Abstract
Background:
Intra-articular injection of hyaluronan (HA) has been suggested to have a disease-modifying effect in osteoarthritis, but little is known about the possible mechanisms.
Objective:
To investigate the effects of HA species of different molecular mass, including 800 kDa (HA800) and 2700 kDa (HA2700), on the expression of aggrecanases (ie, ADAMTS species), which play a key role in aggrecan degradation.
Methods:
The effects of HA species on the expression of ADAMTS1, 4, 5, 8, 9 and 15 in interleukin 1α (IL1α)-stimulated osteoarthritic chondrocytes were studied by reverse transcription PCR and real-time PCR. Expression of ADAMTS4 protein and aggrecanase activity and signal transduction pathways of IL1, CD44 and intracellular adhesion molecule 1 (ICAM1) were examined by immunoblotting.
Results:
IL1α treatment of chondrocytes induced ADAMTS4, and HA800 and HA2700 significantly decreased IL1α-induced expression of ADAMTS4 mRNA and protein. IL1α-stimulated aggrecanase activity in osteoarthritic chondrocytes was reduced by treatment with HA2700 or transfection of small interfering RNA for ADAMTS4. A similar result was obtained when HA2700 was added to explant cultures of osteoarthritic cartilage. HA2700 neither directly inhibited nor bound to ADAMTS4. Downregulation of ADAMTS4 expression by HA2700 was attenuated by treatment of IL1α-treated chondrocytes with antibodies to CD44 and/or ICAM1. The increased phosphorylation of IL1 receptor-associated kinase-1 and extracellular signal-regulated protein kinase1/2 induced by the IL1α treatment was downregulated by enhanced IRAK-M expression after HA2700 treatment.
Conclusion:
These data suggest that HA2700 suppresses aggrecan degradation by downregulating IL1α-induced ADAMTS4 expression through the CD44 and ICAM1 signalling pathways in osteoarthritic chondrocytes.
Aggrecan degradation and subsequent digestion of collagen fibrils make up the central pathway for the destruction of cartilage in osteoarthritis (OA). Collagen degradation is carried out principally by collagen-degrading matrix metalloproteinases (MMPs) such as MMP1, MMP8 and MMP13.1–3 On the other hand, aggrecan-degrading metalloproteinases, called aggrecanases, are considered to play a key role in aggrecan degradation.4 5 Aggrecanases belong to the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) gene family, and ADAMTS1, 4, 5, 8, 9 and 15 are known to have aggrecanase activity.4 6 Studies using ADAMTS4 and ADAMTS5 knockout mice have indicated that ADAMTS5, but not ADAMTS4, has an essential role in aggrecan degradation in mice.7 8 However, because there is little information about the biochemical character, expression patterns or gene promoter structures of mouse ADAMTS4 and ADAMTS5, the data from knockout mice must be interpreted with care and should not be extrapolated to the human disease.9 10 In human chondrocytes, ADAMTS4 is inducible by treatment with cytokines such as interleukin 1 (IL1), but the expression of ADAMTS5 is constitutive.9 11–13 Our recent study also showed that, of the aggrecanase-type ADAMTS species, ADAMTS4 is selectively overexpressed in human osteoarthritic cartilage, with a direct correlation with the degree of cartilage destruction, whereas ADAMTS5 is constitutively expressed in both normal and osteoarthritic cartilage.10 These results suggest that ADAMTS4 is a major aggrecanase in human osteoarthritic cartilage.
Hyaluronan (HA) is widely used to treat OA of the knee by intra-articular injection. The effects are reported to depend on the molecular mass of the HA species,14 15 which can be classified as very low (50 kDa), low (300 kDa), medium (800 kDa) or high (2000–3000 kDa) according to a consensus reached at the 7th International Conference on Hyaluronan (South Carolina, 2007). Symptom-modifying effects of medium and high molecular mass HA, including relief of joint pain, have been demonstrated,14 15 but some experimental and clinical studies have provided evidence that these HA species also have potentially disease-modifying effects.14 16–18 HA of 2700 kDa (HA2700) has been shown to be a potent inhibitor of proteoglycan release from the cell matrix of rabbit chondrocyte cultures.19 Previous studies20 21 have also shown that HA of 800 kDa (HA800) inhibits IL1-stimulated production of MMP1, MMP3 and MMP13 through interaction with CD44, a major receptor for HA on chondrocytes and synoviocytes, although the authors did not search for effects on the signalling pathway of CD44. On the other hand, intra-articular injection of HA800 in a rabbit OA model has been reported to suppress osteoarthritic changes without inhibiting expression of MMP3.22 These data suggest that the chondroprotective effect of medium and high molecular mass HA may be due to inhibition of aggrecanases as well as MMPs. At this juncture, however, little or no information is available on the effects of HA on aggrecanases in human osteoarthritic chondrocytes or cartilage.
In this study, we examined the effects of HA species on the expression of ADAMTS1, 4, 5, 8, 9 and 15 in osteoarthritic chondrocytes stimulated with IL1, one of the most powerful stimulators of proteinase production.23 As HA2700 inhibited IL1-induced expression of ADAMTS4, we also studied the signalling pathways through interaction between HA2700 and HA receptors.
METHODS
Chondrocyte cultures
Non-osteophytic articular cartilage was obtained at arthroplasty from knee or hip joints of patients with OA diagnosed according to the criteria of the American College of Rheumatology.24 Chondrocytes were isolated from the cartilage,25 and the cultured cells were confirmed to be of chondrocyte phenotype by the positive immunostaining of aggrecan and type II collagen.26 Chondrocytes were starved of serum in Dulbecco’s modified Eagle’s medium/Ham’s F-12 (DMEM/F-12) containing 0.2% lactalbumin hydrolysate, and then treated with IL1α in the presence or absence of HA species of different molecular mass, including 1.2 kDa (HAoligo), 300 kDa (HA300), 800 kDa (HA800) and 2700 kDa (HA2700), all of which were gifts from Chugai Pharmaceutical Co (Tokyo, Japan). The molecular mass of HA2700, which is known as Suvenyl (Chugai Pharmaceutical Co) and used to treat knee OA, was determined by the multi-angle laser light scattering method.27 We used 1 ng/ml IL1α and 2.5 mg/ml HA species (except for HAoligo), as these concentrations of IL1α and HA showed a definite stimulatory effect on ADAMTS4 expression and a maximal inhibitory effect on IL1α-induced ADAMTS4 expression, respectively (data not shown). HAoligo was used at a concentration of 250 μg/ml according to data from a previous study.28 The non-cytotoxic effect of these HA species on cultured chondrocytes was confirmed using an MTT assay kit (Nacalai Tesque Corp, Kyoto, Japan).29 Informed consent was obtained from patients according to the hospital ethics guidelines for the experimental use of the samples.
Reverse transcription (RT)-PCR
Total RNA extracted from cultured chondrocytes was reverse-transcribed to cDNA, and PCR amplification by Ex Taq DNA polymerase (Takara Bio, Otsu, Japan) was performed using primers specific for ADAMTS1, ADAMTS4, ADAMTS5, ADAMTS8, ADAMTS9, ADAMTS15, MMP1, MMP2, MMP3, MMP13, membrane type (MT)1-MMP, MT4-MMP, tissue inhibitor of metalloproteinases 3 (TIMP3) and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as described previously.10 30 31 Positive control experiments were carried out as described elsewhere.10
Real-time quantitative PCR
The relative mRNA expression ratios of ADAMTS1, ADAMTS4, ADAMTS5, ADAMTS8, ADAMTS9 and ADAMTS15 to GAPDH were measured in a TaqMan real-time PCR assay as described previously.32 Sequences of the primers and TaqMan probe are provided as supplementary material 1.
Immunoblotting of ADAMTS4
Chondrocytes were cultured in serum-free DMEM/F-12 in the presence or absence of IL1α. Cell lysates and conditioned medium were subjected to sodium dodecyl sulphate/polyacrylamide gel electrophoresis (SDS/PAGE) under reduction,33 and immunoblotted with antibodies to ADAMTS4 (247-3F6 and 250-4F7) or GAPDH (Abcam, Cambridge, UK) as described previously.10 The specificity of monoclonal antibodies to the spacer domain (247-3F6) or a portion of the disintegrin and thrombospondin domains (250-4F7) of human ADAMTS4 has been demonstrated.10 Both antibodies were able to immunoprecipitate ADAMTS4 (supplementary material 2).
Detection of aggrecanase activity in osteoarthritic chondrocytes and articular cartilage
Chondrocytes were cultured for 5 days in serum-free DMEM/F-12 containing 100 μg/ml aggrecan isolated from porcine nasal cartilage34 in the presence or absence of IL1α. Aggrecanase activity was detected by immunoblotting aggrecan fragments in the medium using anti-aggrecan neoepitope (NITEGE373)-specific antibody (2 μg/ml).35 The effects of HA2700, KB-R7785 (a synthetic ADAM inhibitor)32 36 and TIMP3 (a natural inhibitor of aggrecanases)37 38 on the aggrecanase activity of the IL1α-stimulated chondrocytes were evaluated by immunoblotting. Similarly, the effects of HA2700 and KB-R7785 on the activity in osteoarthritic cartilage were examined by immunoblotting after culture of cartilage tissue slices (5×5×1 mm) in serum-free DMEM/F-12 containing 0.2% lactalbumin hydrolysate and IL1α. After the reactions, the samples were freeze-milled under liquid nitrogen and pulverised.25 The concentration of glycosaminoglycan in proteoglycans extracted from the cartilage powder was determined by colorimetric assay.39 The inhibitory activity of KB-R778532 36 on ADAMTS4 was confirmed in an assay of aggrecan digestion using recombinant ADAMTS435 (data not shown).
Transfection of small interfering RNA (siRNA) for ADAMTS4
The siRNA for ADAMTS4 (CCATCAATGGAGATCCGGAGT) (Ambion Inc, Austin, Texas, USA) and non-silencing oligonucleotide (AATTCTCGGAACGTGTCACGT) (Qiagen Inc, Valencia, California, USA) were custom synthesised and transfected to IL1α-stimulated osteoarthritic chondrocytes and cartilage (see supplementary material 3 for detailed conditions). Expression of ADAMTS4 after siRNA transfection was monitored by RT-PCR and immunoblotting, and aggrecanase activity was detected by immunoblotting as describe above.
Blocking of HA2700 binding to chondrocytes with antibodies to HA receptor, and analysis of cell signalling
Chondrocytes were incubated with neutralising CD44 antibody (BD Biosciences, San Diego, California, USA), ICAM1 antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, California, USA) or non-immune IgG (BD Biosciences), and then cultured in serum-free DMEM/F-12 with or without HA2700 in the presence or absence of IL1α. ADAMTS4 expression was monitored by RT-PCR, real-time PCR and immunoblotting. For the study of cell signalling, chondrocytes were cultured under similar conditions, and the cell lysates were subjected to SDS/PAGE for immunoblotting analyses using rabbit polyclonal antibodies to IL1-associated kinase 1 (IRAK1), phospho-IRAK1, IRAK-M, extracellular signal-regulated protein kinase1/2 (ERK1/2) or phospho-ERK1/2 (Cell Signalling Technology Co, Beverly, Massachusetts, USA).32 The effect of mitogen-activated protein kinase kinase (MEK) inhibitor on ERK1/2 phosphorylation and expression of ADAMTS4 was also examined by treating chondrocytes with PD98059 (Calbiochem, San Diego, California, USA).32 Detailed conditions are described in supplementary material 4.
Statistical analysis
Comparisons between two groups were performed by the Mann–Whitney U test. Comparisons among more than three groups were made by the Kruskal–Wallis test. Statistical analyses were carried out using Stat-View statistical software. p<0.05 was considered significant.
RESULTS
Effects of HA on mRNA expression of ADAMTS species, MMPs and TIMP3
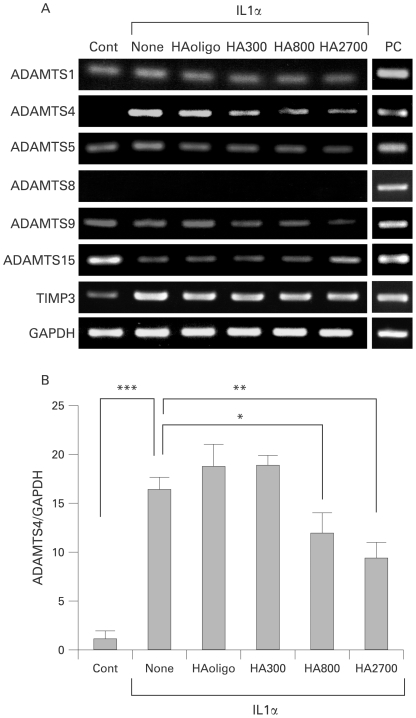
RT-PCR analysis showed that IL1α treatment of osteoarthritic chondrocytes induces expression of ADAMTS4 and stimulates expression of TIMP3 (fig 1A). ADAMTS1, ADAMTS5 and ADAMTS9 were constitutively expressed, and ADAMTS15 expression decreased under stimulation with IL1α. Negligible expression of ADAMTS8 was observed even under IL1α stimulation. When IL1α-stimulated chondrocytes were treated with HA species, the expression of ADAMTS4, ADAMTS5 and ADAMTS9 appeared to decrease in a manner directly related to the molecular mass of the HA used (fig 1A). However, no definite decrease in the expression of ADAMTS1, ADAMTS15 and TIMP3 was obtained by the treatment. Similar inhibitory effects of the HA species on the IL1α-stimulated expression of MMP3, MMP13, MT1-MMP and MT4-MMP were observed (supplementary material 5).
Figure 1.
Effects of hyaluron (HA) species on mRNA expression of ADAMTS species in interleukin (IL)1α-stimulated osteoarthritic chondrocytes. (A) Reverse transcription-PCR analysis of the expression of ADAMTS species and tissue inhibitor metalloproteinase 3 (TIMP3). Total RNA was isolated from the chondrocytes which were treated for 24 h without (Cont) or with IL1α (1 ng/ml) and HA (250 μg/ml for HAoligo and 2.5 mg/ml for other HA species), and reverse-transcribed into cDNA followed by PCR. The experiments were carried out in triplicate, and representative data are shown. Positive controls (PC) are rheumatoid synovial fibroblasts for ADAMTS1, ADAMTS5 and TIMP3, U-251 human glioblastoma cells for ADAMTS4, ADAMTS9 and ADAMTS15, and CaR-1 rectal carcinoma cells for ADAMTS8.10 (B) Real-time PCR analysis of the mRNA expression of ADAMTS4. Relative expression levels of ADAMTS4 in osteoarthritic chondrocytes treated without (Cont) or with IL1α and HA species were normalised to an endogenous control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Bars are mean (SD) (n = 5). *p<0.05; **p<0.01; ***p<0.001.
Real-time PCR showed that the expression level of ADAMTS4 was enhanced 17-fold by IL1α (p<0.001), and the IL1α-induced expression was decreased to 70% and 50% by treatment with HA800 (p<0.05) and HA2700 (p<0.01), respectively, but not with HAoligo or HA300 (fig 1B). No changes in ADAMTS1 expression level were observed in chondrocytes treated or not with IL1α and/or HA species, and negligible expression of ADAMTS8 was detected (supplementary material 6). Although the concentrations of ADAMTS5 and ADAMTS9 in the control and IL1α-treated samples did not differ, they were significantly decreased by treatment with HA2700 (p<0.05) (supplementary material 6). The level of ADAMTS15 expression was significantly decreased after treatment with IL1α (p<0.01), but no effects of HA species on the expression were seen (supplementary material 6).
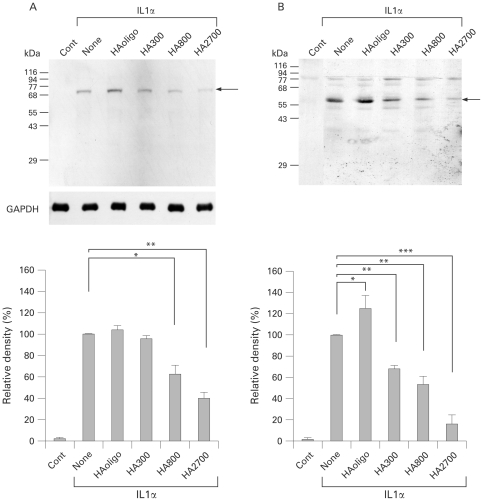
Effects of HA on protein expression of ADAMTS4
On immunoblotting, cell lysates from IL1α-stimulated chondrocytes but not control untreated chondrocytes showed a band of 73 kDa, whereas a major band of 58 kDa was detected in culture medium from IL1α-treated chondrocytes but not control chondrocytes (fig 2A,B). These two ADAMTS4 species were recognised by both antibodies (247-3F6 and 250-4F7). When the IL1α-stimulated chondrocytes were treated with HA species, the bands of ADAMTS4 appeared to decrease in an HA molecular mass-dependent manner (fig 2A,B). Densitometric analysis showed that the 73 kDa band was significantly decreased by treatment with HA800 (p<0.05) and HA2700 (p<0.01), but not with HAoligo or HA300 (fig 2A). The intensity of the 58 kDa band was also significantly decreased with HA300 (p<0.01), HA800 (p<0.01) and HA2700 (p<0.001), although it was significantly increased with HAoligo (p<0.05) (fig 2B).
Figure 2.
Effects of hyaluron (HA) species on ADAMTS4 protein expression in osteoarthritic chondrocytes. Chondrocytes were cultured in the absence (Cont) or presence of interleukin (IL)1α (1 ng/ml) and HA (250 μg/ml for HAoligo and 2.5 mg/ml for other HA species) for 72 h, and then ADAMTS4 protein expression in cell lysates (A) and culture medium (B) was analysed by immunoblotting with ADAMTS4 antibody (0.5 μg/ml; 250-4F7). Arrows indicate ADAMTS4 of molecular mass 73 kDa and 58 kDa. The protein bands were densitometrically analysed and compared. Note that treatment with HA800 and HA2700 significantly decreased the level of expression of ADAMTS4 protein in both cell lysates and culture media. Bars are mean (SD) (n = 5). *p<0.05; **p<0.01; ***p<0.001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
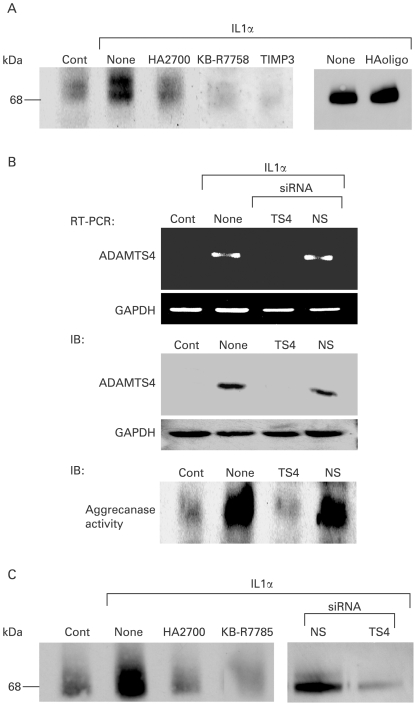
Inhibition of aggrecanase activity with HA2700
Aggrecanase activity was monitored by immunoblotting using aggrecan neoepitope-specific antibody. As shown in fig 3A, non-stimulated chondrocytes showed an immunoreactive band of ∼70 kDa, and the intensity of the band increased after treatment with IL1α. Importantly, the band intensity in the IL1α-treated chondrocytes was reduced to basal level by treatment with HA2700, and almost disappeared on treatment with KB-R7785 or TIMP3, whereas no such inhibition was observed with HAoligo (fig 3A). Transfection of IL1α-stimulated chondrocytes with ADAMTS4 siRNA abrogated the mRNA and protein expression of ADAMTS4, whereas no changes in expression were obtained with non-silencing siRNA (fig 3B). Accordingly, aggrecanase activity observed after IL1α treatment decreased to basal level by transfection of ADAMTS4 siRNA but not non-silencing siRNA (fig 3B). Similar effects of HA2700 and ADAMTS4 siRNA on aggrecanase activity were obtained in osteoarthritic cartilage tissue slices: IL1α-induced aggrecanase activity was reduced to basal level by treatment with HA2700, KB-R7785 or siRNA (fig 3C).
Figure 3.
Inhibition of aggrecanase activity by treatment of osteoarthritic chondrocytes and cartilage tissue with hyaluron (HA)2700. (A) Inhibition of interleukin (IL)1α-stimulated aggrecanase activity in chondrocytes treated with HA2700. Osteoarthritic chondrocytes were stimulated with IL1α (1 ng/ml) and treated with HA2700 (2.5 mg/ml), KB-RR7785 (1 μM), tissue inhibitor of metalloproteinases 3 (TIMP3) (100 nM), HAoligo (250 μg/ml) or buffer alone (None) in the presence of porcine aggrecan (100 μg/ml). Culture media were subjected to immunoblotting using aggrecan neoepitope-specific antibody (2 μg/ml). (B) Inhibition of IL1α-stimulated expression of ADAMTS4 and aggrecanase activity with small interfering RNA (siRNA) for ADAMTS4. Chondrocytes were treated with siRNA for ADAMTS4 (TS4) or non-silencing siRNA (NS), and expression of ADAMTS4 mRNA and protein was determined by RT-PCR and immunoblotting (IB) with ADAMTS4 antibody (0.5 μg/ml; 250-4F7). Inhibition of aggrecanase activity was evaluated by immunoblotting with aggrecan neoepitope antibody (2 μg/ml). (C) Inhibition of IL1α-stimulated aggrecanase activity in osteoarthritic cartilage tissue treated with HA2700. Cartilage tissue slices were treated for 5 days with buffer alone (None), HA2700 (2.5 mg/ml), KB-R7785 (1 μM), non-silencing siRNA (NS) or siRNA for ADAMTS4 (TS4) in the presence of IL1α (1 ng/ml), and then aggrecanase activity was detected by immunoblotting with aggrecan neoepitope antibody. Cont, cartilage tissue without IL1α treatment; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
When the direct action of HA2700 on ADAMTS4 was examined, neither inhibition of ADAMTS4 activity nor binding of ADAMTS4 species to HA2700 was observed (supplementary material 7).
Involvement of CD44 and ICAM1 in inhibitory effect of HA2700 on ADAMTS4 expression
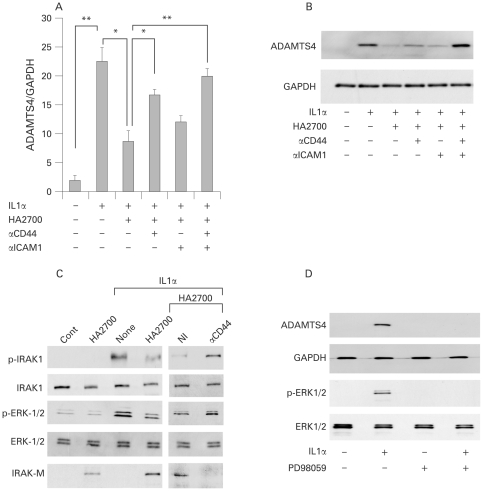
The inhibitory effect of HA2700 on ADAMTS4 expression was partially attenuated by treatment with antibody to CD44 (p<0.05) or ICAM1, and almost completely with both antibodies (p<0.01) (fig 4A), although the antibody showed no effects on IL1α-induced ADAMTS4 expression (data not shown). The effect of the antibody on HA2700-induced inhibition of ADAMTS4 expression was confirmed by immunoblotting of cell lysates (fig 4B).
Figure 4.
Analysis of the involvement of the CD44 and intracellular adhesion molecule 1 (ICAM1) signalling pathways in the HA2700 inhibitory effect on ADAMTS4 expression. (A,B) Effect of CD44 antibody (αCD44) and ICAM1 antibody (αICAM1) on the inhibition of ADAMTS4 expression with HA2700. Osteoarthritic chondrocytes were allowed to react for 1 h with buffer alone, non-immune IgG (20 μg/ml; data not shown), αCD44 (20 μg/ml), αICAM1 (20 μg/ml) or αCD44 (20 μg/ml) + αICAM1 (20 μg/ml), treated with 2.5 mg/ml HA2700 for 24 h, and then cultured for 24 h for the real-time PCR analysis of ADAMTS4 gene expression (A) and for 48 h for immunoblotting analysis of ADAMTS4 protein (B) after the addition of IL1α (1 ng/ml) to the culture medium. Bars are mean (SD) (n = 3). *p<0.05; **p<0.01. (C) Analysis of signalling pathways of IL1 and CD44 under treatment with HA2700. Expression of IL1 receptor-associated kinase-1 (IRAK1), extracellular signal-regulated protein kinase1/2 (ERK1/2) and IRAK-M and phosphorylation of IRAK1 and ERK1/2 were examined by immunoblotting of osteoarthritic chondrocytes. The cells were cultured for 24 h in the absence (Cont and None) or presence (2.5 mg/ml) of HA2700, and then stimulated for 30 min without or with IL1α (1 ng/ml), followed by immunoblotting for p-IRAK1, IRAK1, p-ERK1/2, ERK1/2 and IRAK-M. The effect of αCD44 on HA2700-induced signalling was examined as described above. (D) Involvement of mitogen-activated protein kinase kinase (MEK) in ADAMTS4 expression and phosphorylation of ERK1/2. Chondrocytes were treated for 30 min without or with PD98059 (50 μM). Then, the cells were cultured in the absence or presence of IL1α (1 ng/ml) for 48 h for immunoblotting of ADAMTS4 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or for 30 min for immunoblotting of p-ERK1/2 and ERK1/2. All experiments were performed in triplicate.
To analyse the signalling pathways, we examined phosphorylation of IRAK1 and ERK1/2. The phosphorylation of both molecules was enhanced by IL1α treatment, and the IL1α-induced phosphorylation was downregulated by treatment with HA2700 (fig 4C). HA2700 treatment of IL1α-treated or untreated chondrocytes induced the expression of IRAK-M, a negative regulator of IRAK1, via CD44.40 Inhibition of IL1α-induced phosphorylation of IRAK1 and ERK1/2 was attenuated by treatment of the cells with CD44 antibody, and induction of IRAK-M by HA2700 was abolished by incubation with the antibody (fig 4C). In addition, phosphorylation of ERK1/2 and expression of ADAMTS4 were abrogated by treatment with PD98059, a MEK inhibitor (fig 4D). These results suggest that IRAK-M induced by HA2700 binding to CD44 has a key role in downregulation of the IL1α-stimulated signalling pathway, and MEK is involved in the ERK1/2 phosphorylation.
DISCUSSION
Conflicting data have been reported on the expression of aggrecan-degrading ADAMTS species: some studies have reported that ADAMTS4 is upregulated by IL1, tumour necrosis factor α, oncostatin M and transforming growth factor β,2 12 13 but others have shown that IL1 treatment does not affect ADAMTS4 expression.41 42 Our systematic analyses of ADAMTS4 expression in human osteoarthritic chondrocytes under monolayer culture have provided the first evidence that IL1α selectively induces ADAMTS4 expression, whereas ADAMTS1, ADAMTS5 and ADAMTS9 are constitutively expressed, and ADAMTS8 is negligibly expressed without being affected by IL1α. Treatment of chondrocytes with tumour necrosis factor α and oncostatin M has been reported to downregulate ADAMTS15,13 but our study showed that IL1α also functions as a suppressor of ADAMTS15 expression.
Although previous studies have shown that HA800 suppresses the production of MMP1, MMP3 and MMP13 by osteoarthritic and normal cartilage explants,20 our study is the first to show that 2.5 mg/ml of HA2700 and HA800, which is within the physiological concentration range in synovial fluids (2–4 mg/ml),43 inhibits the mRNA and protein expression of ADAMTS4 in osteoarthritic chondrocytes. The effect of HA is known to depend on its molecular mass.14 15 Indeed, our study shows significant inhibition of ADAMTS4 expression with only HA2700 and HA800, but not with HA300 or HAoligo. When the effects were compared, HA2700 was considered to be the more efficient suppressor of expression of aggrecan-degrading ADAMTS species, as inhibition of ADAMTS4 expression appeared to be greater with HA2700 than with HA800, and HA2700, but not HA800, significantly inhibited the expression of ADAMTS5 and ADAMTS9.
This study shows that IL1α-induced aggrecanase activity in cultured chondrocytes and osteoarthritic cartilage explants is suppressed by treatment with HA2700. Although TIMP3 is an efficient inhibitor of ADAMTS4,37 38 IL1α-stimulated TIMP3 expression appeared to be unchanged by HA2700 treatment. HA2700 did not directly inhibit ADAMTS4 activity or bind to ADAMTS4. siRNA for ADAMTS4 suppressed IL1α-induced aggrecanase activity in cultured chondrocytes and cartilage tissue. Therefore, these data suggest that HA2700 inhibition of aggrecanase activity may be due principally to reduced expression and production of ADAMTS4. On the other hand, our finding that treatment with synthetic inhibitor and TIMP3 appeared to reduce aggrecanase activity to below that of untreated osteoarthritic chondrocytes suggests that such strong inhibition may be due to simultaneous suppression of aggrecanase(s) such as constitutively expressed ADAMTS5.
Previous studies have shown the involvement of CD44 in the HA effects.20 21 44 No previous studies have clarified the intracellular signalling pathways. As binding of HA to CD44 on activated monocytes is known to modulate IL1-induced signalling by inducing the expression of IRAK-M, which suppresses IRAK1,40 we examined the expression of these signalling molecules. We found that HA2700 treatment reduces IL1α-stimulated phosphorylation of IRAK1 and ERK1/2 through induction of IRAK-M. These data suggest that HA2700, at least in part, inhibits the IL1 receptor signalling pathway of IRAK1 and ERK1/2 by the action of IRAK-M (fig 5). However, as the recovery with CD44 antibody was partial, other pathways of HA receptors, including ICAM145 and Toll-like receptor (TRL)2 and 4,46 appear to be involved. In fact, the finding that treatment with both CD44 antibody and ICAM1 antibody attenuated the inhibition suggests the involvement of both CD44 and ICAM1 pathways (fig 5). It is, however, unlikely that TRL2 and TRL4 act as receptors for HA in osteoarthritic chondrocytes, as neither the expression of these molecules nor any effect of their antibodies on the recovery was observed in the chondrocytes (data not shown).
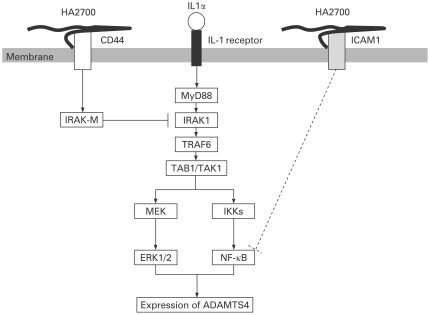
Figure 5.
Hypothesis of the signalling pathways for HA2700 inhibition of ADAMTS4 expression in IL1α-stimulated osteoarthritic chondrocytes. The IL1α signal via IL1 receptor is transduced by IRAK1 and may enhance ADAMTS4 expression through the MAP kinase and NF-κB pathways.47 Binding of HA2700 to CD44 induces IRAK-M, which inhibits IRAK1 and subsequently suppresses phosphorylation of ERK1/2 and finally ADAMTS4 expression. HA2700 also binds to ICAM1 and this binding may inhibit NF-κB48 and subsequently suppress ADAMTS4 expression.
Intra-articular administration of HA2700 and HA800 is widely used as a symptom-modifying treatment for knee OA,14 15 and growing experimental and clinical evidence suggests that HA2700 and HA800 may also modify the structure of diseased joints and slow the rate of OA disease progression. The potential disease-modifying effect seems to be explained by positive regulation of joint repair through stimulation of chondrocyte growth metabolism and synthesis of cartilage matrix and suppression of inflammatory processes.14 However, HA800 has been reported to inhibit the production of MMP1, MMP3 and MMP13 in normal and osteoarthritic cartilage treated with IL1β,20 and the present study shows suppression of ADAMTS4 expression by HA2700 and HA800. Thus, we hypothesise that HA2700 and HA800 protect the cartilage from damage in OA by inhibiting the expression of chondrodegradative metalloproteinases, ie, ADAMTS4 and MMPs. Clinical trials of the disease-modifying effect of HA2700 on OA are necessary.
Acknowledgments
We thank Dr Edward D Harris, Jr, (Stanford University School of Medicine) for reviewing the manuscript. We are also grateful to Ms Mayumi Kishi for her technical assistance.
Footnotes
Funding: Grant-in-Aid from the Ministry of Education, Science and Culture of Japan (18060038 and 19109004) to YO.
Competing interests: None.
Ethics approval: Obtained.
Patient consent: Obtained.
REFERENCES
- 1.Dahlberg L, Billinghurst RC, Manner P, Nelson F, Webb G, Ionescu M, et al. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1). Arthritis Rheum 2000;43:673–82 [DOI] [PubMed] [Google Scholar]
- 2.Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage 2001;9:539–52 [DOI] [PubMed] [Google Scholar]
- 3.Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem 2003;278:45539–45 [DOI] [PubMed] [Google Scholar]
- 4.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J 2005;386:15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, Lohmander LS. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis Cartilage 2006;14:101–13 [DOI] [PubMed] [Google Scholar]
- 6.Okada Y. Proteinases and matrix degradation. In: JHarris ED, Budd RC, Genovese MC, Firestein GS, Sargent JS, eds. Kelley’s textbook of rheumatology. 8th edn.Philadelphia: Elsevier Saunders, 2008 [Google Scholar]
- 7.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005;434:644–8 [DOI] [PubMed] [Google Scholar]
- 8.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 2005;434:648–52 [DOI] [PubMed] [Google Scholar]
- 9.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum 2007;56:575–85 [DOI] [PubMed] [Google Scholar]
- 10.Naito S, Shiomi T, Okada A, Kimura T, Chijiiwa M, Fujita Y, et al. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathol Int 2007;57:703–11 [DOI] [PubMed] [Google Scholar]
- 11.Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum 2002;46:2648–57 [DOI] [PubMed] [Google Scholar]
- 12.Moulharat N, Lesur C, Thomas M, Rolland-Valognes G, Pastoureau P, Anract P, et al. Effects of transforming growth factor-beta on aggrecanase production and proteoglycan degradation by human chondrocytes in vitro. Osteoarthritis Cartilage 2004;12:296–305 [DOI] [PubMed] [Google Scholar]
- 13.Hui W, Barksby E, Young DA, Cawston TE, McKie N, Rowan AD. Oncostatin M in combination with tumour necrosis factor alpha induces a chondrocyte membrane-associated aggrecanase that is distinct from ADAMTS aggrecanase-1 or -2. Ann Rheum Dis 2005;64:1624–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage 2005;13:216–24 [DOI] [PubMed] [Google Scholar]
- 15.Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther 2003;5:54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshioka M, Shimizu C, Harwood FL, Coutts RD, Amiel D. The effects of hyaluronan during the development of osteoarthritis. Osteoarthritis Cartilage 1997;5:251–60 [DOI] [PubMed] [Google Scholar]
- 17.Shimizu C, Yoshioka M, Coutts RD, Harwood FL, Kubo T, Hirasawa Y, et al. Long-term effects of hyaluronan on experimental osteoarthritis in the rabbit knee. Osteoarthritis Cartilage 1998;6:1–9 [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K, Amiel M, Harwood FL, Healey RM, Sonoda M, Moriya H, et al. The long-term effects of hyaluronan during development of osteoarthritis following partial meniscectomy in a rabbit model. Osteoarthritis Cartilage 2000;8:359–65 [DOI] [PubMed] [Google Scholar]
- 19.Shimazu A, Jikko A, Iwamoto M, Koike T, Yan W, Okada Y, et al. Effects of hyaluronic acid on the release of proteoglycan from the cell matrix in rabbit chondrocyte cultures in the presence and absence of cytokines. Arthritis Rheum 1993;36:247–53 [DOI] [PubMed] [Google Scholar]
- 20.Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T. Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum 2004;50:516–25 [DOI] [PubMed] [Google Scholar]
- 21.Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage 2006;14:1237–47 [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Goomer RS, Harwood F, Kubo T, Hirasawa Y, Amiel D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta (IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthritis Cartilage 1999;7:182–90 [DOI] [PubMed] [Google Scholar]
- 23.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res 2004;427:S27–36 [DOI] [PubMed] [Google Scholar]
- 24.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–49 [DOI] [PubMed] [Google Scholar]
- 25.Fujita Y, Shiomi T, Yanagimoto S, Matsumoto H, Toyama Y, Okada Y. Tetraspanin CD151 is expressed in osteoarthritic cartilage and is involved in pericellular activation of pro-matrix metalloproteinase 7 in osteoarthritic chondrocytes. Arthritis Rheum 2006;54:3233–43 [DOI] [PubMed] [Google Scholar]
- 26.Enomoto H, Inoki I, Komiya K, Shiomi T, Ikeda E, Obata K, et al. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am J Pathol 2003;162:171–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yomoto C. Evaluation of molecualr weights of hyaluronate preparations by multi-angle laser light scattering. Bull Natl Inst Health Sci 2003;121:30–3 [PubMed] [Google Scholar]
- 28.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFkappaB and p38 MAP kinase in articular chondrocytes. J Biol Chem 2006;281:17952–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akagi M, Kanata S, Mori S, Itabe H, Sawamura T, Hamanishi C. Possible involvement of the oxidized low-density lipoprotein/lectin-like oxidized low-density lipoprotein receptor-1 system in pathogenesis and progression of human osteoarthritis. Osteoarthritis Cartilage 2007;15:281–90 [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama T, Nakamura H, Otani Y, Kubota T, Fujimoto N, Seiki M, et al. Differences between scirrhous and non-scirrhous human gastric carcinomas from the aspect of proMMP-2 activation regulated by TIMP-3. Clin Exp Metastasis 2004;21:223–33 [DOI] [PubMed] [Google Scholar]
- 31.Konttinen YT, Ainola M, Valleala H, Ma J, Ida H, Mandelin J, et al. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis 1999;58:691–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsui Y, Mochizuki S, Kodama T, Shimoda M, Ohtsuka T, Shiomi T, et al. ADAM28 is overexpressed in human breast carcinomas: implications for carcinoma cell proliferation through cleavage of insulin-like growth factor binding protein-3. Cancer Res 2006;66:9913–20 [DOI] [PubMed] [Google Scholar]
- 33.Takizawa M, Ohuchi E, Yamanaka H, Nakamura H, Ikeda E, Ghosh P, et al. Production of tissue inhibitor of metalloproteinases 3 is selectively enhanced by calcium pentosan polysulfate in human rheumatoid synovial fibroblasts. Arthritis Rheum 2000;43:812–20 [DOI] [PubMed] [Google Scholar]
- 34.Hardingham TE, Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J 1974;139:565–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto G, Shimoda M, Okada Y. ADAMTS4 (aggrecanase-1) interaction with the C-terminal domain of fibronectin inhibits proteolysis of aggrecan. J Biol Chem 2004;279:32483–91 [DOI] [PubMed] [Google Scholar]
- 36.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med 2002;8:35–40 [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto G, Aoki T, Nakamura H, Tanzawa K, Okada Y. Inhibition of ADAMTS4 (aggrecanase-1) by tissue inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4). FEBS Lett 2001;494:192–5 [DOI] [PubMed] [Google Scholar]
- 38.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem 2001;276:12501–4 [DOI] [PubMed] [Google Scholar]
- 39.Chockalingam PS, Zeng W, Morris EA, Flannery CR. Release of hyaluronan and hyaladherins (aggrecan G1 domain and link proteins) from articular cartilage exposed to ADAMTS-4 (aggrecanase 1) or ADAMTS-5 (aggrecanase 2). Arthritis Rheum 2004;50:2839–48 [DOI] [PubMed] [Google Scholar]
- 40.del Fresno C, Otero K, Gomez-Garcia L, Gonzalez-Leon MC, Soler-Ranger L, Fuentes-Prior P, et al. Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. J Immunol 2005;174:3032–40 [DOI] [PubMed] [Google Scholar]
- 41.Koshy PJ, Lundy CJ, Rowan AD, Porter S, Edwards DR, Hogan A, et al. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum 2002;46:961–7 [DOI] [PubMed] [Google Scholar]
- 42.Yamanishi Y, Boyle DL, Clark M, Maki RA, Tortorella MD, Arner EC, et al. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J Immunol 2002;168:1405–12 [DOI] [PubMed] [Google Scholar]
- 43.Dahl LB, Dahl IM, Engstrom-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis 1985;44:817–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brun P, Panfilo S, Daga Gordini D, Cortivo R, Abatangelo G. The effect of hyaluronan on CD44-mediated survival of normal and hydroxyl radical-damaged chondrocytes. Osteoarthritis Cartilage 2003;11:208–16 [DOI] [PubMed] [Google Scholar]
- 45.Lisignoli G, Grassi F, Zini N, Toneguzzi S, Piacentini A, Guidolin D, et al. Anti-Fas-induced apoptosis in chondrocytes reduced by hyaluronan: evidence for CD44 and CD54 (intercellular adhesion molecule 1) invovement. Arthritis Rheum 2001;44:1800–7 [DOI] [PubMed] [Google Scholar]
- 46.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 2005;11:1173–9 [DOI] [PubMed] [Google Scholar]
- 47.Gottipati S, Rao NL, Fung-Leung WP. IRAK1: a critical signaling mediator of innate immunity. Cell Signal 2008;20:269–76 [DOI] [PubMed] [Google Scholar]
- 48.Yasuda T. Hyaluronan inhibits cytokine production by lipopolysaccharide-stimulated U937 macrophages through down-regulation of NF-kappaB via ICAM-1. Inflamm Res 2007;56:246–53 [DOI] [PubMed] [Google Scholar]