Abstract
Introduction
Estrogen depletion after menopause is accompanied by bone loss and architectural deterioration of trabecular bone. The hypothesis underlying this work is that the μMRI-based virtual bone biopsy can capture the temporal changes of scale and topology of the trabecular network and that estrogen supplementation preserves the integrity of the trabecular network.
Materials and Methods
Subjects studied were early postmenopausal women, 45–55 yr of age (N = 65), of whom 32 were on estrogen (estradiol group), and the remainder were not (control group). Early menopause was defined by amenorrhea for 6–24 mo and elevated serum follicle-stimulating hormone (FSH) concentration. The subjects were evaluated with three imaging modalities at baseline and 12 and 24 mo to determine the temporal changes in trabecular and cortical architecture and density. μMRI of the distal radius and tibia was performed at 137 × 137 × 410-μm3 voxel size. The resulting bone volume fraction maps were Fourier interpolated to a final voxel size of 45.7 × 45.7 × 136.7 μm3, binarized, skeletonized, and subjected to 3D digital topological analysis (DTA). Skeletonization converts trabecular rods to curves and plates to surfaces. Parameters quantifying scale included BV/TV, whereas DTA parameters included the volume densities of curves (C) and surface (S)-type voxels, as well as composite parameters: the surface/curve ratio (S/C), and erosion index (EI, ratio of the sum of parameters expected to increase with osteoclastic resorption divided by the sum of those expected to decrease). For comparison, pQCT of the same peripheral locations was conducted, and trabecular density and cortical structural parameters were measured. Areal BMD of the lumbar vertebrae and hip was also measured.
Results
Substantial changes in trabecular architecture of the distal tibia, in particular as they relate to topology of the network, were detected after 12 mo in the control group. S/C decreased 5.6% (p < 0.0005), and EI increased 7.1% (p < 0.0005). Most curve- and profile-type voxels (representative of trabecular struts), increased significantly (p < 0.001). Curve and profile edges resulting from disconnection of rod-like trabeculae increased by 9.8% and 5.1% (p = 0.0001 and <0.001, respectively). Similarly, DXA BMD in the spine and hip decreased 2.6% and 1.3% (p < 0.0001 and <0.005, respectively), and pQCT cortical area decreased 3.6% (p = 0.0001). However, neither trabecular density nor BV/TV changed. Furthermore, none of the parameters measured in the estradiol group were significantly different after 12 mo. Substantial differences in the mean changes from baseline between the estradiol treatment and control groups, in particular after 24 mo, were observed, with relative group differences as large as 13% (S/C, p = 0.005), and the relative changes in the two groups had the opposite sign for most parameters. The observed temporal alterations in architecture are consistent with remodeling changes that involve gradual conversion of plate-like to rod-like trabecular bone along with disconnection of trabecular elements, even in the absence of a net loss of trabecular bone. The high-resolution 3D rendered images provide direct evidence of the above remodeling changes in individual subjects. The radius structural data indicated similar trends but offered no definitive conclusions.
Conclusions
The short-term temporal changes in trabecular architecture after menopause, and the protective effects of estradiol ensuring maintenance of a more plate-like TB architecture, reported here, have not previously been observed in vivo. This work suggests that MRI-based in vivo micromorphometry of trabecular bone has promise as a tool for monitoring osteoporosis treatment.
Key words: menopause, estrogen, hormone replacement, trabecular bone structure, MRI
INTRODUCTION
Depletion of serum estrogen levels after menopause is well known to cause loss in bone mass, which is accompanied by reduced mechanical competence.(1) Because trabecular or spongy bone remodels faster than cortical bone, it responds faster to hormonal changes or treatment with antiresorptive or anabolic drugs than does cortical bone. The focus of prior research has been on quantifying the temporal changes in response to intervention at locations rich in trabecular bone. Recent data suggest that besides BMD, usually measured by X-ray–based densitometric techniques, such as DXA or QCT, “bone quality” is significantly determined by the bone's microarchitecture,(2) which cannot be quantified by densitometric techniques. Several large treatment studies, a case in point being the raloxifene trial, have shown that the large reductions in fracture incidence in response to intervention are incommensurate with the small increments in BMD.(3)
Trabecular bone consists of a complex array of interconnected plates and rods, of ∼100–150 μm thickness. Whereas initially more plate-like, during aging and more so in response to loss in sex steroids, the remodeling equilibrium shifts toward excess resorption, which cannot be offset by new bone formation, and the bone becomes more rod-like.(4) This etiology of bone loss, first proposed by Parfitt et al.,(5) is believed to be caused by repeated osteoclastic pitting during successive remodeling cycles, eventually leading to fenestration of plates and their conversion to rod-like trabeculae, ultimately disconnecting the trabecular elements. There is now substantial evidence for the prevalence of this mechanism from histomorphometry(6) and 3D μCT.(4) The conversion of plates to rods or the disconnection of trabecular rods entails altered topology of the trabecular network. In general, topological changes lead to irreversible loss in mechanical competence even if the original bone volume was restored.(7,8) Structural measures can then be determined by means of stereology from sections or μCT images. However, thus far, it has not been possible to observe remodeling-induced alterations of the trabecular bone scaffold noninvasively.
Knowledge and quantification of the structural changes occurring after menopause in women or loss of testosterone in men is of substantial interest. Although it has been shown that osteoclastic resorption can be slowed with antiresorptive therapy such as hormone replacement(9) or bisphosphonates,(10) along with a net accrual of bone, the notion that some of the topological changes may be reversible, remains controversial.
Recent advances in imaging technology, notably μMRI(11,12) and peripheral high-resolution CT (HR-pQCT),(13,14) along with the development of image processing techniques,(12) now enable visualization and quantification of trabecular bone architecture in vivo at peripheral locations such as the distal radius,(15,16) proximal(17) and distal tibia,(18) and calcaneus.(19,20) For reviews of the subject, see previously published material.(21,22)
We have previously developed an MRI-based method dubbed “virtual bone biopsy” (VBB) for visualizing and quantifying the topological changes of trabecular network architecture in vivo in patients.(12,23) This study is the first to provide detailed insight into the short-term structural changes that occur in the trabecular microarchitecture during early menopause and the protective effect of estrogen supplementation, based solely on noninvasive structural measurements.
MATERIALS AND METHODS
Subjects
Sixty-five subjects were recruited through advertisements, letters, and flyers. Subjects were early menopausal women, 45–55 yr of age, who wished to take or continue estradiol (estradiol group) and those who did not wish to take estradiol (control group). Early menopause was defined by amenorrhea for 6–24 mo and elevated serum follicle-stimulating hormone (FSH) concentration (>30 mIU/ml). Some of the estradiol subjects had had prior hysterectomy.
Subjects were excluded if they had used any medication known to affect bone (bisphosphonates, calcitonin, selective estrogen receptor modulators, diphenylhydantoin, or glucocorticoids), if they had any metabolic bone disease or disease that could result in metabolic bone disease, current alcohol or drug use, or untreated or unstable cardiac, pulmonary, liver, or renal disease, or uncontrolled diabetes. Women in the estrogen group were also excluded if they had a personal history of breast, endometrial, or cervical cancer, or endometrial hyperplasia, or had a first degree relative with a history of breast cancer. If any of these conditions occurred during the study, the subject was withdrawn.
Subjects in the control group had to have a baseline BMD T-score > −2.5 to enter the study and could not have taken any estrogen preparation or selective estrogen receptor modulator within the previous 6 mo. If the T-score dropped below −3.0 at any time during the study, the subject had to be withdrawn.
A dietary history was taken from all subjects, and those whose diets and diet supplements that did not contain at least 1500 mg of calcium and 400 U of vitamin D per day were offered a calcium-vitamin D supplement to increase their total intake to 1500 mg/d. Other supplements of the subject's choice were acceptable.
Of the 65 women enrolled, 8 withdrew before follow-up scans. One control subject began treatment after the 12-mo exam, effectively withdrawing from the study as a control and instead participating as a treatment subject for all three time points. One treatment subject withdrew after 12 mo in the study because of diagnosis of breast cancer. All human subject procedures were conducted under an Institutional Review Board–approved protocol after patients had given written consent.
Study medications
Women who wished to use hormone replacement (typically to alleviate menopausal symptoms), were provided with Vivelle-Dot (Novartis Pharmaceuticals, East Hanover, NJ, USA), an estradiol transdermal system, and Prometrium (Solvay Pharmaceuticals, Marietta, GA, USA), a micronized progesterone. The initial doses were 0.05 mg/d of Vivelle-Dot and 100 mg/d of Prometrium orally. Dose adjustment occurred if the subject experienced side effects that could be dose related. Some women had already taken estrogen before the start of the study using oral preparations. These patients were, on enrollment, switched to the transdermal administration. Patients completed a questionnaire about their calcium and vitamin D ingestion at the outset of the study. Calcium-vitamin D supplementation was provided to women whose daily calcium intake was <1000 mg daily and vitamin D <400 IU. The questionnaire was repeated every 6 mo, and adjustments in doses were made accordingly. Women with a prior hysterectomy received Vivelle-Dot only.
Imaging procedures
The primary imaging modality was μMRI involving customized radiofrequency coils, imaging pulse sequences, reconstruction, processing, and analysis, details of which have been given previously(12,22); thus, only a brief summary is given below. In addition, DXA and pQCT were performed to obtain measures of BMD for reference. Subjects were scanned at three time points: at baseline on enrollment and again after 12 and 24 mo.
μMRI was conducted on a commercial 1.5-T imaging system (General Electric Signa), with the subjects in supine position. Structural imaging of trabecular bone was performed in the metaphysis of the distal tibia and distal radius; both are sites that allow the use of closely coupled RF coils ensuring adequate signal-to-noise at the resolution required to resolve the trabecular network. The radius was scanned with an elliptical transmit-receive bird-cage coil,(24) with the patient's arm parallel to the body axis, the tibia with a two-element surface coil placed anteriorly. A sagittal spin-echo localizer image set was acquired to prescribe the high-resolution 3D FLASE (fast large-angle spin-echo).(24,25) FLASE images were acquired with a flip angle of 140°, TR/TE 80 ms/9.5ms (fractional-echo acquisition), 7.81-kHz bandwidth, field of view of 7 cm × 4 cm (radius) or 7 cm × 5 cm (tibia), with a single acquisition per k-space line. Thirty-two slices were obtained along the partition direction (inferior-superior), 512 × 384 samples were collected (frequency and phase encoding directions, respectively) at the tibia, and 512 × 288 were collected at the radius. Gradient-echo navigator data were taken every 0.2 s to measure temporal displacements in the transverse plane during the scan. Displacements were fed back into the raw data as phase shifts before reconstruction,(24) which yielded a nominal image pixel size of 137 × 137 μm3 in the transverse plane and slice thickness of 410 μm. Total scan times were 16 min for the tibia and 12 min for the radius.
DXA bone densitometry was performed at the lumbar spine and proximal femur (Version 12.3; Hologic, Bedford, MA, USA) with standard positioning techniques yielding measures of areal BMD (g/cm2) for antero-posterior (AP) spine (vertebrae L1–L4) and proximal femur (femoral neck, trochanteric, and intertrochanteric regions). For the spine scan, subjects were positioned supine with the lower legs elevated on a positioning wedge. Lumbar vertebrae 1 through 4 were measured in the AP view and vertebrae 2 through 4 in the lateral view. For the hip scan, subjects were supine and the femur was internally rotated 15–20° with the aid of a positioning device provided by the manufacturer. Furthermore, volumetric trabecular BMD and cortical cross-sectional area and thickness were measured in the distal radius of the nondominant arm and distal tibia by pQCT (XCT2000; Orthometrix, White Plains, NY, USA). A scout scan was performed first, and an axial image from a slice of 2.3-mm thickness and 0.4 mm × 0.4-mm pixel size was collected at 4% from the distal end of the radius or tibia. Trabecular density, cortical thickness, and cross-sectional area were computed.
Image processing and analysis
After acquisition, the μMRI data were transferred and stored on a laboratory-built RAID server. The data underwent a series of processing steps performed under Linux (SUSE 10.0) running on a PC with a 3.2-GHz Intel Pentium 4 processor. The first step consisted of correction for translational motion during the scan using the navigator echoes acquired simultaneously with the image data.(24)
After Fourier transformation of the motion-corrected k-space data yielding 32 contiguous image slices, a semiautomated masking routine was applied to segment the region of interest based on signal-to-noise ratio (SNR) criteria and gross anatomy. Subsequently, a local thresholding procedure was applied to the data the result of which is an image in which the gray value of each voxel is the fractional marrow content so that 100% represents the intensity of pure marrow, from which BV/TV was computed.(26)
The next step in the processing chain involved interpolation of the images by a factor of 3 × 3 × 3 using a 3D sinc function resulting in a 3-fold reduced voxel size in each of the three spatial dimensions. The images were binarized at 80% of the marrow intensity to yield a 3D binary volume that served as input to 3D skeletonization.(27) The skeletonization process reduces by one the dimensionality of trabecular elements, converting trabecular plates to surfaces (S) and struts to curves (C), which is a necessary condition for determining local topology.(28) In the final step, the topology of each voxel was determined by digital topological analysis,(29) yielding the voxel densities of C- and S-type voxels and their mutual junctions (J = Σ{CC, CS, SS}), distinguishing further whether they were located in the interior (I) or edge (E) of the respective curves or surfaces (CI, SI, CE, SE). Profiles are a special class of curves that consist of two-voxel wide surfaces, which represent an intermediate structural type between surfaces and curves in the plate-to-rod bone loss etiology. Analogous to curves, the algorithm distinguishes between profile interior voxels (PI) and profile edges (PE). The process leading to topological voxel classification is shown schematically in Fig. 1. Finally, composite parameters were evaluated: the ratio S/C (the sum of S-type voxel densities divided by C-type voxel densities) and an erosion index (EI, the ratio of voxel densities that decrease divided by those that increase with osteoclastic resorption). Because bone loss typically results in a loss of surface elements and an increase in curve-type voxels, S/C and EI reflect such changes in an amplified manner because numerator and denominator change in opposite direction.
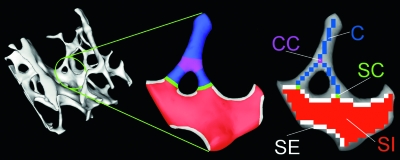
FIG. 1.
Schematic illustration of topological classification: (A) 3D rendition of a trabecular bone network. (B) Expansion of portion circled in A with different trabecular elements highlighted: blue, rods; red, plates; purple, rod–rod junction; green, rod–plate junction; white, plate edges. (C) Skeletonization converts the digitized structure to curves (C) and surfaces (S), allowing distinction of different topological entities that include curve–curve junctions (CC, purple), surface–curve junctions (SC, green), surface edges (SE, white), and surface interior (SI, red).
Figure 2 shows the cascade of processing steps with images from the distal tibia. The resulting virtual cores are for visualization only in that the parameters were derived from the volume of all available slices encompassed by the cortical boundary and meeting the signal-to-noise criteria.
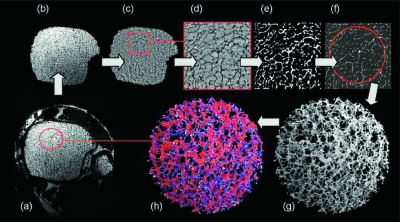
FIG. 2.
Illustration of processing steps for the MRI-based virtual bone biopsy with sample images from the distal tibia: (A) 1 of 32 contiguous cross-sectional images; (B) region of interest segmented with automatic segmentation algorithm; (C) intensity normalized image; (D) sinc-interpolated by factor of 3 × 3 × 3 yielding a voxel size of 46 × 46 × 137 μm3; (E) thresholded at 80% of marrow intensity; (F) skeleton map; (G) 3D core of skeleton; and (H) DTA classification showing surfaces (plates) in red and curves (rods) in blue.
Some examinations did not yield images of adequate quality for analysis, primarily because of motion degradation that could not be removed with the motion correction algorithms available. Three authors (FWW, GAL, and MB) independently reviewed the images from each subject for quality using a procedure described previously.(30) The images were given a number and not identified by subject or treatment group. Each reviewer was asked to decide whether to include a subject based on absolute image quality for each time point and on relative image quality for the entire time series (0–12–24 mo). Image quality was graded from 1 (unable to distinguish gross or macroscopic features) to 10 (phantom grade image). Images with quality scores of 1, 2, or 3 were excluded from the study. Relative image comparison was graded from 1 (exams too different in quality to allow comparison) to 4 (all exams of the subject's time series of images are of similar quality). Exam pairs rated 1 or 2 were excluded from the study. In this manner, of the 55 subjects completing the study, the number of 0–12 and 0–24 mo comparisons was reduced somewhat. Furthermore, some pQCT exams could not be performed because of machine failure. In summary, the numbers of control subjects who provided analyzable tibia data for the baseline to 12 (24)-mo interval for MRI, DXA, and pQCT were 28 (23), 30 (29), and 28 (28), respectively. The corresponding numbers for the estradiol group were 23 (19), 23 (22), and 20 (21). Similarly, the numbers of control subjects who provided analyzable radius data for each modality were 24 (20), 27 (26), and 24 (26), respectively, whereas the corresponding numbers for the estradiol group were 16 (13), 20 (19), and 19 (19).
Statistical analysis
After screening the data for normality, an ANOVA was performed with time (repeated) and treatment (nonrepeated) factors along with inclusion of the treatment × time cross product to assess second-order interaction before testing significance of main effects. The changes from baseline to time points 1 and 2 and from time point 1 to time point 3 were subsequently computed for each subject in each group for the structural and BMD measures selected. When a response variable was associated with significant treatment × time interaction, simple effects over time were assessed within each treatment groups using two-sided paired t-tests; alternatively, treatment group differences of the mean changes between the various time points were evaluated by unpaired t-tests. All statistical analyses were performed using JMP Discovery Software, Version 5.1 (SAS Institute).
RESULTS
Table 1 lists the characteristics of the study subjects. Because enrollment was random by design, the subjects did not differ in age or any of the anthropometric parameters. Eight estradiol patients and four control subjects had undergone hysterectomy and/or oophorectomy before enrollment. Ten estradiol subjects had been taking estradiol before enrollment for duration of 3.7 ± 2.7 (SD) yr. Of the subjects who had not undergone surgery or been on estradiol before the study, there was no difference in months since last menstrual period (LMP) between the two groups.
Table 1.
Patient Characteristics for 33 Control and 26 Estradiol Subjects for Whom Data Were Available for Analysis (Months Since LMP Data Exclude Subjects Who Had Previously Undergone Hysterectomy, Oophorectomy, or Estradiol Before the Study)
| Group | Mean | SD | SE | p | |
| Age (yr) | Control | 52.1 | 2.5 | 0.43 | 0.63 |
| Estradiol | 51.7 | 3.1 | 0.61 | ||
| Weight (kg) | Control | 68.1 | 9.0 | 1.6 | 0.73 |
| Estradiol | 67.3 | 9.7 | 1.9 | ||
| Height (cm) | Control | 164.2 | 5.9 | 1.0 | 0.94 |
| Estradiol | 164.0 | 6.1 | 1.2 | ||
| Months since LMP | Control | 11.4 | 5.6 | 1.0 | 0.29 |
| Estradiol | 13.9 | 6.2 | 2.2 |
The changes from baseline to 12 and 24 mo in μMRI-derived topological and scale parameters, as well as DXA and pQCT densities and cortical structural quantities, are listed in Tables 2 and 3 for control and estradiol subjects, respectively. The changes in the structural parameters for the control group are commensurate with a degradation of the trabecular network as is evident from the changes in topology. The strength of the associations is greater for the changes from baseline to 12 mo. The most notable changes among the simple topological parameters are an increase in curve density by 5.7% (p < 0.0001), with all individual curve-type elements increasing significantly (not tabulated). Similarly, profile edges increased by 5.1% (p < 0.001). The two composite parameters, surface-to-curve ratio and erosion index, change in opposite direction (−5.6 and +7.1%, both p < 0.0005). The observed topological changes are characteristic and fully consistent with a mechanism that involves gradual conversion of plates to rods through fenestration of plates. The increase in curve and profile edges is further indicative of disconnection of existing struts.
Table 2.
Structural Parameters at the Distal Tibia Derived From MMRI and pQCT Images and BMD of the Hip and Spine in Control Subjects at Baseline and 12 and 24 mo, Including Relative Changes of Mean Values From Baseline to Both Follow-Up Time Points
| Parameter |
Mean value |
Baseline to 12 mo |
Baseline to 24 mo |
||||
| Baseline | 12 mo | 24 mo | Relative change (%) | p | Relative change (%) | p | |
| μMRI parameters | |||||||
| BV/TV (%) | 10.62 | 10.55 | 10.64 | −0.427 | 0.61 | −0.326 | 0.69 |
| Skeleton density | 0.0588 | 0.0592 | 0.0592 | 0.809 | 0.25 | −0.116 | 0.88 |
| Surface density | 0.0442 | 0.0438 | 0.0440 | −0.576 | 0.51 | −1.150 | 0.22 |
| Curve density | 8.19 × 10−3 | 8.68 × 10−3 | 8.54 ×10−3 | 5.697 | <0.0001 | 4.111 | 0.01 |
| Junction density | 5.98 × 10−3 | 6.27 × 10−3 | 6.17 × 10−3 | 5.260 | 0.0005 | 2.763 | 0.14 |
| Curve edges | 2.86 × 10−4 | 3.14 × 10−4 | 3.07 × 10−4 | 9.792 | 0.0001 | 9.562 | 0.002 |
| Profile edges | 4.04 × 10−3 | 4.27 × 10−3 | 4.21 ×10−3 | 5.091 | 0.0007 | 3.976 | 0.05 |
| Surface interiors | 00272 | 0.0262 | 0.0266 | −3.016 | 0.02 | −2.681 | 0.04 |
| Surface-to-curve ratio | 5.445 | 5.108 | 5.182 | −5.620 | 0.0004 | −4.596 | 0.01 |
| Erosion index | 0.856 | 0.920 | 0.895 | 7.057 | 0.0004 | 5.178 | 0.009 |
| BMD parameters | |||||||
| Hip BMD (g/cm2) | 0.944 | 0.930 | 0.912 | −1.349 | 0.002 | −3.478 | <0.0001 |
| Spine BMD (g/cm2) | 1.050 | 1.022 | 1.006 | −2.608 | <0.0001 | −4.382 | <0.0001 |
| pQCT parameters | |||||||
| Cortical area (mm2) | 1.17 × 102 | 1.13 × 102 | 1.11 × 102 | −3.615 | <0.0001 | −5.912 | 0.0007 |
| Cortical thickness (mm) | 1.077 | 1.038 | 1.020 | −3.782 | 0.0001 | −5.954 | 0.002 |
| Trab. density (mg/cm3) | 2.40 × 102 | 2.40 × 102 | 2.41 ×102 | 0.0967 | 0.77 | −0.284 | 0.56 |
Table 3.
Structural Parameters at the Distal Tibia Derived From MMRI and pQCT Images and BMD of the Hip and Spine in Estradiol-Treated Subjects at Baseline and 12 and 24 mo, Including Relative Changes of Mean Values From Baseline to Both Follow-Up Time Points
| Parameter |
Mean value |
Baseline to 12 mo |
Baseline to 24 mo |
||||
| Baseline | 12 mo | 24 mo | Relative change (%) | p | Relative change (%) | p | |
| μMRI parameters | |||||||
| BV/TV (%) | 10.18 | 10.40 | 10.86 | 2.415 | 0.10 | 6.859 | 0.01 |
| Skeleton density | 0.0573 | 0.0583 | 0.0607 | 1.821 | 0.08 | 5.685 | 0.01 |
| Surface density | 0.0420 | 0.0428 | 0.0452 | 2.076 | 0.18 | 7.806 | 0.02 |
| Curve density | 8.65 × 10−3 | 8.75 × 10−3 | 8.70 × 10−3 | 1.378 | 0.32 | 0.262 | 0.88 |
| Junction density | 5.92 × 10−3 | 6.09 × 10−3 | 6.48 × 10−3 | 2.362 | 0.14 | 8.919 | 0.01 |
| Curve edges | 3.20 × 10−4 | 3.18 × 10−4 | 3.08 × 10−4 | 0.165 | 0.94 | −2.452 | 0.44 |
| Profile edges | 4.39 × 10−3 | 4.42 × 10−3 | 4.18 × 10−3 | 1.042 | 0.64 | −3.973 | 0.21 |
| Surface interiors | 0.0279 | 0.0253 | 0.0273 | 2.376 | 0.33 | 10.81 | 0.03 |
| Surface-to-curve ratio | 4.892 | 4.919 | 5.253 | 1.294 | 0.61 | 8.632 | 0.07 |
| Erosion index | 0.966 | 0.961 | 0.895 | 0.286 | 0.91 | −6.151 | 0.11 |
| BMD parameters | |||||||
| Hip BMD (g/cm2) | 0.907 | 0.912 | 0.911 | 0.567 | 0.30 | 0.215 | 0.77 |
| Spine BMD (g/cm2) | 1.023 | 1.025 | 1.012 | 0.211 | 0.74 | −0.571 | 0.52 |
| pQCT parameters | |||||||
| Cortical area (mm2) | 1.14 × 102 | 1.13 × 102 | 1.15 × 102 | 0.397 | 0.81 | 2.301 | 0.23 |
| Cortical thickness (mm) | 1.053 | 1.044 | 1.084 | 0.671 | 0.76 | 3.178 | 0.19 |
| Trab. density (mg/cm3) | 2.28 × 102 | 2.30 × 102 | 2.29 × 102 | 0.185 | 0.54 | −0.0800 | 0.85 |
Finally, the structural imaging data provide direct visual evidence of the remodeling-induced changes in the trabecular architecture as shown with the images in Fig. 3.
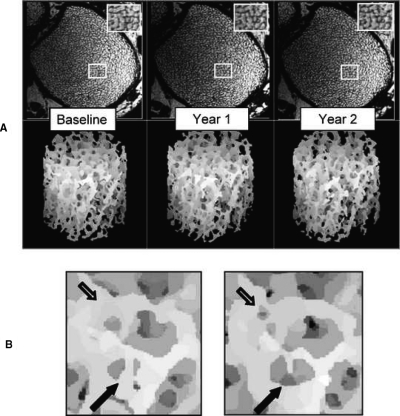
FIG. 3.
(A) Serially volume registered cross-sectional images of the distal tibia of a control subject at three time points along with volume-rendered virtual cores. Note similarity in structural patterns. (B) Magnification of subvolume in regions of A indicated by rectangles. Arrows point to regions where remodeling changes have occurred between 12 and 24 mo; open arrow, a newly formed perforation; filled arrow, enlarged perforation and disconnected trabecula.
Both hip and spine BMD decreased as expected during the first 12-mo observation period (−1.3% and −2.6%, p = 0.002 and p < 0.0001), as did cortical cross-sectional area and thickness measured by pQCT (−3.6 and −3.8%, p < 0.0001 and 0.0001). Interestingly, however, neither BV/TV or trabecular density, measured by μMRI and pQCT, respectively, changed significantly.
Whereas the above trends are maintained at the second time point, the associations are generally weaker, and the magnitude of the effects from baseline to 24 mo in most instances was smaller. Exceptions are the densitometric parameters for which the magnitude of the effect is found to increase over the total observation period, as do the cortical structural parameters.
The corresponding data for the estradiol group are listed in Table 3. The observed changes vary in magnitude but are either not significant or near the threshold of statistical significance. The data, however, strongly support that maintenance of estrogen levels at least preserves the trabecular network and possibly improves it. Indications in support of an improvement are an increase in BV/TV and skeleton density during the 24-mo treatment period (by 6.9% and 5.7%, p = 0.01). Other parameters suggestive of improved network connectivity are the increase in surface edges (by 2.9%, p = 0.01), surface interior densities (by 10.9%, p = 0.03), and junction densities (8.9%, p = 0.01). Notably, however, none of the BMD or cortical structural parameters changed in a manner approaching statistical significance.
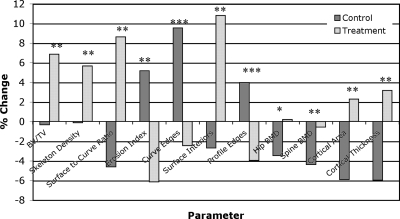
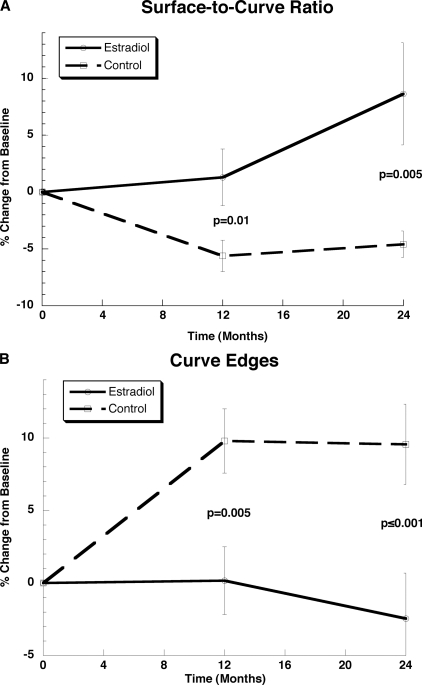
The group differences in the observed changes over time are given in Table 4, and the relative changes from baseline to 24 mo are charted in Fig. 4. Particularly dramatic are the group differences (Δ) in the composite topological parameters, surface-to-curve ratio and erosion index, with Δ = +13.2% (p = 0.005) and −11.3% (p = 0.005), where the observed relative changes have opposite signs as hypothesized. The mean group differences of a number of other structural parameters are also significantly different, lending further support to the hypothesis. Among these are BV/TV and skeleton density (Δ = 7.2% and 5.8%, both p = 0.005), as well as curve and profile edges (Δ = 12.0% and 7.9%, p < 0.01 and 0.05, respectively). Figure 5 charts the temporal evolution of two of the key parameters. Areal BMD also differed highly significantly between groups (p = 0.0002 and 0.003), as did cortical area and thickness (p = 0.002). However, pQCT-derived trabecular density changes were no different in the two groups. Finally, it is noted that, unlike for the within-group changes, the between-group changes are significantly larger for the 0- to 24-mo period than for the corresponding 0- to 12-mo period (Table 4).
Table 4.
Difference (%) in the Temporal Changes From Baseline to 12 and 24 mo, Respectively, Between Estradiol-Treated and Control Subjects for Structural Parameters Measured by MMRI and pQCT in the Distal Tibia and DXA BMD of the Hip and Spine
| Parameter |
Baseline to 12 mo |
Baseline to 24 mo |
||||
| Control | Treatment | p | Control | Treatment | p | |
| μMRI parameters | ||||||
| BV/TV (%) | −0.427 | 2.415 | 0.08 | −0.326 | 6.859 | 0.005 |
| Skeleton density | 0.809 | 1.821 | 0.39 | −0.116 | 5.685 | 0.005 |
| Surface density | −0.576 | 2.076 | .012 | −1.150 | 7.806 | 0.003 |
| Curve density | 5.697 | 1.378 | 0.02 | 4.111 | 0.262 | 0.09 |
| Junction density | 5.260 | 2.862 | 0.29 | 2.763 | 8.919 | 0.09 |
| Curve edges | 9.792 | 0.165 | 0.005 | 9.562 | −2.452 | 0.006 |
| Profile edges | 5.091 | 1.042 | 0.11 | 3.976 | −3.973 | 0.03 |
| Surface interiors | −3.016 | 2.376 | 0.04 | −2.681 | 10.81 | 0.003 |
| Surface-to-curve ratio | −5.620 | 1.294 | 0.01 | −4.596 | 8.632 | 0.005 |
| Erosion index | 7.057 | 0.286 | 0.03 | 5.178 | −6.151 | 0.005 |
| BMD parameters | ||||||
| Hip BMD (g/cm2) | −1.349 | 0.567 | 0.005 | −3.478 | 0.215 | 0.0002 |
| Spine BMD (g/cm2) | −2.608 | 0.211 | 0.0005 | −4.382 | 0.571 | 0.003 |
| pQCT parameters | ||||||
| Cortical area (mm2) | −3.615 | 0.397 | 0.02 | −5.912 | 2.301 | 0.001 |
| Cortical thickness (mm) | −3.782 | 0.671 | 0.04 | −5.954 | 3.178 | 0.002 |
| Trab. density (mg/cm3) | 0.0967 | 0.185 | 0.85 | −0.284 | −0.0800 | 0.76 |
FIG. 4.
Changes in structural and densitometric parameters from baseline to 24 mo for both estradiol-treated and control subjects. Structural parameters were measured in the distal tibia by μMRI and pQCT, BMD of the spine and hip by DXA: *p < 0.0005, **p ≤ 0.005, ***p < 0.05.
FIG. 5.
Temporal changes for two biomechanically relevant parameters: (A) surface-to-curve ratio, representative of the ratio of plate-like to rod-like trabeculae; (B) curve-edges, resulting from disconnection of rod-like trabeculae. Error bars represent SE changes within each group, and p values pertain to group differences.
The μMRI structural data at the radius did not provide a consistent picture. First, the group means of the changes from baseline to either 12 or 24 mo were not different for any of the parameters, although some trends were evident. None of the group differences for the changes from baseline to 24 mo were significant, including BV/TV and the pQCT-derived trabecular density. However, both cortical cross-sectional area and cortical thickness measured at the distal radial site by pQCT differed, showing a reduction for cortical area of 5.5% in the control group versus a gain of 0.9% in the estradiol group (p = 0.0007), along with a reduction in cortical thickness by 6.9% for the controls and a gain of 2.0% for the estradiol group (p = 0.0008).
DISCUSSION
In this work, we provided compelling evidence for the structural changes occurring in trabecular bone within 12 mo at the distal tibia by in vivo MRI of women immediately after menopause and the protective effect of estrogen. The data are the first obtained in vivo by noninvasive imaging showing topological changes secondary to estrogen depletion. We also show for the first time that it may be possible with the new technology to observe and monitor structural changes in individual patients. Using motion correction and registration technology,(31) we showed that it is possible to accurately match up the analysis volume from repeat studies to the baseline and thereby detect subtle remodeling effects.
The structural alterations observed in control subjects not taking estrogen include a gradual transformation of the trabecular network from a plate-like to rod-like architecture as indicated by the decrease in topological surface-to-curve ratio and increase in erosion index (Table 2). The large increase in curve and profile edges (both representative of disconnected struts) by nearly 10% for curve edge (5% for profile edge densities) from baseline to 12 mo is further evidence of the previously hypothesized etiology underlying postmenopausal bone loss. Such a mechanism is paralleled by recently reported observations based on paired bone biopsies.(32) Similar structural effects were observed by serial μCT imaging on ovariectomy in animal models mimicking the effect of postmenopausal gonadal steroid depletion.(33) The structural changes shown in this study in early postmenopausal women were accompanied by a reduction in pQCT-derived cortical area and cortical thickness at the distal tibia and a decrease in areal density measured by DXA at both the hip and the spine. However, neither MRI nor pQCT indicated any loss in either trabecular volume fraction or trabecular density (Table 2; Fig. 4). A recent study based on analysis of paired bone biopsies carried out before and after menopause(32) found similar structural changes, including an increase in the structure model index,(34) suggesting the bone having become less plate-like in its architecture. However, the observed associations were weaker despite the much longer interval between measurements, presumably because of greater heterogeneity because the use of bone biopsies does not allow for identical measurement sites.
The short-term, 12-mo changes in areal BMD were relatively small (−1.3 and −2.6%) compared with those of the architectural parameters (−5.6% and +7.1% for S/C and EI and 9.8% for CE, all p < 0.0005). However, because the variances in the densitometric parameters are generally smaller, the effects were highly significant. Hence, whereas the effect size for structural parameters is generally larger than that for BMD, the measurement precision is currently still lower for the structural measures of topology (5–7%(35) versus 1–2% for BMD). The structural alterations in the control group from baseline to 24 mo were more heterogeneous, although the observed pattern was reproduced, but there was generally no further increase in the magnitude of the observed effects, unlike BMD, which declined further. In fact, the structural changes from baseline to the 24-mo time point were generally lower. It is unclear what could have caused this discrepancy, although it is conceivable that some structural adaptation may have occurred as a means to offset the adverse effects of bone loss. A possible bias caused by the different number of subjects for whom data were available for the 0- to 24-mo analysis (because of drop-outs) can be excluded because we also performed the analysis on the joint dataset for which the results were not significantly different. On the other hand, it is noteworthy that the differences in the temporal changes from 0 to 24 mo between the estradiol and control groups were significantly larger than those from 0 to 12 mo (Table 4; Fig. 4). Also, whereas for a number of parameters the group differences were marginal or not at all significant at 12 mo, they became highly significant for the 0- to 24-mo interval, along with a substantial increase in effect size. Parameters for which such a behavior was noted are TB/TV, skeleton density, EI, and S/C, but also the cortical structural parameters derived from pQCT (Table 4). Similar to the findings in a recent testosterone treatment study,(36) the largest group differences were found for the composite parameters S/C and EI (Δ = 13.2% and 11.3%, respectively). Also dramatic are the opposite changes in curve edges (representative of disconnected trabeculae), increasing in the control group but decreasing in the estradiol group (Fig. 5B)
The above observations are consistent with some of the changes observed in the estradiol group, even though, when evaluated separately (i.e., as within-group differences), they were not or only marginally significant. Among the structural parameters in the estradiol group indicative of an improvement in bone quality was BV/TV, which increased by ∼7% (p = 0.01), as was skeleton density (+5.7%, p = 0.01), surface (+7.8%, p = 0.02), and junction density (+8.9%, p = 0.01), all suggestive of a more connected network. Interestingly, however, none of the densitometric or cortical structural parameters indicated such a trend.
The skeletal effects of hormone supplementation after menopause have been widely studied,(9,37–41) but the data are rather inconsistent, ranging from findings of mere maintenance(9,37) to claims of strong anabolic effects.(39) Virtually all of the above studies (except(41)) were made up of older women, varying in baseline BMD from normal to significantly compromised (T-scores of −2.5 or less), most evaluating changes in BMD and dynamic histomorphometry, which makes comparison with the present data in early postmenopausal women questionable.
Because, as stated in the Materials and Methods section, 10 of the 23 estradiol subjects had already been taking estradiol at the initiation of the study, it could be argued that these would respond in a manner different from the remainder of the group. Examination of the temporal changes from baseline to 12 and 24 mo between prior and new hormone replacement users suggested the changes for some of the key parameters (BV/TV and S/C) to be different in new estradiol users, but the differences were not significant. At best, these observations would indicate that the observed treatment effects are underestimated.
The lack of commensurate trabecular structural changes at the distal radius is unexpected. Neither trabecular density measured by pQCT or MRI-derived BV/TV changed in either the control or the estradiol group. On the other hand, both cortical area and thickness were reduced in the control group after 24 mo (by 5.5% and 6.9%, p = 0.0002 and 0.0005, respectively), with no significant increase in the estradiol group. There were no significant differences in MRI image quality between the two anatomic sites studied even though the radius is generally more prone to patient motion.(31) In prior work from the authors' laboratory,(16,42) as well as by other investigators,(13,43) the radius was found to be a useful site to differentiate patients with fractures from their unfractured peers. However, these studies were all cross-sectional and did not involve intervention. Although these findings are surprising, it would be premature to conclude the tibia to be superior as an indicator of treatment efficacy. Nevertheless, the tibia undergoes greater loading than the radius during daily activities but the discrepancy in the behavior of the two measurement sites demands further scrutiny.
There are only a few studies in the literature examining relatively short-term effects of antiresorptive or anabolic therapy by in vivo structural imaging.(36,44–46) Pothuaud et al.(44) purported to show a treatment effect in patients treated with idoxifen, a selective estrogen-receptor modulator, based on μMRI data analyzed with a 3D line graph method. Chesnut et al.(45) reported data on postmenopausal women treated with salmon calcitonin for 2 yr, comparing them with placebo and showing effects of treatment measured in terms of the conventional 2D structural parameters based on μMRI in the distal radius. The data indicated substantial improvement in trabecular microarchitecture in some (albeit not all) of the distal radius, or preservation in the treatment group compared with significant deterioration in the placebo control group. Neither structural measurements in the calcaneus or paired bone biopsies showed consistent differences in architecture between the mean changes observed between groups. Of note is that significant associations were obtained only in the more proximal regions of the distal radial metaphysis, which could explain why in the current work the distal radius did not show quantifiable temporal changes. In another study using methodology similar to the one of this study, Benito et al.(36) showed large effects on the topology of the trabecular architecture in the distal tibia in hypogonadal men treated with testosterone over a period of 2 yr.
In summary, our data suggest μMRI-based structure analysis to be a viable technique for the evaluation of the effectiveness of therapeutic intervention and for providing detailed insight into structural manifestations of postmenopausal bone loss. Among the current limitations of the technology are the relatively long scan times, which exacerbate subject motion-induced artifacts, as well as the sensitivity of the processing and analysis algorithms to variations in signal-to-noise ratio. Both adversely affect measurement precision and therefore statistical power. It is expected, however, that improvements in radiofrequency coil technology, operation at higher magnetic field strength, parallel imaging methods and advanced motion correction techniques will eventually overcome the present limitations of the method.
ACKNOWLEDGMENTS
This study was supported by National Institute of Health Grants R01 AR41443, RO1 49553, T32 EB000814, T32 DK07006, and UL1 RR024134, CTRC University of Pennsylvania School of Medicine, and Novartis Pharmaceuticals.
Footnotes
Dr Wehrli is Chairman of the Scientific Advisory Board of MicroMRI Inc. and owns stock in this company. He also is co-inventor of technology licensed to MicroMRI Inc. Dr Saha owns stock in MicroMRI Inc. and is co-inventor of technology licensed to MicroMRI Inc. All other authors state that they have no conflicts of interest.
REFERENCES
- 1.Ettinger B, Genant HK, Cann CE. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med. 1985;102:319–324. doi: 10.7326/0003-4819-102-3-319. [DOI] [PubMed] [Google Scholar]
- 2.Riggs BL, Melton LJ., III Bone turnover matters: The raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J Bone Miner Res. 2002;17:11–14. doi: 10.1359/jbmr.2002.17.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res. 2002;17:1–10. doi: 10.1359/jbmr.2002.17.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: Microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 5.Parfitt AM, Mathews CHE, Villanueva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983;72:1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amling M, Posl M, Ritzel H, Hahn M, Vogel M, Wening VJ, Delling G. Architecture and distribution of cancellous bone yield vertebral fracture clues. A histomorphometric analysis of the complete spinal column from 40 autopsy specimens. Arch Orthop Trauma Surg. 1996;115:262–269. doi: 10.1007/BF00439050. [DOI] [PubMed] [Google Scholar]
- 7.Kinney JH, Ladd AJC. The relationship between three-dimensional connectivity and the elastic properties of trabecular bone. J Bone Miner Res. 1998;13:839–845. doi: 10.1359/jbmr.1998.13.5.839. [DOI] [PubMed] [Google Scholar]
- 8.Guo XE, Kim CH. Mechanical consequence of trabecular bone loss and its treatment: A three-dimensional model simulation. Bone. 2002;30:404–411. doi: 10.1016/s8756-3282(01)00673-1. [DOI] [PubMed] [Google Scholar]
- 9.Vedi S, Compston JE. The effects of long-term hormone replacement therapy on bone remodeling in postmenopausal women. Bone. 1996;19:535–539. doi: 10.1016/s8756-3282(96)00227-x. [DOI] [PubMed] [Google Scholar]
- 10.Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997;100:1475–1480. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majumdar S. Magnetic resonance imaging of trabecular bone structure. Top Magn Reson Imaging. 2002;13:323–334. doi: 10.1097/00002142-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Wehrli FW, Song HK, Saha PK, Wright AC. Quantitative MRI for the assessment of bone structure and function. NMR Biomed. 2006;19:731–764. doi: 10.1002/nbm.1066. [DOI] [PubMed] [Google Scholar]
- 13.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6805–6815. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 14.Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ., III Effects of sex and age on bone microstructure at the ultradistal radius: A population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21:124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majumdar S, Genant HK, Grampp S, Newitt DC, Truong V-H, Lin JC, Mathur A. Correlation of trabecular bone structure with age, bone, mineral density, and osteoporotic status: In vivo studies in the distal radius using high-resolution magnetic resonance imaging. J Bone Miner Res. 1997;12:111–118. doi: 10.1359/jbmr.1997.12.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Wehrli FW, Hwang SN, Ma J, Song HK, Ford JC, Haddad JG. Cancellous bone volume and structure in the forearm: Noninvasive assessment with MR microimaging and image processing. Radiology. 1998;206:347–357. doi: 10.1148/radiology.206.2.9457185. [DOI] [PubMed] [Google Scholar]
- 17.Beuf O, Ghosh S, Newitt DC, Link TM, Steinbach L, Ries M, Lane N, Majumdar S. Magnetic resonance imaging of normal and osteoarthritic trabecular bone structure in the human knee. Arthritis Rheum. 2002;46:385–393. doi: 10.1002/art.10108. [DOI] [PubMed] [Google Scholar]
- 18.Benito M, Gomberg B, Wehrli FW, Weening RH, Zemel B, Wright AC, Song HK, Cucchiara A, Snyder PJ. Deterioration of trabecular architecture in hypogonadal men. J Clin Endocrinol Metab. 2003;88:1497–1502. doi: 10.1210/jc.2002-021429. [DOI] [PubMed] [Google Scholar]
- 19.Link TM, Vieth V, Matheis J, Newitt D, Lu Y, Rummeny EJ, Majumdar S. Bone structure of the distal radius and the calcaneus vs BMD of the spine and proximal femur in the prediction of osteoporotic spine fractures. Eur Radiol. 2002;12:401–408. doi: 10.1007/s003300101127. [DOI] [PubMed] [Google Scholar]
- 20.Boutry N, Cortet B, Dubois P, Marchandise X, Cotten A. Trabecular bone structure of the calcaneus: Preliminary in vivo MR imaging assessment in men with osteoporosis. Radiology. 2003;227:708–717. doi: 10.1148/radiol.2273020420. [DOI] [PubMed] [Google Scholar]
- 21.Genant HK, Jiang Y. Advanced imaging assessment of bone quality. Ann NY Acad Sci. 2006;1068:410–428. doi: 10.1196/annals.1346.038. [DOI] [PubMed] [Google Scholar]
- 22.Wehrli FW. Structural and functional assessment of trabecular and cortical bone by micro magnetic resonance imaging. J Magn Reson Imaging. 2007;25:390–409. doi: 10.1002/jmri.20807. [DOI] [PubMed] [Google Scholar]
- 23.Wehrli FW, Saha PK, Gomberg BR, Song HK. Noninvasive assessment of bone architecture by magnetic resonance micro-imaging-based virtual bone biopsy. Proc IEEE. 2003;91:1520–1542. [Google Scholar]
- 24.Song HK, Wehrli FW. In vivo micro-imaging using alternating navigator echoes with applications to cancellous bone structural analysis. Magn Reson Med. 1999;41:947–953. doi: 10.1002/(sici)1522-2594(199905)41:5<947::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Wehrli FW, Song HK. Fast 3D large-angle spin-echo imaging (3D FLASE) Magn Reson Med. 1996;35:903–910. doi: 10.1002/mrm.1910350619. [DOI] [PubMed] [Google Scholar]
- 26.Vasilic B, Wehrli FW. A novel local thresholding algorithm for trabecular bone volume fraction mapping in the limited spatial resolution regime of in-vivo MRI. IEEE Trans Med Imaging. 2005;24:1574–1585. doi: 10.1109/TMI.2005.859192. [DOI] [PubMed] [Google Scholar]
- 27.Manzanera A, Bernard TM, Preteux P, Longuet B. N-dimensional skeletonization: A unified mathematical framework. J Electron Imaging. 2002;11:25–37. [Google Scholar]
- 28.Saha PK, Gomberg BR, Wehrli FW. Three-dimensional digital topological characterization of cancellous bone architecture. Int J Imaging Syst Technol. 2000;11:81–90. [Google Scholar]
- 29.Gomberg BG, Saha PK, Song HK, Hwang SN, Wehrli FW. Application of topological analysis to magnetic resonance images of human trabecular bone. IEEE Trans Med Imaging. 2000;19:166–174. doi: 10.1109/42.845175. [DOI] [PubMed] [Google Scholar]
- 30.Ladinsky GA, Vasilic B, Popescu AM, Wald M, Zemel BS, Snyder PJ, Loh L, Song HK, Saha PK, Wright AC, Wehrli FW. Trabecular structure quantified with the MRI-based virtual bone biopsy in postmenopausal women contributes to vertebral deformity burden independent of areal vertebral BMD. J Bone Miner Res. 2008;23:64–74. doi: 10.1359/JBMR.070815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasilic B, Ladinsky GA, Saha PK, Wehrli FW. Micro-MRI-based image acquisition and processing system for assessing the response to therapeutic intervention. Proceedings of the SPIE. The International Society for Optical Engineering. International Society for Optical Engineering; February 11–16, 2006; San Diego, CA, USA. 2006. [Google Scholar]
- 32.Akhter MP, Lappe JM, Davies KM, Recker RR. Transmenopausal changes in the trabecular bone structure. Bone. 2007;41:111–116. doi: 10.1016/j.bone.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Boyd SK, Davison P, Muller R, Gasser JA. Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone. 2006;39:854–862. doi: 10.1016/j.bone.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Hildebrand T, Rüegsegger P. A new method for the model independent assessment of thickness in three-dimensional images. J Microsc. 1997;185:67–75. [Google Scholar]
- 35.Gomberg BR, Wehrli FW, Vasilic B, Weening RH, Saha PK, Song HK, Wright AC. Reproducibility and error sources of micro-MRI-based trabecular bone structural parameters of the distal radius and tibia. Bone. 2004;35:266–276. doi: 10.1016/j.bone.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, Wright AC, Zemel B, Cucchiara A, Snyder PJ. Effect of testosterone replacement on bone architecture in hypogonadal men. J Bone Miner Res. 2005;20:1785–1791. doi: 10.1359/JBMR.050606. [DOI] [PubMed] [Google Scholar]
- 37.Vedi S, Croucher PI, Garrahan NJ, Compston JE. Effects of hormone replacement therapy on cancellous bone microstructure in postmenopausal women. Bone. 1996;19:69–72. doi: 10.1016/8756-3282(96)00108-1. [DOI] [PubMed] [Google Scholar]
- 38.Harris ST, Eriksen EF, Davidson M, Ettinger MP, Moffett Jr AH, Jr, Baylink DJ, Crusan CE, Chines AA. Effect of combined risedronate and hormone replacement therapies on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2001;86:1890–1897. doi: 10.1210/jcem.86.5.7505. [DOI] [PubMed] [Google Scholar]
- 39.Khastgir G, Studd J, Holland N, Alaghband-Zadeh J, Fox S, Chow J. Anabolic effect of estrogen replacement on bone in postmenopausal women with osteoporosis: Histomorphometric evidence in a longitudinal study. J Clin Endocrinol Metab. 2001;86:289–295. doi: 10.1210/jcem.86.1.7161. [DOI] [PubMed] [Google Scholar]
- 40.Weinstein RS, Parfitt AM, Marcus R, Greenwald M, Crans G, Muchmore DB. Effects of raloxifene, hormone replacement therapy, and placebo on bone turnover in postmenopausal women. Osteoporos Int. 2003;14:814–822. doi: 10.1007/s00198-003-1434-z. [DOI] [PubMed] [Google Scholar]
- 41.Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: Four-year randomized study. Am J Med. 1995;99:36–42. doi: 10.1016/s0002-9343(99)80102-8. [DOI] [PubMed] [Google Scholar]
- 42.Wehrli FW, Gomberg BR, Saha PK, Song HK, Hwang SN, Snyder PJ. Digital topological analysis of in vivo magnetic resonance microimages of trabecular bone reveals structural implications of osteoporosis. J Bone Miner Res. 2001;16:1520–1531. doi: 10.1359/jbmr.2001.16.8.1520. [DOI] [PubMed] [Google Scholar]
- 43.Majumdar S, Link TM, Augat P, Lin JC, Newitt D, Lane NE, Genant HK. Trabecular bone architecture in the distal radius using magnetic resonance imaging in subjects with fractures of the proximal femur. Magnetic Resonance Science Center and Osteoporosis and Arthritis Research Group. Osteoporos Int. 1999;10:231–239. doi: 10.1007/s001980050221. [DOI] [PubMed] [Google Scholar]
- 44.Pothuaud L, Newitt DC, Lu Y, MacDonald B, Majumdar S. In vivo application of 3D-line skeleton graph analysis (LSGA) technique with high-resolution magnetic resonance imaging of trabecular bone structure. Osteoporos Int. 2004;15:411–419. doi: 10.1007/s00198-003-1563-4. [DOI] [PubMed] [Google Scholar]
- 45.Chesnut CH, III, Majumdar S, Newitt DC, Shields A, Van Pelt J, Laschansky E, Azria M, Kriegman A, Olson M, Eriksen EF, Mindeholm L. Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: Results from the QUEST study. J Bone Miner Res. 2005;20:1548–1561. doi: 10.1359/JBMR.050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graeff C, Timm W, Nickelsen TN, Farrerons J, Marin F, Barker C, Gluer CC. Monitoring teriparatide associated changes in vertebral microstructure by high-resolution computed tomography in vivo: Results from the EUROFORS study. J Bone Miner Res. 2007;22:1426–1433. doi: 10.1359/jbmr.070603. [DOI] [PubMed] [Google Scholar]