control of the tetracycline-regulated promoter (TetOp)(33) was kindly provided by Dr Eric Nestler (University of Texas Southwestern Medical Center, Dallas, TX, USA). To generate the pTetOp-Δ2ΔFosB construct used for transgene expression, Δ2ΔFosB cDNA was amplified by PCR using the forward primer 5`-GAGCTGAAGCTTTCCATGGCCCAGTCC-3` containing a HindIII site and the reverse primer 5`-GTGCCGATATCTCCCCTCTCCTCCTCA-3` containing a EcoRV site. The PCR fragment was cut with HindIII/EcoRV and used to replace the ΔFosB insert, which was released from the pTetOp-ΔFosB construct at the corresponding sites.
Generation of transgenic mice
The linearized TetOp-Δ2ΔFosB DNA fragment containing the promoter, open reading frame, SV40 intron, and poly(A)+ signal was gel-purified and microinjected into the pronuclei of oocytes from (SJL × C57BL6) F2 mouse ova. Viable embryos were implanted into pseudopregnant recipients and allowed to develop to term. Founders were identified by isolating tail DNA using the Tissue DNeasy kit (Qiagen, Valencia, CA, USA), and detecting the transgene by PCR, using the forward primer 5`-TCCATGGCCCAGTCCCAGGGG-3` and the reverse primer 5`-GCCTGAAGTCGATCTGTCAGCTCC-3`, which were also used for routine genotyping of the mice. ENO2-Δ2ΔFosB transgenic mice were generated by crossing mice that express the tetracycline transactivator (tTA) under the control of the enolase2 (ENO2, also known as neuron-specific enolase or NSE; provided by Dr Eric Nestler),(32,34) with the newly generated TetOp-Δ2ΔFosB mice. For all experiments, the bitransgenic ENO2-Δ2ΔFosB mice were bred as heterozygotes and monotransgenic ENO2-tTA littermates were used as controls. ENO2-ΔFosB mice were generated as described previously.(17,18,32,34,35) All animal protocols were approved by the Yale University Institutional Animal Care and Use Committee.
Histomorphometric analysis
All mice used for analysis were injected with calcein (20 mg/kg; Sigma, Valencia, CA, USA) and demeclocycline (20 mg/ml; Sigma) at 10 and 3 days before death to label bone mineralization fronts. Femur and tibia were fixed in 3.7% formaldehyde/PBS and embedded by standard procedures in methylmethacrylate resin.(36) Five-micrometer toluidine blue-stained and 10-μm unstained sections were used for standard histomorphometric analysis(37) using the Osteomeasure system (Osteometrics, Atlanta, GA, USA). All measurements were performed in a blind fashion. Sections of proximal tibia were stained by the Von Kossa procedure as described elsewhere.(38)
Quantification of abdominal fat and serum leptin levels
Ten-week-old ENO2-Δ2ΔFosB mice and control littermates were anesthetized with Metofane (Medical Developments, Melbourne, Australia) and bled by cardiac puncture. Serum leptin levels were measured immediately using the QuantikineM RIA kit (R&D Systems, Minneapolis, MN, USA). Abdominal adipose tissue was removed and weighed.
Cell culture and transfection
Primary calvarial cell cultures used to analyze Δ2ΔFosB expression were prepared from control, ENO2-ΔFosB, and ENO2-Δ2ΔFosB mice using standard techniques.(39) Cultures of primary bone marrow cells were established from control and ENO2-Δ2ΔFosB mice by plating the marrow flushed from tibia and femur in DMEM supplemented with 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin (complete medium). Nonadherent cells were removed after 5 days, and the adherent cells were trypsinized, replated, and allowed to reach confluency. Complete medium supplemented with 50 μg/ml ascorbic acid, 5 mM β-glycerophosphate, and 10 nM dexamethasone (all from Sigma) was added at confluency (day 0) to induce osteoblast differentiation, and the cultures were maintained under these conditions for the indicated number of days.
C2C12 cells (ATCC, Manassas, VA, USA) were maintained as described previously.(17) The osteoblastic phenotype was induced by incubating in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 5% FBS (Invitrogen) and 300 ng/ml recombinant human BMP-2 (rhBMP-2, kindly provided by ProSkelia Pharmaceuticals) for up to 48 h. For transient transfections, cells were transfected using FuGENE6 (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions and harvested 48–72 h after transfection. The generation of the stable transfectants used in these studies has been described previously.(17)
RNA extraction and Northern blotting
Total RNA was extracted from cells using TRIZOL (Invitrogen) according to standard procedures.(40) Twenty micrograms was resolved in 1% denaturing agarose/formaldehyde gels and transferred onto Hybond-N nylon membranes (Amersham, Piscataway, NJ, USA), as described.(41) [32P]random labeled cDNA probes were purified on Nick columns (Promega, Madison, WI, USA), and membranes were incubated at 42°C overnight in hybridization buffer (50% formamide, 5× SSPE, 5× Denhardt, 0.1% SDS). Membranes were washed at 55°C in 0.5× SSC/0.1% SDS before being exposed to X-ray film. 18S rRNA was used as an internal control.
Western blot analysis
Cells were lysed in modified RIPA (mRIPA) buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% NP40, 0.25% sodium deoxycholate, 1 mM EGTA) supplemented with 1 mM NaF, 1 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml pepstatin, and 1 mM phenylmethylsulfonylfluoride. The lysates were cleared by centrifugation, and the protein concentration was determined by the BCA assay (Pierce, Pittsburgh, PA, USA). Nuclear extracts were prepared by the method of Schreiber.(42) Total cell lysates (50 μg) were analyzed by electrophoresis on SDS-polyacrylamide gels (Invitrogen) and transferred onto nitrocellulose membranes (Whatman, Florham Park, NJ, USA). Western blotting was performed using standard techniques, and immunoreactive bands were detected by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ, USA). All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) except for anti-GFP (Clontech), anti-FLAG M2 (Sigma), anti-pSmad1 (Upstate Biotech, Charlottesville, VA, USA), and anti-Xpress (Invitrogen).
In vitro transcribed and translated proteins
FosB, ΔFosB, and Δ2ΔFosB cDNA in the pcDNA3.1 vector were transcribed and translated in vitro using the TNT Coupled Reticulocyte Lysate System (Promega), as described by the manufacturer. In vitro transcribed/translated proteins were quantified by Western blotting using the polyclonal rabbit anti-FosB antibody (Santa Cruz Biotechnology).
Binding to GST fusion proteins
The glutathione-S-tranferase (GST) fusion proteins were produced and purified by binding to glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech.) as previously described.(19) For interaction studies, JunD proteins were produced by in vitro transcription-translation and incubated with GST fusion proteins bound to glutathione-Sepharose beads overnight at 4°C. The samples were washed three times in mRIPA, the beads were sedimented and boiled in 10 μl of loading buffer, and the complexes were analyzed by Western blotting.
EMSA
AP-1–binding double-stranded probes (forward oligonucleotide: GTCGACGTGAGTCAGCGCGC; reverse oligonucleotide: GGGCGCGCTGACTCACGT) were labeled with 15 μCi [32P]dCTP using the Amersham Random Prime kit and purified on NICK G50 Sephadex columns. Approximately equal amounts of in vitro–transcribed FosB, ΔFosB, Δ2ΔFosB, and JunD proteins were incubated with labeled probe (25,000 cpm) in 340 mM KCl, 50 mM MgCl2, 1 mM DTT, and 3 μg/ml poly-dIdC (Sigma) at room temperature for 30 min. Samples were separated on 6% nondenaturing polyacrylamide gels at 200 V for 2 h. Gels were dried and autoradiographed.
Luciferase reporter assay
HEK-293 cells (ATCC) were plated at a density of 1 × 105 in 6-well plates and transfected using FuGENE 6 (Roche, Indianapolis, IN, USA) with constructs encoding a 6X-TRE-luciferase reporter (6X-TRE-luc; Clontech) to measure AP-1 activity or a BMP-responsive Xvent2-luciferease reporter (Xvent2-luc) to measure BMP/Smad activity. For AP-1 activity, cells were also transfected with pcJunD and cDNAs encoding the ΔFosB isoforms (pcΔFosB2i3i and pcΔ2ΔFosB) at the indicated concentrations. For BMP/Smad activity, cells were transfected with a cDNA encoding the constitutively active form of BMP receptor IA (ALK3) in the presence or absence of cDNA encoding Smad1-FLAG, Xpress-Smad4, and the ΔFosB isoforms at the indicated concentrations. Transfection efficiency was assessed by co-transfecting an SV40 Renilla luciferase construct (Promega); the total amount of transfected DNA was maintained at 1.25 μg/well by adding empty vector (pcDNA3.1). Dual-Luciferase assay (Promega) was performed according to the manufacturer’s instructions.
Confocal microscopy
For immunofluorescence analysis, cells were cultured on glass coverslips. Cultured cells were fixed in 3.7% (vol/vol) formaldehyde in PBS for 10 min at room temperature. Coverslips were blocked and permeabilized in PBS containing 0.1% BSA, 0.05% saponin, and 5% normal goat serum (Roche) for 30 min at room temperature, followed by incubation with anti-pSmad1 (Upstate, Lake Placid, NY, USA) for 1 h at room temperature. Samples were washed in PBS-BSA-saponin, incubated with fluorescein-conjugated secondary antibody (Invitrogen) for 1 h at room temperature in the dark, and mounted with Fluorosave fluorescent mounting media (Calbiochem, San Diego, CA, USA). Cells were examined using a Zeiss LSM 510 scanning laser confocal microscope. Computer images were recorded, and composites of time courses were compiled, enhanced, and pseudocolorized for fluorescence intensity using Adobe Photoshop.
Statistical analysis
The data are represented as mean ± SD or mean ± SE as indicated. Statistical analysis was performed using Student’s t-test or ANOVA. p < 0.05 was considered statistically significant.
RESULTS
Truncating FosB progressively reduces its transactivating potential
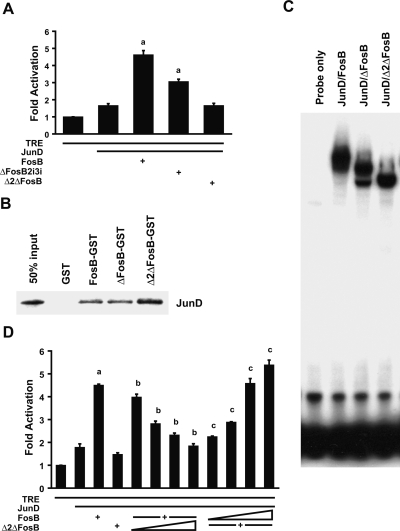
We previously showed that the doubly truncated Δ2ΔFosB induced the expression of several osteoblast marker genes(17) even though it lacks both of the known transactivation domains in FosB, raising the question of whether Δ2ΔFosB contains another unidentified AP-1–transactivating element. To address this question, we determined the relative JunD-dependent AP-1–transactivating abilities of FosB, ΔFosB, and Δ2ΔFosB by measuring the activation of an AP-1 reporter gene (6X-TRE-luc) in HEK293 cells co-transfected with cDNAs encoding the reporter gene, JunD, and FosB, ΔFosB, or Δ2ΔFosB (Fig. 2A). None of the FosB isoforms activated the reporter when expressed without JunD (data not shown). The activity with JunD alone was ∼60% higher than background, and FosB increased the JunD-dependent activity by ∼5-fold. Deletion of first the C-terminal transactivation domain (to generate ΔFosB) and the FHD (to generate Δ2ΔFosB) progressively decreased the activation of the reporter. Δ2ΔFosB failed to enhance the activity of the reporter gene over that obtained with JunD alone, indicating that, whereas ΔFosB can contribute to the transactivation of AP-1–responsive promoters to some degree, the core Δ2ΔFosB protein lacks that ability. The failure of Δ2ΔFosB to further activate the reporter was not because of an inability of Δ2ΔFosB to bind JunD (Fig. 2B) or of the resulting dimer to bind DNA (Fig. 2C).
FIG. 2.
Δ2ΔFosB lacks intrinsic transactivating activity. (A) HEK293 cells were co-transfected with cDNAs encoding the 6X-TRE-luciferase reporter gene (0.5 μg), JunD (0.125 μg), and FosB (0.125 μg), ΔFosB2i3i (0.125 μg), or Δ2ΔFosB (0.125 μg) as indicated, and the luciferase activity was measured. FosB increased luciferase expression 5-fold above that induced by JunD alone. ΔFosB2i3i increased expression by only 3-fold, whereas the expression when Δ2ΔFosB was present was not different from JunD. Data are presented as mean ± SE; a p < 0.01 relative to JunD alone. (B) GST, GST-FosB, GST-ΔFosB, and GST-Δ2ΔFosB fusion proteins bound to glutathione-Sepharose beads were incubated with in vitro-translated JunD and the bound proteins were processed for Western blotting. All of the FosB isoforms bound to JunD. (C) The interaction of JunD-FosB, JunD-ΔFosB, or JunD-Δ2ΔFosB with a TRE-containing oligonucleotide was analyzed in an electromobility shift assay. The three FosB isoforms formed complexes with JunD that bound equally well to the TRE. (D) Activation of the 6X-TRE-luciferase reporter gene by different ratios of FosB and Δ2ΔFosB was measured. HEK293 cells were co-transfected with cDNAs encoding the 6X-TRE-luciferase reporter gene (0.5 μg), JunD (0.125 μg), and FosB (0.125 μg) or Δ2ΔFosB (0.125 μg) as indicated. Increasing the ratio of Δ2ΔFosB to FosB by increasing the amount of Δ2ΔFosB cDNA (0.0125, 0.25, 0.5, or 0.75 μg) co-transfected with 0.125 μg FosB cDNA progressively reduced the activity of the reporter gene, whereas increasing the amount of FosB cDNA (0.0125, 0.25, 0.5, or 0.75 μg) co-transfected with 0.125 μg Δ2ΔFosB cDNA progressively increased the activity. Data are presented as mean ± SE; a p < 0.01 relative to JunD alone; b p < 0.01 relative to JunD plus FosB; c p < 0.01 relative to JunD plus Δ2ΔFosB.
Because Δ2ΔFosB binds JunD and the complex binds to DNA but fails to enhance JunD transcriptional activity on TREs, we next examined the effect of Δ2ΔFosB on the ability of full-length FosB and JunD to transactivate the AP-1 reporter gene when the two FosB isoforms were co-expressed. Co-transfecting increasing amounts of Δ2ΔFosB cDNA with constant amounts of JunD and FosB cDNA caused a progressive decrease in reporter gene activity, and conversely, increasing the amount of FosB cotransfected with constant amounts of JunD and Δ2ΔFosB progressively increased the reporter gene activity (Fig. 2D), indicating that Δ2ΔFosB competitively displaces FosB from the JunD-containing AP-1 complex, thereby antagonizing FosB-induced gene activation. Thus, Δ2ΔFosB can influence AP-1–dependent gene expression despite its lack of intrinsic transactivating activity by acting in a dominant-negative manner to displace other FosB isoforms and possibly other Fos family members(43) from transcriptionally active complexes. Although ΔFosB is also capable of antagonizing FosB activity,(16,26,44) the residual FHD-dependent intrinsic AP-1 transactivation activity (Fig. 2A) prevents it from being a pure antagonist to the effects of FosB and raises the question of whether the FHD contributes to the induction of bone formation by ΔFosB.
Overexpression of Δ2ΔFosB in transgenic mice induces osteosclerosis and reduces fat
To test whether Δ2ΔFosB was sufficient to induce osteosclerosis in vivo, we generated transgenic mice that expressed the Δ2ΔFosB isoform under the control of the TetOp promoter and crossed them with the ENO2-tTA mice used in our previous studies.(17) Consistent with our earlier results, the bitransgenic offspring expressed Δ2ΔFosB in a number of tissues, including bone (Fig. 3A), where this promoter drives expression in osteoblasts but not osteoclasts.(17) Western blot analysis of the FosB isoforms in primary calvarial osteoblasts from the Δ2ΔFosB-expressing mice (Fig. 3B) showed a high level of the 27-kDa Δ2ΔFosB isoform (center lane), whereas both ΔFosB and Δ2ΔFosB isoforms were detected in cells obtained from the original ENO2-ΔFosB bitransgenic mice (right lane). In comparison, the level of expression of endogenous FosB isoforms in osteoblasts from control littermates appeared low at this short exposure (left lane).
FIG. 3.
Expression of the Δ2ΔFosB protein in tissues of the ENO2-Δ2ΔFosB mice. (A) Western blot analysis of Δ2ΔFosB expression in tissues isolated from ENO2-Δ2ΔFosB transgenic mice (Δ2) and littermate controls (C). (B) Western blot analysis of FosB isoforms in extracts from primary calvarial osteoblasts derived from ENO2-ΔFosB and ENO2-Δ2ΔFosB transgenic mice and littermate controls.
Like the ENO2-ΔFosB transgenic mice, there was no alteration of the skeleton, including the skull at birth, and the osteosclerotic phenotype developed progressively postnatally. To determine whether the osteosclerotic phenotype of the original ENO2-ΔFosB transgenic mice was fully recapitulated in ENO2-Δ2ΔFosB transgenic mice, proximal tibias from 10-wk-old mice were sectioned and analyzed by standard histological and histomorphometric procedures. The changes in the ENO2-Δ2ΔFosB transgenic bones were essentially identical to the previously described changes in the bones from the ENO2-ΔFosB mice, which express both the ΔFosB and the Δ2ΔFosB isoforms.(17,18,35) Mineralized tissue, visualized by von Kossa stain, was grossly increased in the ENO2-Δ2ΔFosB mice relative to control littermates to a degree similar to that seen in the ENO2-ΔFosB transgenic animals (Fig. 4A). A detailed histomorphometric analysis of the mice confirmed that, similar to ΔFosB-expressing mice, the trabecular bone volume of ENO2-Δ2ΔFosB transgenic mice was increased by >4-fold over control values (Fig. 4B). The bone formation rate (Fig. 4C) and osteoblast number (Fig. 4D) were strikingly higher in the ENO2-Δ2ΔFosB transgenic mice than in control littermates, whereas osteoclast number remained unchanged (Fig. 4E), indicating that the ENO2-Δ2ΔFosB transgenic mice, like the ENO2-ΔFosB transgenic animals,(17) are osteosclerotic. All these changes were strikingly similar to what is observed in ENO2-ΔFosB mice (Fig. 4).
FIG. 4.
The bone and fat phenotypes of the ENO2-Δ2ΔFosB transgenic mice are similar to those of the ENO2-ΔFosB mice. (A) Von Kossa–stained sections of proximal tibias from 10-wk-old mice that express the ENO2-ΔFosB transgene or the ENO2-Δ2ΔFosB transgene, and nontransgenic littermate. (B–E) Histomorphometric analysis of proximal tibias from ENO2-Δ2ΔFosB and ENO2-ΔFosB transgenic mice and control littermates at 10 wk of age: (B) trabecular bone volume (BV/TV), (C) bone formation rate (BFR/TV), (D) osteoblast number (ObN/Tar), and (E) osteoclast number (OcN/Tar). Data are presented as mean ± SD; a p < 0.01. (F) Western blot analysis of the osteoblastic transcription factor Runx2 in primary bone marrow stromal cell cultures established from ENO2-Δ2ΔFosB transgenic mice and control littermates. Actin was used as an internal loading control. (G) Abdominal fat pad weight and (H) serum leptin levels of 10-wk-old ENO2-Δ2ΔFosB and ENO2-ΔFosB transgenic mice and control littermates. Data are presented as mean ± SD; a p < 0.01.
Finally, we examined the expression of the essential osteoblast differentiation factor Runx2 in differentiating bone marrow mesenchymal stromal cells from the Δ2ΔFosB-expressing mice and their control littermates. Marrow stromal cells were cultured in ascorbic acid, β-glycerophosphate, and dexamethasone for 8 and 16 days, at which times the cells were lysed, and the lysates were analyzed by Western blot for Runx2. Runx2 expression was increased at both time points (Fig. 4F), consistent with the previously reported effect in transfected mesenchymal cells.(17)
In addition to the osteosclerotic bone phenotype, the ENO2-ΔFosB mice exhibit a marked reduction in fat and serum leptin levels.(17) We therefore quantified adipose mass and serum leptin in the ENO2-Δ2ΔFosB mice and found that both the abdominal adipose tissue (Fig. 4G) and serum leptin levels (Fig. 4H) were significantly reduced, as in the ENO2-ΔFosB mice. Similar bone and fat phenotypes were observed in two different founder lines.
Thus, the doubly truncated and AP-1 transactivation–deficient Δ2ΔFosB isoform has the same effects on bone development and fat accumulation as the larger ΔFosB isoform when overexpressed in vivo, indicating that the N-terminal sequences of ΔFosB, including the FHD and its intrinsic AP-1 transactivating activity are dispensable. Therefore, in seeking specific mechanisms that mediate the ΔFosB-induced increase in bone formation, we sought to identify cell autonomous changes in function that are induced by overexpressing Δ2ΔFosB.
ΔFosB and Δ2ΔFosB enhance Smad-dependent transactivation of the BMP-responsive Xvent2 promoter
BMP-induced Smad-dependent gene regulation is a key osteogenic regulatory mechanism(30,31) that induces the expression of several key osteoblast genes, including runx2, collagen type I, and osteocalcin. The fact that both ΔFosB and Δ2ΔFosB induce the increased expression of all three of these genes(17) suggested that the truncated FosB isoforms might act, at least in part, by enhancing the activity of this important osteogenic regulatory pathway.
AP-1 components have been reported to interact with Smads and enhance Smad-dependent transcriptional responses.(44–47) We therefore examined the effect of ΔFosB and Δ2ΔFosB on Smad-dependent activation of the Xvent2-luciferase reporter gene (Xvent-luc), which is directly activated by Smads downstream of BMP-induced signaling(48,49) (Fig. 5). Cells were co-transfected with the following cDNAs: the Xvent2-luc reporter, a constitutively active form of the ALK3 BMP receptor, and various combinations of Smad1-FLAG plus Xpress-Smad4, and Δ2ΔFosB or ΔFosB2i3i (producing only ΔFosB and not Δ2ΔFosB). Co-expression of ALK3 and Smads 1 and 4 increased reporter activity by 2-fold or more, as expected. Expressing ΔFosB in the absence of Smads increased reporter activity to a degree similar to the increase obtained by Smads1 and 4, whereas Δ2ΔFosB had no effect, suggesting that the residual AP-1 activity of ΔFosB, although decreased, can activate the Xvent promoter independent of Smads. ΔFosB also increased the ALK3/Smad1,4-dependent reporter activity 3-fold, consistent with previous reports of cooperation between Smads and AP-1 factors.(44,46,47) Interestingly, Δ2ΔFosB induced a smaller but significant increase in the ALK3/Smad1,4-dependent reporter activity, showing that the truncated FosB proteins cooperate with Smads independent of AP-1 activity. When JunD was also overexpressed, as in the experiments with the 6X-TRE-luc reporter, both ΔFosB and Δ2ΔFosB induced responses that were significantly greater than those induced by JunD alone, both in the presence and in the absence of Smads 1 and 4, despite the absence of a TRE motif in the Xvent promoter and in contrast to the inability of Δ2ΔFosB to increase JunD-induced activation of the 6X-TRE-luc reporter, but the presence of JunD did not alter the relative abilities of the two truncated FosB isoforms to increase Smad-induced activation of the Xvent-luc reporter. Thus, these results suggest that Δ2ΔFosB can enhance Smad transcriptional activity despite its lack of intrinsic AP-1 transactivating activity.
FIG. 5.
Δ2ΔFosB transactivates the BMP-responsive Xvent2 promoter. HEK293 cells were co-transfected with cDNAs encoding the Xvent2-luciferase reporter gene, a constitutively active ALK3 BMP receptor, Smads 1 and 4, and ΔFosB2i3i (0.125 μg) or Δ2ΔFosB (0.125 μg) as indicated, and the luciferase activity was measured. ΔFosB2i3i increased luciferase expression to the same extent as Smads 1 and 4, whereas Δ2ΔFosB had no activity. Expressing ΔFosB with the Smads increased expression by >2-fold over that with the Smads alone, whereas Δ2ΔFosB had an intermediate effect. Data are presented as mean ± SE; a p < 0.01 relative to ALK3 alone; b p < 0.05 relative to ALK3 plus Smads1 and 4; bb p < 0.01 relative to ALK3 plus Smads1 and 4.
ΔFosB and Δ2ΔFosB modulate Smad1 expression
Activation of the BMP receptors leads to the phosphorylation of Smads 1, 5, and 8 (the R-Smads), the binding of the phosphorylated R-Smads to Smad4, and the translocation of the resulting complex to the nucleus.(30,31,50–52) Thus, in addition to changes in BMP signaling that would result from an increase in the expression of BMP and/or BMP receptors, BMP signaling and the expression of BMP-responsive genes could be enhanced by changes in the expression of one or more of the Smads that increased the ratio of R-Smads to I-Smads. We therefore determined whether overexpressing ΔFosB and Δ2ΔFosB affected the basal or BMP-induced expression of some of the components of the BMP/Smad signaling pathway.
To elucidate the effects of the truncated FosB isoforms on expression of components of the BMP/Smad pathway, we first determined whether the effects of overexpressing ΔFosB or Δ2ΔFosB on the expression of BMP-2 or the BMPIa and BMPIb receptors. C2C12 cells that were stably transfected with ΔFosB or Δ2ΔFosB were grown to confluence and treated with rhBMP-2 for 0, 1, 1.5, and 2 h. Total RNA was extracted, and gene expression was analyzed by Northern blot. Neither ΔFosB nor Δ2ΔFosB induced changes in the mRNAs that encode BMP-2 or BMP receptors relative to matched controls, regardless of whether the cells were treated with BMP or not (data not shown), indicating that the truncated FosB isoforms were not activating a BMP autocrine loop. Furthermore, co-expression of Noggin in the absence of added BMP2 did not alter Smad1 mRNA expression relative to control (data not shown).
We examined the effect of ΔFosB and Δ2ΔFosB on the expression of Smad1. In the absence of BMP-2 treatment, the truncated FosB isoforms had little effect on the amounts of Smad1 mRNA, and BMP treatment had little or no effect on Smad1 mRNA in the absence of the overexpressed FosB isoforms, consistent with an earlier report.(53) To determine the effects of the individual ΔFosB isoforms on Smad1 expression, C2C12 cells were transiently transfected with cDNAs encoding Δ2ΔFosB, wildtype ΔFosB, or ΔFosB2i3i, in which the methionines at codons 50 and 79 were mutated to isoleucine so that only ΔFosB was generated (Fig. 6A). As in the stably transfected cells, none of the ΔFosB isoforms affected the amount of Smad1 mRNA in the absence of BMP-2 treatment, and BMP-2 failed to alter the amount of Smad1 mRNA in the untransfected cells and induced only a slight increase in Smad1 mRNA in the ΔFosB2i3i-transfected cells. In contrast, BMP-2 induced similar significant increases in the Smad1 mRNA levels in the cells transfected with WT ΔFosB or Δ2ΔFosB. Thus, Δ2ΔFosB, which lacks intrinsic AP-1 transactivating potential, increased Smad1 expression in the presence of BMP-2. In all these assays, Smad 5 was not affected.
FIG. 6.
ΔFosB isoforms promote the phosphorylation of Smad1 and its translocation to the nucleus. (A) C2C12 cells that were transiently transfected with empty vector (C), ΔFosB (ΔF), ΔFosB2i3i (2i3i), or Δ2ΔFosB (Δ2) were cultured with (right) or without (left) 300 ng/ml rhBMP-2 for 2 h. RNA was extracted and Northern blotted for Smad1. Ethidium bromide staining of 18S rRNA was used to evaluate loading. (B) C2C12 cells that were stably transfected with either empty vector (C), ΔFosB (ΔF), or Δ2ΔFosB (Δ2) were treated with 300 ng/ml rhBMP-2 for the indicated times. RNA was extracted and Northern blotted with a Smad6 probe. (The ΔFosB lanes were run on two separate parts of the gel and the partial images merged to create the figure.) Ethidium bromide staining of 18S rRNA (data not shown) served as an internal control. (C) C2C12 cells stably transfected with ΔFosB2i3i (2i3i), Δ2ΔFosB (Δ2), or empty vector (C) were treated with 300 ng/ml BMP-2 for the indicated times. Whole cell extracts were prepared and Western blotted for Smad6 (top panel). Actin was used as a loading control. (D) C2C12 cells stably transfected with ΔFosB (ΔF), Δ2ΔFosB (Δ2), or empty vector (C) were treated with 300 ng/ml BMP-2 for the indicated times. Nuclear extracts were prepared and Western blotted for pSmad1 (top). JunD was used as a loading control, because we have previously shown that neither treatment with BMP-2 nor overexpressing ΔFosB isoforms alters JunD protein levels.(17) (E) C2C12 cells stably transfected with empty vector (C, top), ΔFosB (ΔF, middle), and Δ2ΔFosB (Δ2, bottom) were treated with rhBMP-2 for 0, 2, or 4 h and fixed and stained for pSmad1 (orange). Confocal images were obtained and processed.
We analyzed the effects of ΔFosB and Δ2ΔFosB on BMP-2–induced expression of inhibitory Smad6 downstream of BMP receptor activation.(54–56) Interestingly, BMP-induced Smad6 expression was not detectably affected by ΔFosB but was markedly reduced in cells expressing Δ2ΔFosB (Figs. 6B and 6C). Thus, not only did Δ2ΔFosB increase Smad1 expression in BMP-2–treated cells but it also reduced the BMP-induced expression of inhibitory Smad6, thereby potentially further favoring BMP responses.
Δ2ΔFosB promotes BMP-induced Smad1 phosphorylation and nuclear translocation
The increased expression of Smad1 and decreased expression of Smad6 in the BMP-treated cells transfected with Δ2ΔFosB would be expected to promote Smad-dependent signaling from BMP receptors to the nucleus. We therefore characterized the effects of the two ΔFosB isoforms on the BMP-2–induced nuclear translocation of phosphorylated Smad1 (pSmad1). C2C12 cells stably transfected with ΔFosB, Δ2ΔFosB, or empty vector were treated with rhBMP-2 for 0, 2, and 4 h. Nuclear extracts were prepared and analyzed by Western blot with anti-pSmad1 (Fig. 6D). As expected, BMP-2 induced a progressive increase in the nuclear levels of pSmad1 in cells transfected with empty vector. More importantly, the nuclear levels of pSmad1 expression in BMP-2–treated ΔFosB- and Δ2ΔFosB-overexpressing cells were higher than those in the cells transfected with empty vector under both basal and BMP-2-stimulated conditions, with the highest levels occurring in the Δ2ΔFosB-overexpressing cells.
The increased levels of nuclear pSmad1 in the ΔFosB- and Δ2ΔFosB-overexpressing cells were confirmed by immunofluorescence microscopy (Fig. 6E). Increased pSmad1 was clearly detected in the nuclei of the cells transfected with the empty vector by 4 h after addition of BMP-2. The BMP-induced nuclear staining of pSmad1 in the ΔFosB- and Δ2ΔFosB-overexpressing cells at 2 and 4 h exceeded that in the vector-transfected cells. Furthermore, nuclear pSmad1 levels were elevated in the Δ2ΔFosB-transfected cells even in the absence of exogenous BMP-2. Thus, the overexpressed truncated FosB isoforms indeed promote the activity of the BMP/Smad pathway. The greater effect of Δ2ΔFosB, and in particular the induction of a distinct nuclear staining of pSmad1 even in the absence of exogenous BMP-2 suggest that this isoform, which lacks AP-1 transcriptional activity, may enhance the responses to an endogenous BMP autocrine loop.
We therefore interpret this data to show that Δ2ΔFosB enhances BMP-induced responses, despite its lack of intrinsic AP-1–dependent transcriptional activity. Thus, increased Smad 1 expression and increased Smad1 phosphorylation and nuclear translocation, together with Smad4 and possibly Δ2ΔFosB, may enhance BRE-dependent transcription of osteoblast target genes, ultimately increasing bone formation.
DISCUSSION
We and others have previously shown that overexpression of the AP-1 transcription factors ΔFosB and Fra-1 cause severe osteosclerosis because of cell autonomous effects on osteoblast bone-forming activity.(12,17) ΔFosB is a truncated isoform of the AP-1 transcription factor FosB that affects gene expression and cell function.(16,26,44,57) Elucidation of the mechanisms by which ΔFosB promotes bone formation is complicated by the fact that the use of alternative initiation sites during translation of the ΔFosB mRNA produces at least two isoforms: ΔFosB (FosB 1–237) and Δ2ΔFosB (FosB 79–237). Osteogenic stimuli induce changes in the relative levels of full length FosB, ΔFosB, and Δ2ΔFosB,(20,21) and we have shown that the endogenous levels of ΔFosB and Δ2ΔFosB proteins increase and decrease, respectively, during the progression of in vitro differentiation of primary mouse calvarial osteoblasts, whereas the endogenous level of full-length FosB protein seems to be stable,(17) suggesting that the truncated FosB isoforms may play different physiological roles in osteoblast development and function. Δ2ΔFosB retains the basic domain and leucine zipper that mediate the formation of heterodimers with Jun proteins and subsequent binding to DNA, but it has no known transactivating domains and consequently, as shown here (Fig. 2A), lacks the intrinsic ability to enhance gene activation by Jun-containing AP-1 complexes binding to TREs. In fact, Δ2ΔFosB antagonizes the AP-1 activity of FosB (but not JunD) in a dominant-negative manner, whereas ΔFosB is only a partial antagonist that retains a reduced AP-1 transactivation activity (Fig. 1A). Thus, by studying the function of Δ2ΔFosB, one can determine whether the residual AP-1 transactivation function of ΔFosB plays a meaningful role in inducing the bone and fat phenotype observed in ΔFosB transgenic mice.(17–19)
Despite its lack of AP-1 activity and its dominant-negative effect on FosB-mediated transactivation from a TRE-containing promoter, Δ2ΔFosB shares the ability of ΔFosB to upregulate the transcription of several osteoblast marker genes (runx2, osteocalcin, and collagen I) in vitro,(17) a finding confirmed here in cultured bone marrow stromal cells from the Δ2ΔFosB mice. More importantly, the phenotype we obtained by overexpressing Δ2ΔFosB in mice clearly shows that the doubly truncated Δ2ΔFosB isoform recapitulates the effects of ΔFosB on bone formation in vivo as well as in vitro. Thus, neither the C-terminal transactivation domain, which is deleted in ΔFosB,(22,24,58) nor the N-terminal Fos homology domain, which has also been implicated in transcriptional activation(27,28,59) and is deleted in Δ2ΔFosB, are needed to induce the striking osteoblastic and adipocytic phenotypes of the ENO2-ΔFosB mice. Indeed, the in vivo and in vitro data we report here clearly establish that the induction of osteosclerosis does not require any intrinsic AP-1–transactivating activity of the FosB isoforms. In contrast, overexpression of FosB does not seem to alter the skeleton.(11) Taken together, these results suggest that the Fos proteins with reduced (ΔFosB) or absent (Δ2ΔFosB) AP-1 transcriptional activity may increase bone formation by displacing transcriptionally active c-Fos and/or FosB from AP-1 or other transcription complexes in a dominant-negative manner and/or by enhancing the transcriptional activity of factors other than AP-1. It becomes important to identify the regulatory mechanisms that are altered by the truncated Fos proteins to result in such a profound increase in bone formation.
Our earlier observation that overexpressing the truncated FosB isoforms increased the expression of several downstream targets of the BMP/Smad signaling pathway(17) suggested that the truncated FosB isoforms might promote bone formation, at least in part, by enhancing the activity of this important regulatory pathway. Interestingly, and highly relevant to our studies here, AP-1 proteins form complexes with Smads and enhance TGFβ- and BMP-induced gene transcription,(44–46,60) further supporting the possibility that the truncated FosB proteins may increase in vivo bone formation at least in part by modulating BMP-Smad signaling. The ΔFosB proteins could potentially increase the activity of the BMP/Smad pathway by any of several mechanisms, including upregulating the expression of BMPs or BMP receptors to enhance an autocrine stimulus, altering the relative levels of expression of R-Smads and inhibitory Smads, increasing the translocation of activated R-Smads into the nucleus or modulating the Smad-induced regulation of BMP target gene expression at the promoter level. Although we did not find evidence for a contribution from increased amounts of BMP or BMP receptors, we found that ΔFosB and Δ2ΔFosB can regulate Smad1 expression, enhance Smad 1/4 transcriptional activity, and favor Smad1 phosphorylation and translocation to the nucleus, while interfering with the BMP-induced expression of the inhibitory Smad6.
The significantly increased Smad1 expression levels induced by Δ2ΔFosB and to a lesser extent by ΔFosB (Fig. 6A), together with lower levels of inhibitory Smad6, would be expected to favor BMP signaling. Our observation that Δ2ΔFosB increased the phosphorylation of Smad1 and the nuclear accumulation of pSmad1, even in the absence of exogenously added BMPs (Fig. 6), indicates that it does indeed positively modulate BMP/Smad signaling. In these experiments, ΔFosB also increased pSmad1 and its nuclear translocation but to a lesser extent than Δ2ΔFosB. In interpreting this observation, one cannot exclude the possibility that the observed effects of ΔFosB are caused in part by ΔFosB’s partial antagonistic activity on FosB and c-Fos transactivation(43,44) and in part to the increased levels of expression of Δ2ΔFosB that is always observed when expressing wildtype ΔFosB. Consistent with this possibility, the ΔFosB mutant (ΔFosB 2i3i) that cannot generate Δ2ΔFosB failed to increase Smad 1 expression (Fig. 6A).
We conclude that the osteosclerotic phenotype of the Δ2ΔFosB mouse indicates that the AP-1 transcriptional activity of ΔFosB is not needed for the induction of high bone mass. In fact, the osteosclerosis induced by the overexpression of the truncated FosB proteins (and by the other transcriptionally inactive Fos family members(3,12)) could be the result of a dominant-negative action of these proteins on key FosB- and/or c-Fos–dependent mechanisms that moderate bone formation. We found that both truncated FosB isoforms, and Δ2ΔFosB in particular, can affect the BMP/Smad pathway by at least two distinct mechanisms. First, the truncated FosB proteins modulate Smad 1 and Smad6 expression levels. Second, they regulate Smad1 activity, as shown by the effects of the ΔFosB proteins on the nuclear accumulation of phospho-Smad1. These findings favor the interpretation that Δ2ΔFosB enhances BMP signaling and increases osteoblast target gene expression by altering Smad1 expression, phosphorylation, and translocation to the nucleus, possibly contributing to the observed increase in bone formation both in vitro and in vivo.
As much as these positive effects on BMP signaling most probably contribute to the high bone formation observed in mice overexpressing ΔFosB or Δ2ΔFosB, they may not be the whole explanation of the phenotype. We have also identified other transcription factors that bind both ΔFosB and Δ2ΔFosB, including C/EBPβ(19) and Runx2 (unpublished data), both of which have important transcriptional regulatory functions in mesenchymal cell differentiation,(61–67) as well as a novel zinc finger-containing factor, Zfp521, that is highly expressed in developing bones (unpublished data). However, whereas it is likely that at least some of these interactions with other transcription factors also participate in the induction of bone formation by the truncated FosB isoforms and by the Fra proteins, this study highlights the facts that the intrinsic AP-1 activity of ΔFosB is dispensable for the cell autonomous effects on osteoblasts and that truncated FosB proteins are capable of enhancing BMP signaling in osteoblasts, thereby contributing to the osteosclerotic phenotype observed in transgenic mice overexpressing ΔFosB or Δ2ΔFosB.
Interestingly, we recently obtained evidence (G Rowe, T Green, L Neff, C Choi, H Sauto, M Kueiburg, S Chakravarty, WC Horne, GI Shulman, EJ Nestler, and R Baron, unpublished data) that expression of ΔFosB in the hypothalamus also favors bone formation by a mechanism that is independent of the cell autonomous effects of ΔFosB in osteoblasts shown here and in our previous reports(17,19) and that is most likely independent of the BMP signaling pathway. This pro-osteogenic action of ΔFosB in the hypothalamus also seems to be a consequence of the absence of the AP-1 transactivation domain of FosB. Interestingly, a similar and even stronger increase in bone formation occurred when a JunD construct that lacked the transactivation domain was expressed, suggesting that the endogenous expression of the naturally truncated FosB isoforms may also alter central regulation in a manner that promotes bone formation.
In summary, overexpression of the further truncated and AP-1 transcriptionally inactive Δ2ΔFosB isoform of FosB favors BMP signaling and increases bone formation, recapitulating the skeletal phenotype of ΔFosB overexpressing mice. These results establish that the osteosclerosis induced by truncated FosB isoforms does not require intrinsic FosB AP-1 transcriptional activity.
ACKNOWLEDGMENTS
This work was supported by a postdoctoral fellowship from the Brown Alexander Cox Foundation to GS, a UNCF-Merck Dissertation Fellowship to GCR, a postdoctoral fellowship from the Danish Research Council to MK, and a grant from the National Institutes of Health (AR48218) to RB. The work was also supported by a grant from NIH to the Yale Core Center for Musculoskeletal Diseases (AR46032). The authors thank Dr William Horne for help in writing this manuscript. Dr Eric Nestler is thanked for initially providing us with ENO2-tTA and TetOp-ΔFosB transgenic mice. We also thank Karen Ford and Wayne Grant for excellent technical assistance.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Parfitt AM. Targeted and nontargeted bone remodeling: Relationship to basic multicellular unit origination and progression. Bone. 2002;30:5–7. doi: 10.1016/s8756-3282(01)00642-1. [DOI] [PubMed] [Google Scholar]
- 2.Jilka RL. Biology of the basic multicellular unit and the pathophysiology of osteoporosis. Med Pediatr Oncol. 2003;41:182–185. doi: 10.1002/mpo.10334. [DOI] [PubMed] [Google Scholar]
- 3.Wagner EF. Functions of AP1 (Fos/Jun) in bone development. Ann Rheum Dis. 2002;61(Suppl II):ii40–ii42. doi: 10.1136/ard.61.suppl_2.ii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005;208:126–140. doi: 10.1111/j.0105-2896.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 5.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 6.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 7.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 8.Kenner L, Hoebertz A, Beil T, Keon N, Karreth F, Eferl R, Scheuch H, Szremska A, Amling M, Schorpp-Kistner M, Angel P, Wagner EF. Mice lacking JunB are osteopenic due to cell-autonomous osteoblast and osteoclast defects. J Cell Biol. 2004;164:613–623. doi: 10.1083/jcb.200308155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71:577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z-Q, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- 11.Grigoriadis AE, Schellander K, Wang ZQ, Wagner EF. Osteoblasts are target cells for transformation in c-fos transgenic mice. J Cell Biol. 1993;122:685–701. doi: 10.1083/jcb.122.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jochum W, David J-P, Elliott C, Wutz A, Plenk H, Jr, Matsuo K, Wagner EF. Increased bone formation and osteosclerosis in mice overexpressing the transcription factor Fra-1. Nat Med. 2000;6:980–984. doi: 10.1038/79676. [DOI] [PubMed] [Google Scholar]
- 13.Hoebertz A, Eferl R, Karreth F, Schilling AF, Priemel M, Amling M, Wagner EF. Fra-2:A novel regulator of bone remodeling. J Bone Miner Res. 2003;18(S2):S3. [Google Scholar]
- 14.Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 15.Gruda MC, van Amsterdam J, Rizzo CA, Durham SK, Lira S, Bravo R. Expression of FosB during mouse development: Normal development of FosB knockout mice. Oncogene. 1996;12:2177–2185. [PubMed] [Google Scholar]
- 16.Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- 17.Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R. Overexpression of ΔFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–990. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 18.Sims NA, Sabatakos G, Chen J-S, Kelz MB, Nestler EJ, Baron R. Regulating ΔFosB expression in adult Tet-Off-ΔFosB transgenic mice alters bone formation and bone mass. Bone. 2002;30:32–39. doi: 10.1016/s8756-3282(01)00622-6. [DOI] [PubMed] [Google Scholar]
- 19.Kveiborg M, Sabatakos G, Chiusaroli R, Wu M, Philbrick WM, Horne WC, Baron R. ΔFosB induces osteosclerosis and decreases adipogenesis by two independent cell-autonomous mechanisms. Mol Cell Biol. 2004;24:2820–2830. doi: 10.1128/MCB.24.7.2820-2830.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sciaudone M, Gazzerro E, Priest L, Delany AM, Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–5639. doi: 10.1210/en.2003-0463. [DOI] [PubMed] [Google Scholar]
- 21.Inoue D, Kido S, Matsumoto T. Transcriptional induction of FosB/ΔFosB gene by mechanical stress in osteoblasts. J Biol Chem. 2004;279:49795–49803. doi: 10.1074/jbc.M404096200. [DOI] [PubMed] [Google Scholar]
- 22.Wisdom R, Yen J, Rashid D, Verma IM. Transformation by FosB requires a trans-activation domain missing in FosB2 that can be substituted by heterologous activation domains. Genes Dev. 1992;6:667–675. doi: 10.1101/gad.6.4.667. [DOI] [PubMed] [Google Scholar]
- 23.Chen R-H, Juo PC-H, Curran T, Blenis J. Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. Oncogene. 1996;12:1493–1502. [PubMed] [Google Scholar]
- 24.Skinner M, Qu S, Moore C, Wisdom R. Transcriptional activation and transformation by FosB protein require phosphorylation of the carboxyl-terminal activation domain. Mol Cell Biol. 1997;17:2372–2380. doi: 10.1128/mcb.17.5.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobrzanski P, Noguchi T, Kovary K, Rizzo CA, Lazo PS, Bravo R. Both products of the fosB gene, FosB and its short form, FosB/SF, are transcriptional activators in fibroblasts. Mol Cell Biol. 1991;11:5470–5478. doi: 10.1128/mcb.11.11.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mumberg D, Lucibello FC, Schuermann M, Muller R. Alternative splicing of fosB transcripts results in differentially expressed mRNAs encoding functionally antagonistic proteins. Genes Dev. 1991;5:1212–1223. doi: 10.1101/gad.5.7.1212. [DOI] [PubMed] [Google Scholar]
- 27.Jooss KU, Funk M, Muller R. An autonomous N-terminal transactivation domain in Fos protein plays a crucial role in transformation. EMBO J. 1994;13:1467–1475. doi: 10.1002/j.1460-2075.1994.tb06401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wisdom R, Verma IM. Proto-oncogene FosB: The amino terminus encodes a regulatory function required for transformation. Mol Cell Biol. 1993;13:2635–2643. doi: 10.1128/mcb.13.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: Stable variants of ΔFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 31.Wan M, Cao X. BMP signaling in skeletal development. Biochem Biophys Res Commun. 2005;328:651–657. doi: 10.1016/j.bbrc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Kelz MB, Zeng G, Sakai N, Steffen C, Shockett PE, Picciotto MR, Duman RS, Nestler EJ. Transgenic animals with inducible, targeted gene expression in brain. Mol Pharmacol. 1998;54:495–503. doi: 10.1124/mol.54.3.495. [DOI] [PubMed] [Google Scholar]
- 33.Shockett P, Difilippantonio M, Hellman N, Schatz DG. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci USA. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang Y-J, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 35.Kveiborg M, Chiusaroli R, Sims NA, Wu M, Sabatakos G, Horne WC, Baron R. The increased bone mass in ΔFosB transgenic mice is independent of circulating leptin levels. Endocrinology. 2002;143:4304–4309. doi: 10.1210/en.2002-220420. [DOI] [PubMed] [Google Scholar]
- 36.Sims NA, Clement-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest. 2000;106:1095–1103. doi: 10.1172/JCI10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 38.Baron R, Vignery A, Neff L, Silverglate A, Santa Maria A. Processing of undecalcified bone specimens for bone histomorphometry. In: Recker RR, editor. Bone Histomorphometry: Techniques and Interpretation. vol 1. Boca Raton, FL, USA: CRC Press; 1983. pp. 13–35. [Google Scholar]
- 39.Bellows CG, Heersche JNM, Aubin JE. Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev Biol. 1990;140:132–138. doi: 10.1016/0012-1606(90)90060-v. [DOI] [PubMed] [Google Scholar]
- 40.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–534. [PubMed] [Google Scholar]
- 41.Sambrooke J, Fritsch EF, Maniatis T. Plainview, NY, USA: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 42.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yen J, Wisdom RM, Tratner I, Verma IM. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc Natl Acad Sci USA. 1991;88:5077–5081. doi: 10.1073/pnas.88.12.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamura Y, Hua X, Bergelson S, Lodish HF. Critical role of Smads and AP-1 complex in transforming growth factor-β-dependent apoptosis. J Biol Chem. 2000;275:36295–36302. doi: 10.1074/jbc.M006023200. [DOI] [PubMed] [Google Scholar]
- 45.Liberati NT, Datto MB, Frederick JP, Shen X, Wong C, Rougier-Chapman EM, Wang X-F. Smads bind directly to the Jun family of AP-1 transcription factors. Proc Natl Acad Sci USA. 1999;96:4844–4849. doi: 10.1073/pnas.96.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai C-F, Cheng S-L. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-β in normal human osteoblastic cells. J Biol Chem. 2002;277:15514–15522. doi: 10.1074/jbc.M200794200. [DOI] [PubMed] [Google Scholar]
- 47.Liang C-L, Chen J-L, Hsu Y-P, Ou JT, Chang Y-S. Epstein-Barr virus BZLF1 gene is activated by transforming growth factor-β through cooperativity of Smads and c-Jun/c-Fos proteins. J Biol Chem. 2002;277:23345–23357. doi: 10.1074/jbc.M107420200. [DOI] [PubMed] [Google Scholar]
- 48.Onichtchouk D, Gawantka V, Dosch R, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. The Xvent-2 homeobox gene is part of the BMP-4 signalling pathway controlling dorsoventral patterning of Xenopus mesoderm. Development. 1996;122:3045–3053. doi: 10.1242/dev.122.10.3045. [DOI] [PubMed] [Google Scholar]
- 49.Candia AF, Watabe T, Hawley SHB, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KWY. Cellular interpretation of multiple TGF-β signals: Intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development. 1997;124:4467–4480. doi: 10.1242/dev.124.22.4467. [DOI] [PubMed] [Google Scholar]
- 50.Miyazono K. Positive and negative regulation of TGF-β signaling. J Cell Sci. 2000;113:1101–1109. doi: 10.1242/jcs.113.7.1101. [DOI] [PubMed] [Google Scholar]
- 51.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 52.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T. Smad1 and Smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun. 1997;238:574–580. doi: 10.1006/bbrc.1997.7325. [DOI] [PubMed] [Google Scholar]
- 54.Afrakhte M, Moren A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin C-H, Heldin N-E, ten Dijke P. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family members. Biochem Biophys Res Commun. 1998;249:505–511. doi: 10.1006/bbrc.1998.9170. [DOI] [PubMed] [Google Scholar]
- 55.Hata A, Lagna G, Massague J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takase M, Imamura T, Sampath TK, Takeda K, Ichijo H, Miyazono K, Kawabata M. Induction of Smad6 mRNA by bone morphogenetic proteins. Biochem Biophys Res Commun. 1998;244:26–29. doi: 10.1006/bbrc.1998.8200. [DOI] [PubMed] [Google Scholar]
- 57.Tahara K, Tsuchimoto D, Tominaga Y, Asoh S, Ohta S, Kitagawa M, Horie H, Kadoya T, Nakabeppu Y. ΔFosB, but not FosB, induces delayed apoptosis independent of cell proliferation in the Rat1a embryo cell line. Cell Death Differ. 2003;10:496–507. doi: 10.1038/sj.cdd.4401173. [DOI] [PubMed] [Google Scholar]
- 58.Metz R, Kouzarides T, Bravo R. A C-terminal domain in FosB, absent in FosB/SF and Fra-1, which is able to interact with the TATA binding protein, is required for altered cell growth. EMBO J. 1994;13:3832–3842. doi: 10.1002/j.1460-2075.1994.tb06694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucibello FC, Neuberg M, Jenuwein T, Muller R. Multiple regions of v-Fos protein involved in the activation of AP1-dependent transcription: Is trans-activation crucial for transformation. New Biol. 1991;3:671–677. [PubMed] [Google Scholar]
- 60.Zhang Y, Feng X-H, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature. 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- 61.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 62.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao Y-H, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 63.Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JHM, Owen MJ, Mertelsmann R, Zabel BU, Olsen BR. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 64.Lane MD, Tang Q-Q, Jiang M-S. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun. 1999;266:677–683. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- 65.Gutierrez S, Javed A, Tennant DK, van Rees M, Montecino M, Stein GS, Stein JL, Lian JB. CCAAT/enhancer-binding proteins (C/EBP) β and δ activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J Biol Chem. 2002;277:1316–1323. doi: 10.1074/jbc.M106611200. [DOI] [PubMed] [Google Scholar]
- 66.Shirakawa K, Maeda S, Gotoh T, Hayashi M, Shinomiya K, Ehata S, Nishimura R, Mori M, Onozaki K, Hayashi H, Uematsu S, Akira S, Ogata E, Miyazono K, Imamura T. CCAAT/enhancer-binding protein homologous protein (CHOP) regulates osteoblast differentiation. Mol Cell Biol. 2006;26:6105–6116. doi: 10.1128/MCB.02429-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hata K, Nishimura R, Ueda M, Ikeda F, Matsubara T, Ichida F, Hisada K, Nokubi T, Yamaguchi A, Yoneda T. A CCAAT/enhancer binding protein β isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol Cell Biol. 2005;25:1971–1979. doi: 10.1128/MCB.25.5.1971-1979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]