Abstract
Introduction
B-cell leukemia/lymphoma 2 (Bcl2) is a proto-oncogene best known for its ability to suppress cell death. However, the role of Bcl2 in the skeletal system is unknown. Bcl2 has been hypothesized to play an important anti-apoptotic role in osteoblasts during anabolic actions of PTH. Although rational, this has not been validated in vivo; hence, the impact of Bcl2 in bone remains unknown.
Materials and Methods
The bone phenotype of Bcl2 homozygous mutant (Bcl2 −/−) mice was analyzed with histomorphometry and μCT. Calvarial osteoblasts were isolated and evaluated for their cellular activity. Osteoclastogenesis was induced from bone marrow cells using RANKL and macrophage-colony stimulating factor (M-CSF), and their differentiation was analyzed. PTH(1-34) (50 μg/kg) or vehicle was administered daily to Bcl2 +/+ and Bcl2 −/− mice (4 days old) for 9 days to clarify the influence of Bcl2 ablation on PTH anabolic actions. Western blotting and real-time PCR were performed to detect Bcl2 expression in calvarial osteoblasts in response to PTH ex vivo.
Results
There were reduced numbers of osteoclasts in Bcl2 −/− mice, with a resultant increase in bone mass. Bcl2 −/− bone marrow–derived osteoclasts ex vivo were significantly larger in size and short-lived compared with wildtype, suggesting a pro-apoptotic nature of Bcl2 −/− osteoclasts. In contrast, osteoblasts were entirely normal in their proliferation, differentiation, and mineralization. Intermittent administration of PTH increased bone mass similarly in Bcl2 +/+ and Bcl2 −/− mice. Finally, Western blotting and real-time PCR showed that Bcl2 levels were not induced in response to PTH in calvarial osteoblasts.
Conclusions
Bcl2 is critical in osteoclasts but not osteoblasts. Osteoclast suppression is at least in part responsible for increased bone mass of Bcl2 −/− mice, and Bcl2 is dispensable in PTH anabolic actions during bone growth.
Key words: PTH, B-cell leukemia/lymphoma 2, anabolic, osteoclasts, osteoblasts
INTRODUCTION
The proto-oncogene, B-cell leukemia/lymphoma 2 (Bcl2), was originally identified in patients with follicular B-cell lymphoma where Bcl2 is translocated to juxtapose with the immunoglobulin heavy chain locus, resulting in constitutive accumulation of their messenger RNA.(1) The oncogenic nature of Bcl2 was shown in transgenic mice with lymphocyte targeted Bcl2 gain of function where an extended B-cell lifespan and malignant lymphoma were noted.(2) Accumulating evidence indicates that Bcl2 suppresses apoptosis in certain cells of the myeloid, lymphoid, and neuronal lineages.(3,4) A gene targeted mouse model in which Bcl2 was inactivated showed a significant role of Bcl2 in development of the lymphoid system.(5) Bcl2 deficient (Bcl2 −/−) mice are viable at birth with a normal hematopoietic component initially, but are much smaller than their littermates, and with time, the number of lymphocytes is markedly decreased from peripheral blood because of apoptosis.(5) The exact mechanism for Bcl2 −/− dwarfism is unknown. It could be attributed to the delay of growth plate turnover because Bcl2 plays a role in growth plate chondrocytes.(6) However, considering that Bcl2 −/− mice do not have a short-limb skeletal phenotype, but extreme smallness with normal body proportions, it is unlikely that the dwarfism is solely caused by a chondrodysplasia. Hence, Bcl2 ablation likely has an impact on skeletal growth through its action in osteoclasts and/or osteoblasts.
Regulation of bone cell apoptosis holds tremendous therapeutic value for the treatment of osteoporosis.(7) In osteoporosis, osteoclast activity typically predominates over osteoblast activity, resulting in a net decrease in bone mass. Therefore, suppression of osteoclast activity by promoting osteoclast apoptosis might be one strategy to increase bone mass. Similarly, enhancement of osteoblast activity by extending the lifespan of osteoblasts could result in a net increase in bone mass. Because Bcl2 is associated with apoptosis, deregulated osteoclast and osteoblast apoptosis may contribute to development of the Bcl2 −/− dwarfism. Osteoclasts are multinucleated cells derived from the myeloid lineage.(8) Recent findings suggest that early stage B lymphopoiesis may have a regulatory effect in osteoclastogenesis.(9,10) Furthermore, the subset of bone marrow B cells has been reported to hold potential to differentiate into osteoclasts in vitro.(11,12) Growing evidence indicates that osteoblasts play a pivotal role in the maintenance of hematopoietic stem cell (HSC) niche and myeloid differentiation.(13,14) Evidence shows that osteoblasts are necessary for the maturation of bone marrow B lymphopoiesis.(15) Therefore, a close relationship may exist between the B-lymphoid lineage and osteoclastogenesis. Because Bcl2 −/− mice have defects in lymphopoiesis, one may speculate that osteoclast activity may also be deregulated. Bcl2 has been reported to be an important regulator of apoptosis in osteoblasts as well.(16,17) Notably, Bcl2 has been hypothesized to be a key molecule that mediates PTH-induced anti-apoptotic effects in osteoblasts.(18) PTH increases bone mass when administered intermittently,(19) and because of these anabolic actions, is now used for the treatment of postmenopausal osteoporosis in the United States.(20) However, the molecular mechanisms underlying the anabolic actions of PTH administration are unclear. One proposed mechanism is that PTH extends the lifespan of osteoblasts by upregulating Bcl2 in a Runx2-dependent manner.(18,21) Activation of Bcl2 would turn mature osteoblasts into long-living osteoblasts, resulting in enhanced bone formation. Although such a hypothesis is supported by in vitro studies,(18,22) it has not been confirmed through in vivo experiments. Hence, the role of Bcl2 as a pivotal gene in apoptosis of osteoblasts during the anabolic actions of PTH in bone has not been validated.
In this study, the impact of Bcl2 ablation was assessed on skeletal development to clarify the role of Bcl2 in osteoclasts and osteoblasts. We further tested the hypothesis that Bcl2 plays a critical function in the anabolic actions of PTH during bone growth. The study found that Bcl2 plays a pivotal role in osteoclasts but not osteoblasts and that Bcl2 is dispensable for the anabolic actions of PTH during bone growth.
MATERIALS AND METHODS
Phenotypic characterization of Bcl2−/− mice and TUNEL staining
Experimental protocols were approved, and all animals were treated in accordance with the guidelines of the University Committee on Use and Care of Animals of the University of Michigan. Mice heterozygous mutant for Bcl2 (B6;129S2-Bcl2tm1Sjk) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Tail DNA genotyping was performed by PCR in the presence of Bcl2 (5`-CTTTGTGGAACTGTACGGCCCCAGCATGCG-3`; 5`-ACAGCCTGCAGCTTTGTTTCATGGTACATC-3`) and neo sequences (5`-TCTGGACGAAGAGCATCAGGG-3`; 5`-CAAGCAGGCATCGCCATG-3`). Genotypes were confirmed by Western blotting of tissue lysate from the kidney. Microradiography of mice at day 11 was performed to evaluate the gross skeletal pattern. Body weight of mice was recorded daily from day 4 to day 13 after birth. For histological evaluation, paraffin sections were generated from the kidney and stained with H&E. Spleen from 13-day-old mice was paraffin-embedded and used to detect apoptosis using the In situ Cell Death Detection Assay (Roche, Penberg, Germany) following the manufacturer's instructions. Briefly, the sections were incubated with proteinase K. After rinsing with PBS, TUNEL reaction mixture was applied. The sections were counterstained with hematoxylin.
Flow cytometry
The bone marrow from the long bones of the day 13 mice was flushed with saline. Splenocytes were mechanically isolated by gently scraping spleen and passing cells through a needle. Mononuclear cells (1 × 106) were incubated with a combination of FITC-conjugated anti-mouse CD3 antibody and R-phycoerythrin (R-PE)–conjugated anti-mouse CD19 antibody, FITC-conjugated IgG and R-PE–conjugated IgG were used for isotype controls. Cell counting of CD3+ and CD19+ cells was performed using the BD FACSVantage system (BD Biosciences, Mountain View, CA, USA). All antibodies used in flow cytometry were purchased from BD Biosciences.
Tibias of Bcl2−/− mice, osteoclast perimeter, and TRACP5b
Formalin-fixed tibias of day 13 mice were decalcified in 10% EDTA, embedded in paraffin, and processed for H&E and TRACP staining. The Leukocyte Acid Phosphatase assay system (Sigma, St Louis, MO, USA) was used for TRACP staining. Digital photomicrographs of stained sections were histomorphometrically analyzed using Image-Pro Plus v4 (Media Cybernetics, Silver Spring, MD, USA). Because Bcl2 −/− mice were smaller than Bcl2 +/+, a standardized area of interest was used for measuring bone area of tibias. The distance between the proximal and distal growth plates was first measured to calculate the proximal one third of the bone organ between the growth plates. This proximal one-third region was assessed for bone area (%). Osteoclast number and trabecular bone perimeter were calculated in the proximal tibia to determine osteoclast perimeters (#/mm). TRACP5b (a marker enzyme of bone resorption) was measured in the serum of day 13 mice using the MouseTRACP Assay (IDS, Boldon, UK).
Osteoclastogenesis and Bcl2 inhibitor ex vivo
The bone marrow of day 13 mice was flushed, and mononuclear cells were prepared by Ficoll gradient centrifugation. Cells were plated at 3 × 105 cells/cm2 in 24-well plates in α-MEM (Invitrogen, Grand Island, NY, USA) supplemented with 10% FBS containing penicillin/streptomycin. Osteoclastogenesis was induced with 50 ng/ml RANKL (R&D Systems, Minneapolis, MN, USA) and 50 ng/ml macrophage-colony stimulating factor (M-CSF; R&D Systems). Medium was changed every 2 days. At days 5 and 9, cells were stained for TRACP activity. Number and total area (%) of TRACP+ multinucleated cells (three or more nuclei per cell) were analyzed.
Flushed bone marrow from adult C57BL6 mice was cultured in Iscove's modified Dulbecco's medium (Invitrogen) supplemented with 20% FBS and 5 ng/ml of Flt3 ligand (R&D Systems) for 7 days as described.(23) The amplified hematopoietic cells were collected and plated at 1.8 × 105 cells/cm2 in 24-well plates in α-MEM. Osteoclastogenesis was induced with RANKL and M-CSF. Medium was changed every 2 days. At day 4, cells were treated with a Bcl2 inhibitor (30 μM HA14-1; Tocris Biosciences, Ellisville, MO, USA) or vehicle (DMSO), and TRACP staining was performed at day 5 to enumerate osteoclasts.
Calvarial osteoblasts ex vivo
Calvariae of neonatal mice were dissected and subjected to four sequential 30-min digestions in collagenase A (2 mg/ml; Roche) with 0.25% trypsin. Cell fractions 2–4 were collected and plated in α-MEM. For enumeration, calvarial osteoblasts were plated at 1 × 104 cells/cm2 in 24-well plates. Cell number was determined at days 1, 4, 7, and 10 by trypan blue exclusion and hemocytometer enumeration.
Calvarial osteoblasts were plated at 5 × 104 cells/cm2 in 6-well plates in α-MEM. Medium was replaced with mineralization medium (α-MEM, 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate) and cultured for 28 days. At day 29, cell layers were extracted with 15% trichloroacetic acid overnight. Insoluble material was removed by low-speed centrifugation. Supernatants were assayed for calcium with a commercially available kit (Pointe Scientific, Canton, MI, USA). DNA in pellets was extracted with the high salt method and quantified. Calcium was normalized to cellular DNA and expressed as units per microgram DNA. Alternatively, cell layers were stained for mineral using the von Kossa method. Briefly, cells were fixed in ethanol followed by incubation with 5% silver nitrate solution in the dark and exposed to a bright light.
Western blotting
The protein lysates from the kidneys were used to detect Bcl2 levels. SDS-PAGE was performed using 16% polyacrylamide gels to separate proteins and transferred to nitrocellulose membranes. The blots were blocked and incubated with mouse anti-Bcl2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C, and washed, followed by 1:10,000 horseradish peroxidase–conjugated anti-mouse IgG (Amersham Biosciences, Piscataway, NJ, USA). The protein bands were visualized by autoradiography using an enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). The blot was stripped and reprobed with mouse anti-α-tubulin (Sigma).
Calvarial osteoblasts were plated at 1 × 104 cells/cm2 in α-MEM. At 70% confluency, cells were synchronized by serum starvation and cultured in α-MEM for 24 h. Collected cells were lysed in RIPA buffer, and protein concentration was determined. SDS-PAGE was performed, and proteins were blotted to nitrocellulose membranes. Mouse anti-cyclin D1 (Santa Cruz Biotechnology) was used for a primary antibody. After autoradiography, the blot was stripped and reprobed with anti-actin (Santa Cruz Biotechnology). Autoradiographs were digitized, and densitometry was performed to quantitatively measure the intensity of the bands using Image-Pro Plus.
Calvarial osteoblasts were treated with PTH(1-34) (10−7 M; Bachem, Torrence, CA, USA) to examine the impact of PTH on Bcl2. Ascorbic acid (50 μg/ml) was added to the culture medium 24 h before PTH treatment. Cells were treated with PTH for 0, 1, 2, 6, and 24 h. Western blotting to detect Bcl2 levels and densitometry was performed using mouse anti-Bcl2 and anti-α-tubulin (Sigma) antibodies.
Quantitative real-time PCR
Total RNA was extracted from cells using the RNeasy mini kit (Qiagen, Valencia, CA, USA). First-strand cDNA was synthesized using the SuperScript First-strand system (Invitrogen). Quantitative real-time PCR was performed using an iCycler IQ (BioRad, Hercules, CA, USA) with SYBRGreen mix (Invitrogen). Samples were run in triplicate, and results were normalized to GAPDH expression. The sets of primers used for real-time PCR were as follows: Bcl2, 5`-GGAAGGTAGTGTGTGTGG-3` and 5`-ACTCCACTCTCTGGGTTCTTGG-3`; Runx2, 5`-CTGTGGTAACCGTCATGGCC and 5`-GGAGCTCGGCGGAGTAGTTC-3`; JunB, 5`-ATCAGCTACCTCCCACATGCA-3` and 5`-TACGGTCTGCGGTTCCTCTT-3`.
Apoptosis assays
Thymocytes were mechanically isolated from the thymus of day 13 mice by gently scraping thymi in α-MEM and passing cells through a needle. Thymocytes were plated at 1 × 106 cells/ml in α-MEM and treated with dexamethasone (1 μM; Sigma) for 2 h. Calvarial osteoblasts were plated at 4 × 104 cells/cm2 in α-MEM. Cells were treated with either dexamethasone (1 μM) for 3 days or staurosporine (0.1 μM; Sigma) for 24 h. Apoptosis was estimated by relative cell number using trypan blue exclusion. Alternatively, DNA fragmentation was analyzed using the TACS DNA laddering assay (R&D Systems) following the manufacturer's instructions. Five micrograms of genomic DNA was separated on a 1.2% agarose gel and laddering visualized by ethidium bromide staining.
Intermittent PTH administration and vossicle model
Bcl2 +/+ and Bcl2 −/− mice (4 days old) received daily administration of either hPTH(1-34) (50 μg/kg) or vehicle (saline) for 9 days.(24) Because Bcl2 −/− mice typically die at ∼14 days of age, a 9-day PTH treatment was used in this study. Mice were killed at day 13.
The vertebrae of Bcl2 −/− mice were implanted into immunocompromised mice, and PTH was administered daily to evaluate the effect of PTH on implant growth as described before.(25) Bcl2 +/+ and Bcl2 −/− (7 days old) mice were used as vertebrae donors, and immunocompromised mice (Foxn1nu; Harlan, Indianapolis, IN, USA) were used as recipients. The lumbar vertebrae were removed and sectioned into single vertebral bodies (vossicles). Recipients were anesthetized with intraperitoneal injections of ketamine (90 mg/kg) and xylazine (5 mg/kg). The vossicles were subcutaneously implanted in the dorsal surface of recipients. Daily subcutaneous injection of either hPTH(1-34) (80 μg/kg) or vehicle was initiated a day after the surgery for 21 days. At day 22, vossicles were removed.
Histomorphometry and μCT
The tibias were removed and fixed in 10% formalin. The tibias were processed, paraffin embedded, sectioned, and stained for H&E and TRACP. Bone area (%) in the proximal one third of the bone organ between the growth plates, osteoblast perimeter (#/mm), and osteoclast perimeter (#/mm) in the metaphyseal compartment of the proximal tibias were assessed using Image-Pro Plus. μCT scanning was performed using an eXplore Locus SP cone beam MicroCT system (GE Healthcare Biosciences, London, Ontario, Canada). Image reconstruction was performed on 18-μm voxels, and a threshold was generated to select a mineralized tissue region using the MicroView Analysis+ (GE healthcare Biosciences). Bone volume fraction in the proximal one third of the bone organ between the growth plates was assessed.
Vossicles were decalcified, paraffin embedded, and processed with H&E staining for histomorphometry to measure total bone area (%). Alternatively, μCT scanning was carried out, and images were reconstructed in the same manner as described for the tibias. The trabecular region of the vossicles was delineated from the cortical shell and cartilage to assess the effect of PTH on trabecular bone.
Statistics
All data were analyzed for equality of variances. For parametric data, independent t-tests for two groups and ANOVA for multiple groups were performed. Tukey's test was used as a posthoc test. For nonparametric data, Kruskal-Wallis test was used. All statistical analysis was conducted with SPSS v12 (SPSS, Chicago, IL, USA). An α level of 0.05 was used. Results are presented as mean ± SE unless specified.
RESULTS
Validation and characterization of Bcl2−/− mice
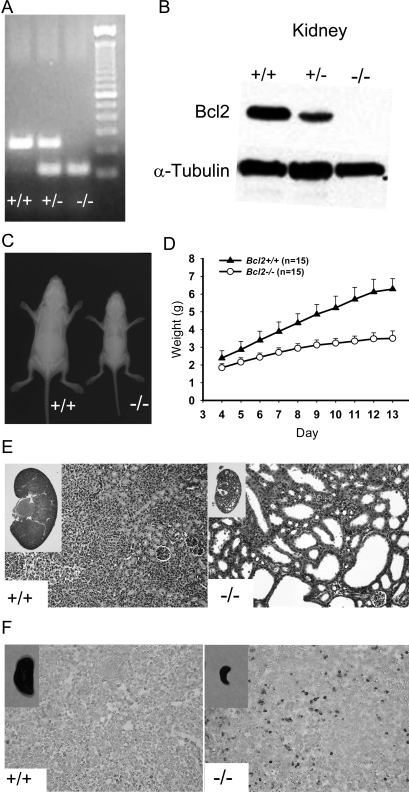
Targeted deletion of the Bcl2 gene was validated by PCR of tail DNA (Fig. 1A). Negative expression of Bcl2 protein was confirmed in Bcl2 −/− kidney (Fig. 1B). Bcl2 −/− mice typically fail to survive >2 wk after birth. The body size of Bcl2 −/− mice at birth was similar to Bcl2 +/+; however, at day 11, it was evident that Bcl2 −/− mice were significantly smaller than Bcl2 +/+ (Fig. 1C). Body weight was measured from day 4 to day 13, and average weight was plotted. Bcl2 −/− mice gained little weight after day 7, whereas Bcl2 +/+ mice gained weight consistently during the observation period (Fig. 1D). Kidney and spleen were harvested from 13-day-old mice for histological evaluation. H&E staining of Bcl2 −/− kidneys showed severe generalized cystic lesions in the entire kidney compared with Bcl2 +/+ (Fig. 1E). TUNEL staining of spleen sections was performed to detect apoptotic cells. A large number of TUNEL+ cells were detected in Bcl2 −/− spleen sections, whereas very few TUNEL+ cells were found in Bcl2 +/+ spleen sections (Fig. 1F).
FIG. 1.
Characterization of Bcl2 −/− mice. (A) Tail DNA genotyping. (B) The absence of Bcl2 protein was confirmed by Western analysis. (C) At day 11, Bcl2 −/− mice were significantly smaller than their littermates. (D) Weight change over 9 days starting at day 4. Bcl2 −/− mice gained on average as little as 1 g in weight, whereas Bcl2 +/+ gained >3.5 g. Results are presented as mean ± SD. (E) H&E staining of the kidney sections, ×200. Bcl2 −/− mice develop severe poly-cystic kidneys. (F) TUNEL staining of the spleen sections, ×200. Dark brown–stained cells indicate apoptosis. Numerous apoptotic splenocytes were observed in Bcl2 −/− spleen.
Hematopoietic component of Bcl2−/− bone marrow was altered
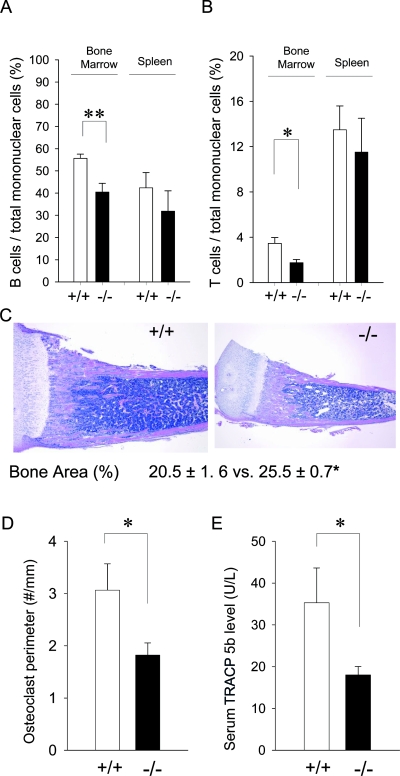
To characterize lymphocytes in the bone marrow and spleen, flow cytometric analysis was performed. Results are summarized in Figs. 2A and 2B. The ratio of B cells to total mononuclear cells in Bcl2 −/− bone marrow was significantly reduced compared with Bcl2 +/+. There was a tendency of reduced numbers of B cells in Bcl2 −/− versus Bcl2 +/+ spleens, but this was not statistically significant. A similar pattern was observed for T lymphocytes. The T-cell ratio to total mononuclear cells in Bcl2 −/− was considerably smaller than that in Bcl2 +/+, whereas no statistical difference was found between Bcl2 +/+ and Bcl2 −/− in T cells in the spleen. Thus, lymphopoiesis in the bone marrow but not in the spleen was impaired in Bcl2 −/− mice.
FIG. 2.
Altered hematopoietic component, decreased osteoclast density, and increased bone mass in Bcl2 −/− bone. Mononuclear cells from the bone marrow and spleen were stained with CD3 and CD19 antibodies. Flow cytometry was performed to detect CD3+ and CD19+ cells (n > 6 /group). (A) The B-cell ratio was significantly reduced in Bcl2 −/− vs. Bcl2 +/+ bone marrow, whereas in the spleen, no significant difference was observed. (B) The T-cell ratio was significantly reduced in Bcl2 −/− bone marrow compared with that of Bcl2 +/+ but not in the spleen. (C) The proximal one third of the bone organ between the growth plates was histomorphometrically analyzed. Significantly higher bone areas (%) were found in Bcl2 −/− tibias than Bcl2 +/+ (n = 5/group). (D) Osteoclast numbers per linear perimeter were significantly less in Bcl2 −/− than Bcl2 +/+ (n > 8/group). (E) Similarly, serum TRACP5b levels in Bcl2 −/− mice were considerably lower than that in Bcl2 +/+ (n > 7/group). *p < 0.05, **p < 0.01 (Bcl2 −/− vs. Bcl2 +/+).
Increased bone mass and decreased osteoclast number in Bcl2−/− tibias
Representative photomicrographs of tibias from day 13 mice are shown in Fig. 2C. Histomorphometric analysis of the metaphyseal compartment showed that Bcl2 −/− tibias had significantly greater percent bone areas (25.5 ± 0.7%; amount of bone tissue per total bone organ) compared with Bcl2 +/+ (20.5 ± 1.6%). No difference was noted in osteoblast perimeter in the metaphyseal compartment of the proximal tibias between Bcl2 +/+ (17.2 ± 2.0 #/mm) and Bcl2 −/− (15.5 ± 1.6 #/mm). TRACP staining showed considerably lower osteoclast number in Bcl2 −/− tibias versus Bcl2 +/+ (Fig. 2D). Consistent with this, serum TRACP5b levels were significantly lower in Bcl2 −/− compared with Bcl2 +/+ (Fig. 2E). These results indicate that osteoclast activity was compromised in Bcl2 −/− bone.
Altered osteoclastogenesis in Bcl2−/− bone marrow ex vivo
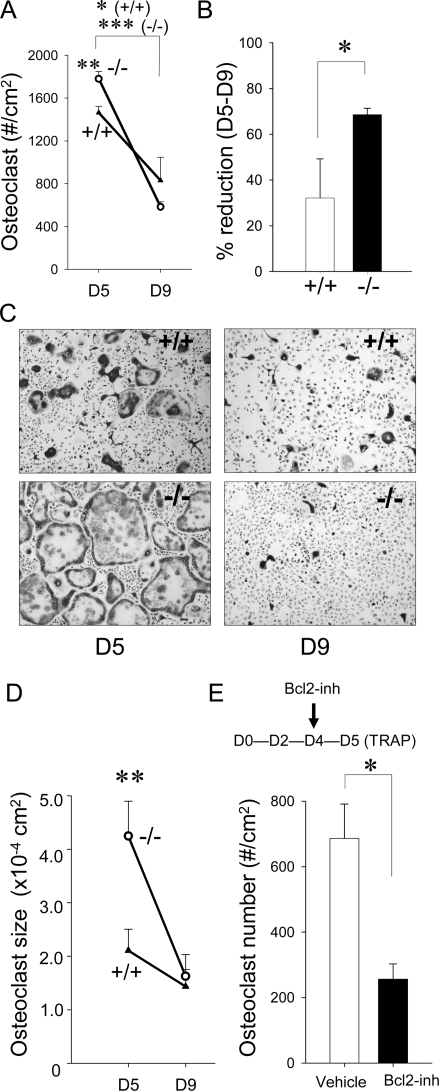
Because Bcl2 −/− mice exhibited significantly fewer osteoclasts, the role of Bcl2 in osteoclastogenesis and osteoclast survival was studied. Osteoclastogenesis was induced in bone marrow mononuclear cell cultures using RANKL and M-CSF. A significant difference was found between Bcl2 +/+ and Bcl2 −/− during a course of osteoclastogenesis. Enumeration of osteoclasts showed that, at day 5, significantly more osteoclasts were present in Bcl2 −/− cell cultures (Fig. 3A). However, the number of osteoclasts significantly dropped during the period of day 5 to day 9 in Bcl2 −/− versus Bcl2 +/+ cultures (Fig. 3B). Another prominent difference was osteoclast size. Bcl2 −/− osteoclasts were much larger than Bcl2 +/+ osteoclasts at day 5 (Figs. 3C and 3D). The size of Bcl2 −/− osteoclasts was approximately two times larger than that of Bcl2 +/+. At day 9, the size was similar between Bcl2 +/+ and Bcl2 −/−, because the Bcl2 −/− osteoclasts were much smaller than at day 5.
FIG. 3.
Bcl2 −/− osteoclasts were large and short lived ex vivo. Osteoclastogenesis was induced from bone marrow mononuclear cells with RANKL and M-CSF. (A) At day 5, osteoclast numbers per square centimeter were significantly higher in Bcl2 −/− than Bcl2 +/+. At day 9, no difference was detected between Bcl2 +/+ and Bcl2 −/− (n > 5/group). (B) Bcl2 −/− osteoclast number dropped from day 5 to day 9 more significantly than Bcl2 +/+ osteoclast number (n > 5/group). (C) Representative micrographs of TRACP-stained bone marrow cell cultures. The size of Bcl2 −/− osteoclasts was larger than Bcl2 +/+ at day 5. At day 9, Bcl2 −/− osteoclasts were much smaller than at day 5. No difference was noted in osteoclast size between genotypes at day 9. (D) Surface area of osteoclasts per well was determined and normalized by cell numbers per well. At day 5, Bcl2 −/− osteoclasts were significantly larger than Bcl2 +/+ osteoclasts. However, at day 9, osteoclasts from both genotypes were small, and no difference was found between genotypes (n > 5/group). (E) The Bcl2 inhibitor HA14-1 significantly suppressed osteoclastogenesis in wildtype hematopoietic cell cultures (n = 3/group). *p < 0.05, **p < 0.01, ***p < 0.001.
Bcl2 inhibitor impaired osteoclastogenesis ex vivo
Wildtype osteoclasts and pre-osteoclasts were treated with the Bcl2 inhibitor (antisense oligonucleotide, HA14-1) to further explore the role of Bcl2 in osteoclasts. The Flt3 ligand-expanded hematopoietic cells from C57BL6 mice were cultured with RANKL and M-CSF for 5 days. Cells were treated with HA14-1 24 h before TRACP staining. HA14-1 significantly suppressed osteoclast number compared with vehicle-treated cells (Fig. 3E).
Role of Bcl2 in osteoblasts ex vivo
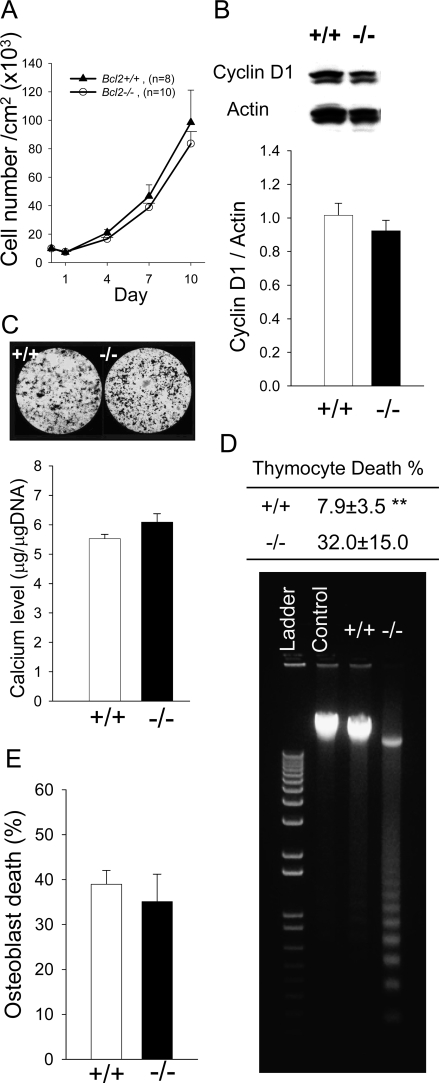
To elucidate how Bcl2 deletion affects the cellular activities of osteoblasts ex vivo, enumeration, proliferation, and mineralization assays were carried out with calvarial osteoblasts. Enumeration of Bcl2 −/− osteoblasts over time were comparable to that of Bcl2 +/+ (Fig. 4A). Western blot analysis for the cell cycle gene, cyclin D1, showed that cyclin D1 was similar in Bcl2 −/− to that in Bcl2 +/+ (Fig. 4B). Densitometric analysis showed no statistical difference in cyclin D1 expression between Bcl2 +/+ and Bcl2 −/−, suggesting that their proliferation activities were equivalent. Bcl2 −/− osteoblasts formed mineralized nodules similar to Bcl2 +/+ osteoblasts (Fig. 4C). To quantify mineralization, calcium levels were normalized by total DNA. Statistical analysis showed that there was no difference in mineralization activity between Bcl2 +/+ and Bcl2 −/− osteoblasts. These ex vivo assays indicated that the absence of Bcl2 did not play a major role in normal osteoblast enumeration, proliferation, or mineralization.
FIG. 4.
Characterization of calvarial osteoblasts. (A) Calvarial osteoblasts were plated at 10,000 cells/cm2, and cell numbers were determined every 3 days. No difference was detected in cell numbers over time between genotypes. (B) Cell growth was synchronized and protein was extracted after serum stimulation for analysis. No significant difference was found in cyclin D1 levels between Bcl2 +/+ and Bcl2 −/− osteoblasts (n > 7/group). (C) Cells were cultured in mineralization medium for 28 days. Representative images of Von Kossa staining are shown. Calcium levels normalized by total DNA were statistically analyzed (n > 10/group). No difference was noted in calcium levels between Bcl2 +/+ and Bcl2 −/− cultures. (D) Thymocytes were treated with dexamethasone for 2 h. Cell death was estimated by relative cell number vs. control. Significant cell death was noted in Bcl2 −/− thymocytes (n > 5/group). Considerable DNA fragmentation occurred in Bcl2 −/− thymocytes compared with Bcl2 +/+. (E) Calvarial osteoblasts were treated with staurosporine for 24 h. Cell number was determined with trypan blue exclusion. No difference was found in numbers of nonviable cells between Bcl2 +/+ and Bcl2 −/− osteoblasts (n > 5/group). **p < 0.01.
Bcl2 is critical in thymocyte but not in osteoblast apoptosis
To assess the role of Bcl2 in apoptosis, thymocytes and osteoblasts were stimulated with dexamethasone and staurosporine. Dexamethasone induced significantly greater apoptosis in Bcl2 −/− thymocytes compared with Bcl2 +/+ (Fig. 4D). DNA laddering confirmed that dexamethasone induced considerable DNA fragmentation in Bcl2 −/− thymocytes. Staurosporine for 24 h induced apoptosis in osteoblasts of both genotypes, but there was no significant difference in cell death rate between Bcl2 +/+ and Bcl2 −/− (Fig. 4E).
Intermittent PTH administration increased bone mass in Bcl2−/− tibias
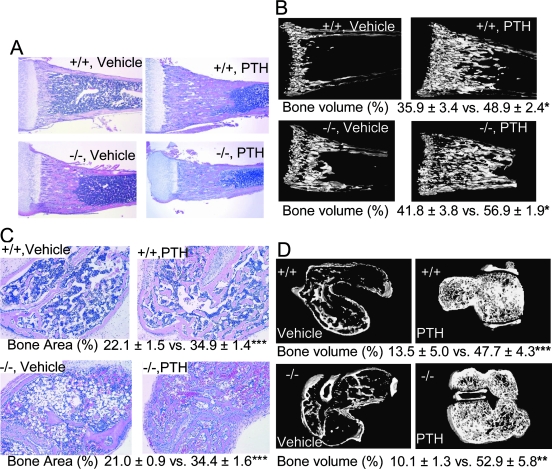
To elucidate the impact of Bcl2 on PTH anabolic actions in intact mice, PTH was administered intermittently to Bcl2 +/+ and Bcl2 −/− mice. Intermittent PTH administration increased bone mass of the proximal tibias significantly regardless of genotype (Fig. 5A). μCT scanning confirmed that Bcl2 −/− mice responded to intermittent PTH in an anabolic mode (Fig. 5B). A significantly higher bone volume fraction was noted in the tibias of both PTH-administered Bcl2 +/+ and Bcl2 −/− mice compared with respective vehicle controls. Consistently, osteoblast perimeter (#/mm) was significantly higher in both PTH administered Bcl2 +/+ and Bcl2 −/− mice (42.6 ± 1.5 and 40.4 ± 1.0, respectively) compared with respective controls (17.2 ± 2.0 and 15.5 ± 1.6). No difference was found between Bcl2 +/+ and Bcl2 −/− with respect to PTH anabolic actions; both genotypes responded similarly to intermittent PTH. These in vivo experiments showed that daily PTH administration for 9 days dramatically increased bone mass in the tibias regardless of genotype.
FIG. 5.
Intermittent PTH increased bone mass in Bcl2 −/− tibias and vossicles. PTH was administered daily to Bcl2 +/+ and Bcl2 −/− mice from day 4 to day 12, and the proximal one third of each tibia was selected for analysis. (A) Representative micrographs of H&E-stained tibias. Substantial trabecular bone was noted in PTH-administered groups regardless of genotype (n > 15/group). (B) Representative μCT images with summary data below. In both genotypes, considerably more trabecular bone was found in PTH-administered groups vs. controls (n = 5/group). Regardless of genotype, intermittent PTH administration induced significantly higher bone volume fraction than vehicle. (C) Vertebrae from Bcl2 +/+ and Bcl2 −/− mice were subcutaneously implanted into athymic mice, and PTH was administered daily for 21 days. Representative micrographs of H&E-stained vossicle sections with summary data below. Enhanced trabeculation was found in PTH-administered groups regardless of genotype. Controls exhibit sparse trabeculation in both genotypes. Bone area (%) was significantly higher in the PTH-administered group than vehicle control regardless of genotype. No difference was found in bone area (%) between Bcl2 +/+ and Bcl2 −/−, irrespective of treatment (n > 23/group). (D) Representative μCT images with summary statistics below. Intermittent PTH administration significantly enhanced bone in vossicles of both genotypes. Little trabecular structure was noted in controls regardless of genotype (n = 4/group). *p < 0.05, **p < 0.01, ***p < 0.001.
Daily PTH administration enhanced bone formation in Bcl2−/− vossicles
Because Bcl2 −/− mice develop kidney failure, endocrine dysfunction in Bcl2 −/− mice could influence the outcome of in vivo PTH therapy. To rule out the possible effect of kidney failure, a vossicle implant experiment was performed.(25) Using this model, the interaction between hematopoietic (host origin) and mesenchymal cells (donor origin) can be assessed in addition to obviating the potential systemic impact of the kidney phenotype and subsequent lethality of Bcl2 ablation.
H&E staining of vossicle sections showed enhanced trabecular bone in PTH-administered mice, regardless of genotype (Fig. 5C). PTH administration enhanced bone in vossicles with fine trabeculation regardless of genotype, whereas vossicles in the vehicle control group exhibited considerably less trabecular bone in both genotypes. The result of μCT scanning of vossicles confirmed that intermittent PTH administration increased bone mass in both Bcl2 +/+ and Bcl2 −/− vossicles. Whereas the vossicles from PTH-administered mice had dense trabecular structure, vossicles from vehicle-treated mice had little trabecular structure (Fig. 5D). To study the effect of PTH on trabecular bone, cartilage, and cortical shells were excluded from μCT data for analysis. Trabecular bone volume fraction (%) of vossicles in PTH-administered mice was significantly higher than that of vehicle. The degree of such increase was similar between Bcl2 +/+ and Bcl2 −/−. Thus, intermittent PTH administration significantly increased net bone mass in both Bcl2 +/+ and Bcl2 −/− vossicles.
PTH effect on Bcl2 in calvarial osteoblasts
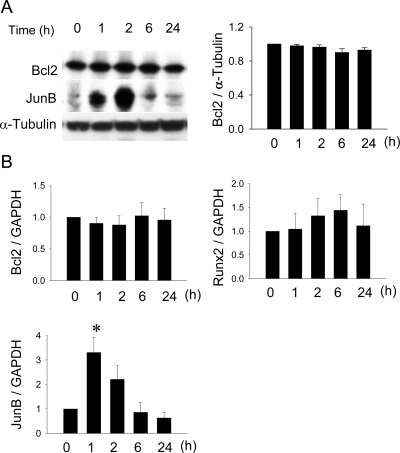
It has been reported that PTH induces Bcl2 in a Runx2-dependent manner in the osteoblastic cell line, OB-6.(18) To determine whether PTH induces Bcl2 in primary osteoblasts, calvarial osteoblasts from Bcl2 +/+ mice were treated with PTH and analyzed for Bcl2 protein expression. As a positive control for the PTH response in osteoblasts, the immediate early gene, JunB, was also analyzed. The result of Western blotting showed that Bcl2 was not induced in calvarial osteoblasts (Fig. 6A). PTH-induced JunB expression followed a pattern typical to that previously published.(26) JunB expression was high a few hours after PTH stimulation and significantly decreased after 6 h. Such a typical expression pattern of JunB by PTH treatment confirms that calvarial osteoblasts responded to PTH. The result of protein analysis was further verified by assessing the relative expression of Bcl2, Runx2, and JunB at the RNA level using real-time PCR. There was no significant upregulation of Bcl2 or Runx2 in response to PTH in osteoblasts, whereas JunB was significantly induced after 1 h PTH treatment (Fig. 6B).
FIG. 6.
Lack of PTH-mediated increase in Bcl2 ex vivo. Calvarial osteoblasts were treated with PTH (10−7 M) for the indicated time course. (A) Protein was extracted and analyzed for Bcl2. JunB expression was detected to verify PTH activity in osteoblasts. Densitometry was carried out and analyzed (n = 5 for each). No significant difference in PTH-induced Bcl2 expression was found during the time course, whereas PTH-induced JunB expression followed an expected pattern. (B) Total RNA was extracted and analyzed for Bcl2, Runx2, and JunB using quantitative real-time PCR. PTH did not induce Bcl2 nor Runx2, but significantly higher JunB expression was noted with 1 h of PTH treatment.
DISCUSSION
Bcl2 −/− mice had increased bone mass and altered bone marrow hematopoietic components versus wildtype mice. The ratios of both B cells and T cells to total mononuclear cells were significantly reduced in Bcl2 −/− mice and decreased osteoclast numbers were found in Bcl2 −/− bone. Consistently, serum TRACP5b levels were significantly lower in Bcl2 −/− mice. Thus, lymphopoiesis and osteoclastogenesis were both weakened in Bcl2 −/− bone marrow. There was no alteration in proliferation or mineralization of Bcl2 −/− osteoblasts, suggesting Bcl2 is not critical for the osteoblast phenotype. Boot-Handford et al.(27) reported that Bcl2 −/− mice had increased osteoblast numbers, and the shape of osteoblasts was more cuboidal than wildtype. In this study, no noticeable difference was observed in osteoblast numbers or morphology. This discrepancy in observation could reflect a difference in mouse strain or techniques used. The quantitative measures used in this study were also more extensive than those used in the former study.
Significant differences were identified in osteoclastogenesis ex vivo. Bcl2 −/− osteoclastogenesis was rapid and robust, but also vanished rapidly thereafter, whereas Bcl2 +/+ osteoclastogenesis developed more gently and remained active for a longer period than Bcl2 −/−. Moreover, when the burst of Bcl2 −/− osteoclastogenesis took place, osteoclasts were much larger compared with Bcl2 +/+ counterparts. This distinctive feature of Bcl2 −/− osteoclastogenesis suggests a critical role of Bcl2 in regulation of osteoclast fusion and apoptosis. Osteoclasts typically have a short lifespan.(28) Apoptosis occurs rapidly after the removal of trophic factors such as M-CSF in vitro.(29) Although the significance of osteoclast fusion remains mostly unknown, osteoclast survival is considered to depend on continued replenishment by fusion.(28) It is known that Bcl2 activation promotes survival of certain cells in the myeloid and lymphoid lineages.(3) Because osteoclasts are of hematopoietic origin and share some features with bone marrow macrophage/monocytes,(30) Bcl2 activation may contribute to osteoclast survival as well. Indeed, our ex vivo finding that the Bcl2 inhibitor significantly suppressed osteoclast survival supports a critical role of Bcl2 in osteoclast survival. Therefore, the cell autonomous change in Bcl2 −/− osteoclasts is likely responsible at least in part for the increased bone mass in Bcl2 −/− mice. McGill et al.(31) reported that microphthalmia (Mitf) regulates Bcl2 in melanocytes and osteoclasts and that the osteopetrotic phenotype of Mitfmi/mi mice is partially attributed to deregulation of Bcl2. In that report, the osteopetrotic phenotype of Bcl2 −/− mice was shown with no insight to explain the phenotypic change. Our result is accordant with their findings and provides analytical explanation. Impaired osteoclastogenesis could result from lymphopenia because lymphocytes are sources of RANKL.(9,11) Because lymphopoiesis of Bcl2 −/− mice is normal at birth(5) and hence lymphopenia developed in a short period in this study (maximum 13 days), it is unlikely that acute lymphopenia solely caused the increased bone mass by suppressing osteoclastogenesis.
Bcl2 is a crucial gene in thymocyte apoptosis but may not play an important role in osteoblast apoptosis stimulated by dexamethasone or staurosporine. Dexamethasone induced significantly higher apoptosis in Bcl2−/− thymocytes compared with Bcl2 +/+, whereas no noticeable dexamethasone-induced apoptosis was observed in osteoblasts from either genotype during 3 days of incubation (data not shown). Glucocorticoids are widely used for the treatment of inflammatory and autoimmune diseases and lymphomas. They are known to suppress the host immune response and induce apoptosis in lymphocytes.(32) In bone, the long-term use of glucocorticoids often results in bone loss.(33) It has been shown that dexamethasone treatment induces apoptosis in osteoblasts in vivo(16,34) and in vitro.(35) Our result that Bcl2−/− thymocytes were susceptible to dexamethasone-induced apoptosis is consistent with a previous report,(5) but the observation in primary osteoblasts was not. This is likely because dexamethasone is a mild apoptosis inducer compared with other chemotherapeutic agents such as etoposide.(36) Therefore, staurosporine was used to induce osteoblast apoptosis. Staurosporine induced apoptosis in osteoblasts; however, no significant difference was noted between Bcl2 +/+ and Bcl2 −/−. This suggests that the absence of Bcl2 does not affect apoptosis in osteoblasts. It is possible that the activation of apoptosis-related genes may be redundant in osteoblasts. Alternative genes may be upregulated to compensate for the functional loss of Bcl2 to maintain the anti-apoptotic to apoptotic ratio in a cell. Further research is needed to elucidate the mechanism of osteoblast apoptosis.
Bcl2 has been proposed as a candidate that renders osteoblasts anti-apoptotic in a Runx2-dependent manner in response to PTH, thereby enhancing bone formation.(18) Intermittent PTH administration to Bcl2 −/− mice in this study dramatically increased bone mass in their trabecular compartments, at a comparable level to that seen in wildtype littermates. Because this study did not focus specifically on apoptosis of osteoblasts but the overall role of Bcl2 in PTH anabolic action, it is difficult to address whether apoptosis of osteoblasts plays a role in PTH actions. Nonetheless, an inference can be made from the in vivo part of this study that Bcl2 is not likely the critical mediator of anti-apoptotic signaling in osteoblasts nor does PTH activate Bcl2 to inhibit osteoblast apoptosis. Because PTH has been shown to have pro- and anti-apoptotic actions in osteoblasts,(21,37) the hypothesis that osteoblast apoptosis plays a role in PTH anabolic action(21) is rational, and it has been anticipated that the inhibition of osteoblast apoptosis would lead to a clinical strategy to increase the bone mass.(7) It is possible that different results could be obtained in a model of osteoporosis such as sex steroid withdrawal compared with the bone growth models used in this study. On the other hand, several lines of evidence have indicated that osteoblast apoptosis is an important component of mineralization in vitro and normal bone formation in vivo.(17,38) It was shown that intermittent PTH administration transiently increased numbers of apoptotic osteoblasts and osteocytes in rat trabecular bone, but no protection from apoptosis was observed during the course of PTH treatment.(39) This study found that PTH did not regulate the apoptosis-related gene Bcl2. Similar findings were shown in a microarray study that compared gene expression in bone between continuous and intermittent PTH administration.(40) This microarray study failed to detect upregulation of Bcl2 and other apoptosis-related genes in the intermittent PTH group. Lindsay et al.(41) studied the effect of PTH treatment on bone of patients with osteoporosis and found that enhanced bone formation was associated with an increase in osteoblast apoptosis. Our finding that Bcl2 is not essential in PTH action in vivo is consistent with these studies. Moreover, our in vivo finding is reinforced by our ex vivo result where PTH did not induce Bcl2 or Runx2 in calvarial osteoblasts.
This study used a global knockout mouse model of Bcl2. Polycystic kidney disease is a prominent feature in Bcl2 −/− mice. Kidney failure induces renal osteodystrophy in which bone is generally osteopenic because of enhanced osteoclast activity.(42) In this study, Bcl2 −/− mice showed higher bone mass with decreased osteoclast numbers. Sorensen et al.(43) reported that kidneys from newborn Bcl2 −/− mice were not cystic but small, and as the mice grew, the kidneys became cystic. Therefore, the systemic effect of acute renal failure in our Bcl2 −/− mice was not likely significant at day 13. Rather, cell autonomous changes in osteoclasts caused by Bcl2 ablation likely played a major role in the bone phenotype of Bcl2 −/− mice. Because the kidney is a crucial organ for calcium reabsorption that is partly under the hormonal control of PTH, Bcl2−/− mice may have fluctuating endogenous PTH levels that may affect the experimental outcome. However, in the vossicle implant system, the hematopoietic components come from the host (athymic mice). Hence, no effect of fluctuating endogenous PTH or compromised kidney function would be expected in the vossicle response in this study. Daily PTH administration increased bone mass in vossicles significantly regardless of genotype. The consideration that Bcl2 +/+ stromal progenitors could migrate into vossicles from the host through blood vessels is possible; however, we found in a similar model system that PTH was unable to mobilize host cells to implants.(44) Therefore, the result from the vossicle experiment reinforces our finding that Bcl2 is dispensable in PTH anabolic actions. Interestingly, considering that the intact Bcl2 −/− mice responded to PTH with increased bone, this study also validated that PTH anabolic actions are not dependent on the presence of a normally functioning kidney or physiologic increases in body weight/size. Despite that the mice failed to normally increase body weight during growth, they maintained a robust skeletal response to PTH.
This study showed that Bcl2 is critical in maturation of osteoclasts but not in osteoblasts. Suppressed osteoclasts in Bcl2 −/− bone marrow are at least in part responsible for the high bone mass of Bcl2 −/− mice. Evidence was also found that Bcl2 is dispensable in PTH anabolic action in bone.
ACKNOWLEDGMENTS
The authors thank Akihiro Yamashita for osteoclast analysis. This study was supported by the National Institutes of Health DK53904, CA093900, the University of Michigan musculoskeletal Core Center P30-AR46024, and OVPR #5534.
Footnotes
Dr McCauley receives unrelated clinical research grant support from Eli Lilly and has stock in Amgen. All other authors state that they have no conflicts of interest.
REFERENCES
- 1.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18) Nature. 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- 3.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 4.Garcia I, Martinou I, Tsujimoto Y, Martinou JC. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science. 1992;258:302–304. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- 5.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 6.Amling M, Neff L, Tanaka S, Inoue D, Kuida K, Weir E, Philbrick WM, Broadus AE, Baron R. Bcl-2 lies downstream of parathyroid hormone-related peptide in a signaling pathway that regulates chondrocyte maturation during skeletal development. J Cell Biol. 1997;136:205–213. doi: 10.1083/jcb.136.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manolagas SC. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N, Udagawa N, Takami M, Suda T. Cells of bone, osteoclast generation. In: Bilezikian JP, Gaisz LG, Rodan GA, editors. Principles of Bone Biology. vol. 1. San Diego, CA, USA: Academic Press; 2002. pp. 109–126. [Google Scholar]
- 9.Miyaura C, Onoe Y, Inada M, Maki K, Ikuta K, Ito M, Suda T. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: Similarity to estrogen deficiency. Proc Natl Acad Sci USA. 1997;94:9360–9365. doi: 10.1073/pnas.94.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horowitz MC, Xi Y, Pflugh DL, Hesslein DG, Schatz DG, Lorenzo JA, Bothwell AL. Pax5-deficient mice exhibit early onset osteopenia with increased osteoclast progenitors. J Immunol. 2004;173:6583–6591. doi: 10.4049/jimmunol.173.11.6583. [DOI] [PubMed] [Google Scholar]
- 11.Manabe N, Kawaguchi H, Chikuda H, Miyaura C, Inada M, Nagai R, Nabeshima Y, Nakamura K, Sinclair AM, Scheuermann RH, Kuro-o M. Connection between B lymphocyte and osteoclast differentiation pathways. J Immunol. 2001;167:2625–2631. doi: 10.4049/jimmunol.167.5.2625. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Shibata T, Ikeda K, Watanabe K. Generation of bone-resorbing osteoclasts from B220+ cells: Its role in accelerated osteoclastogenesis due to estrogen deficiency. J Bone Miner Res. 2001;16:2215–2221. doi: 10.1359/jbmr.2001.16.12.2215. [DOI] [PubMed] [Google Scholar]
- 13.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 14.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87:518–524. [PubMed] [Google Scholar]
- 15.Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman R, Emerson SG. Osteoblasts support B lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 16.Gohel A, McCarthy MB, Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–5347. doi: 10.1210/endo.140.11.7135. [DOI] [PubMed] [Google Scholar]
- 17.Pantschenko AG, Zhang W, Nahounou M, McCarthy MB, Stover ML, Lichtler AC, Clark SH, Gronowicz GA. Effect of osteoblast-targeted expression of bcl-2 in bone: Differential response in male and female mice. J Bone Miner Res. 2005;20:1414–1429. doi: 10.1359/JBMR.050315. [DOI] [PubMed] [Google Scholar]
- 18.Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 19.Dobnig H, Turner RT. The effects of programmed administration of human parathyroid hormone fragment (1-34) on bone histomorphometry and serum chemistry in rats. Endocrinology. 1997;138:4607–4612. doi: 10.1210/endo.138.11.5505. [DOI] [PubMed] [Google Scholar]
- 20.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 21.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubrij I, Ali AA, Chambers TM, Berryhill SB, Liu X, Roberson P, O'Brien CA, Weinstein RS, Manolagas SC, Jilka RL. Decreased osteoblast apoptosis and increased bone formation in implants of marrow-derived osteoblast progenitors overexpressing bcl-2: In vivo evidence for a pivotal role of apoptosis in bone formation. J Bone Miner Res. 2003;18(S1):S136. [Google Scholar]
- 23.Servet-Delprat C, Arnaud S, Jurdic P, Nataf S, Grasset MF, Soulas C, Domenget C, Destaing O, Rivollier A, Perret M, Dumontel C, Hanau D, Gilmore GL, Belin MF, Rabourdin-Combe C, Mouchiroud G. Flt3+ macrophage precursors commit sequentially to osteoclasts, dendritic cells and microglia. BMC Immunol. 2002;3:1–15. doi: 10.1186/1471-2172-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demiralp B, Chen HL, Koh AJ, Keller ET, McCauley LK. Anabolic actions of parathyroid hormone during bone growth are dependent on c-fos. Endocrinology. 2002;143:4038–4047. doi: 10.1210/en.2002-220221. [DOI] [PubMed] [Google Scholar]
- 25.Koh AJ, Demiralp B, Neiva KG, Hooten J, Nohutcu RM, Shim H, Datta NS, Taichman RS, McCauley LK. Cells of the osteoclast lineage as mediators of the anabolic actions of parathyroid hormone in bone. Endocrinology. 2005;146:4584–4596. doi: 10.1210/en.2005-0333. [DOI] [PubMed] [Google Scholar]
- 26.Berry JE, Ealba EL, Pettway GJ, Datta NS, Swanson EC, Somerman MJ, McCauley LK. JunB as a Downstream Mediator of PTHrP Actions in Cementoblasts. J Bone Miner Res. 2006;21:246–257. doi: 10.1359/JBMR.051024. [DOI] [PubMed] [Google Scholar]
- 27.Boot-Handford RP, Michaelidis TM, Hillarby MC, Zambelli A, Denton J, Hoyland JA, Freemont AJ, Grant ME, Wallis GA. The bcl-2 knockout mouse exhibits marked changes in osteoblast phenotype and collagen deposition in bone as well as a mild growth plate phenotype. Int J Exp Pathol. 1998;79:329–335. doi: 10.1046/j.1365-2613.1998.790411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks SC, Jr, Seifert MF. The lifespan of osteoclasts: Experimental studies using the giant granule cytoplasmic marker characteristic of beige mice. Bone. 1985;6:451–455. doi: 10.1016/8756-3282(85)90223-6. [DOI] [PubMed] [Google Scholar]
- 29.Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med. 1993;178:1733–1744. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurihara N, Chenu C, Miller M, Civin C, Roodman GD. Identification of committed mononuclear precursors for osteoclast-like cells formed in long term human marrow cultures. Endocrinology. 1990;126:2733–2741. doi: 10.1210/endo-126-5-2733. [DOI] [PubMed] [Google Scholar]
- 31.McGill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, Lin YL, Ramaswamy S, Avery W, Ding HF, Jordan SA, Jackson IJ, Korsmeyer SJ, Golub TR, Fisher DE. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707–718. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 32.Marchetti MC, Di Marco B, Cifone G, Migliorati G, Riccardi C. Dexamethasone-induced apoptosis of thymocytes: Role of glucocorticoid receptor-associated Src kinase and caspase-8 activation. Blood. 2003;101:585–593. doi: 10.1182/blood-2002-06-1779. [DOI] [PubMed] [Google Scholar]
- 33.Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: Pathogenesis and management. Ann Intern Med. 1990;112:352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 35.Pagel CN, de Niese MR, Abraham LA, Chinni C, Song SJ, Pike RN, Mackie EJ. Inhibition of osteoblast apoptosis by thrombin. Bone. 2003;33:733–743. doi: 10.1016/s8756-3282(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 36.Davies JH, Evans BA, Jenney ME, Gregory JW. In vitro effects of chemotherapeutic agents on human osteoblast-like cells. Calcif Tissue Int. 2002;70:408–415. doi: 10.1007/s002230020039. [DOI] [PubMed] [Google Scholar]
- 37.Chen HL, Demiralp B, Schneider A, Koh AJ, Silve C, Wang CY, McCauley LK. Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. J Biol Chem. 2002;277:19374–19381. doi: 10.1074/jbc.M108913200. [DOI] [PubMed] [Google Scholar]
- 38.Lynch MP, Capparelli C, Stein JL, Stein GS, Lian JB. Apoptosis during bone-like tissue development in vitro. J Cell Biochem. 1998;68:31–49. [PubMed] [Google Scholar]
- 39.Stanislaus D, Yang X, Liang JD, Wolfe J, Cain RL, Onyia JE, Falla N, Marder P, Bidwell JP, Queener SW, Hock JM. In vivo regulation of apoptosis in metaphyseal trabecular bone of young rats by synthetic human parathyroid hormone (1-34) fragment. Bone. 2000;27:209–218. doi: 10.1016/s8756-3282(00)00309-4. [DOI] [PubMed] [Google Scholar]
- 40.Onyia JE, Bidwell J, Herring J, Hulman J, Hock JM. In vivo, human parathyroid hormone fragment (hPTH 1-34) transiently stimulates immediate early response gene expression, but not proliferation, in trabecular bone cells of young rats. Bone. 1995;17:479–484. doi: 10.1016/8756-3282(95)00332-2. [DOI] [PubMed] [Google Scholar]
- 41.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22:495–502. doi: 10.1359/jbmr.070104. [DOI] [PubMed] [Google Scholar]
- 42.Malluche H, Faugere MC. Renal bone disease 1990: An unmet challenge for the nephrologist. Kidney Int. 1990;38:193–211. doi: 10.1038/ki.1990.187. [DOI] [PubMed] [Google Scholar]
- 43.Sorenson CM, Rogers SA, Korsmeyer SJ, Hammerman MR. Fulminant metanephric apoptosis and abnormal kidney development in bcl-2-deficient mice. Am J Physiol. 1995;268:F73–F81. doi: 10.1152/ajprenal.1995.268.1.F73. [DOI] [PubMed] [Google Scholar]
- 44.Pettway GJ, Schneider A, Koh AJ, Widjaja E, Morris MD, Meganck JA, Goldstein SA, McCauley LK. Anabolic actions of PTH (1-34): Use of a novel tissue engineering model to investigate temporal effects on bone. Bone. 2005;36:959–970. doi: 10.1016/j.bone.2005.02.015. [DOI] [PubMed] [Google Scholar]