Abstract
Purpose: To establish reference values of the ratios of flow velocity in the middle cerebral artery (VMCA) and the terminal portion of the internal carotid artery (VtICA) to flow velocity in the extracranial portion of internal carotid artery (VICA) in children with sickle cell disease (SCD).
Materials and Methods: Institutional ethics committee approval and parental informed consent were obtained for this prospective HIPAA-compliant study. Sixty-eight children (38 female; mean age, 7.7 years ± 3.3; range, 2–14 years) with HbSS genotype, without neurologic deficits and no history of stroke, were enrolled. Final study population comprised 56 (mean age 8.0 ± 3.3 years, 26 females) children who underwent magnetic resonance (MR) angiography, which excluded intracranial arterial narrowing, transcranial color-coded duplex ultrasonography (US), and carotid US to determine VMCA/VICA and VtICA/VICA ratios from angle-corrected and uncorrected velocities. Tolerance interval estimates were used to calculate reference ranges and linear regression was used to quantify associations of Doppler parameters with age adjusted for hemoglobin and hematocrit.
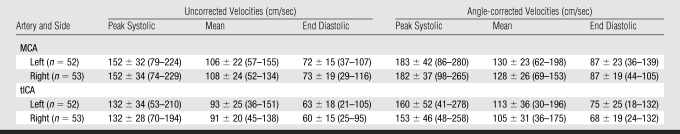
Results: Reference ranges in centimeters per second for mean angle-corrected VMCA on the left and right sides were 62–198 and 69–153; those for VtICA were 30–196 and 36–175; and those for VICA were 18–116 and 15–95, respectively. Reference ranges for mean angle-corrected VMCA/VICA ratio on the left and right sides were 1.2–4.0 and 0.4–3.4 and those for VtICA/VICA ratio were 0.5–2.9 and 0.5–2.7, respectively. VMCA, VtICA, and VtICA/VICA ratio were not age dependent, contrary to VICA and VMCA/VICA ratio, after controlling for hematocrit and hemoglobin.
Conclusion: The study provides reference limits for VMCA, VtICA, VICA, and velocity ratios obtained from children with SCD.
© RSNA, 2009
Children with sickle cell disease (SCD) who develop intracranial arterial narrowing are at high risk of stroke (1). Transcranial Doppler ultrasonography (US) is a noninvasive method extensively used to detect narrowing based on flow velocity thresholds (1,2). Chronic transfusions can substantially reduce the risk of stroke in children with high velocities at transcranial Doppler US (1), yet even 70% of patients with high velocity who do not undergo transfusions do not develop stroke (3). Chronic transfusions are costly and associated with substantial morbidity and some mortality from transfusion-related infections, alloimmunization, and hemosiderosis (4,5). Children with high hyperemic velocity due to anemia may not have the same benefits from the transfusion therapy as those with arterial narrowing. Transcranial Doppler US screening should be improved since currently more patients may be exposed to substantial risk than the number that benefit from transfusion.
False-positive findings at transcranial Doppler US can be a result from poor reliability of velocity measurements and influence of many biologic factors on flow velocities (6–10). Standardization of transcranial Doppler US procedures among centers is problematic because efforts to reproduce recommended velocity values have shown differences (2,11,12). Using transcranial color-coded duplex US, which is more reliable in velocity measurements than “blind” transcranial Doppler US, we found substantial variability in velocities (range, > 100 cm/sec) in children with SCD without any arterial narrowing and neurologic deficits (12). This indicates that biologic factors must have greater influence on velocity than previously assumed (7,9,13–16).
Transcranial Doppler US screening uses the premise that unilateral velocity elevation indicates narrowing, whereas bilateral velocity increase represents bilateral narrowing, hyperemia, or both (1). Large bilateral differences in flow velocities in children without narrowing suggest that unilateral velocity increase is also possible in hyperemia (12). Children without narrowing but with high velocity are currently labeled as at high risk of stroke by using transcranial Doppler US criteria (1,3).
Middle cerebral artery (MCA) spasm can be accurately detected by using the ratio of intracranial velocities to corresponding velocities in the ipsilateral internal carotid artery (ICA) (17). Velocity ratios can also be used to discriminate narrowing from hyperemia. Thus, the purpose of our study was to prospectively establish tolerance intervals of the ratios of flow velocity in the MCA (VMCA) and the terminal portion of the ICA (VtICA) to flow velocity in the extracranial portion of ICA (VICA) in children with SCD.
MATERIALS AND METHODS
Study Group
Institutional ethics committee approval was obtained for this prospective cross-sectional study compliant with Health Insurance Portability and Accountability Act, and informed parental consent (with participant's assent for those 7 years and older) was obtained. The study group consisted of 68 children (mean age, 7.7 years ± 3.3 [standard deviation]; range, 2–14 years). There were 38 girls (mean age, 7.1 years ± 3.3; range, 2–13 years) and 30 boys (mean age, 8.2 years ± 3.1; range, 3–14 years). The names were selected from the Comprehensive Sickle Cell Center at The Children's Hospital of Philadelphia by using the following inclusion criteria: (a) homozygous for hemoglobin S (HbSS), confirmed by means of isoelectric focusing with DNA-based confirmatory testing or parental studies; (b) ages 2–14 years; (c) no neurologic deficits; and (d) no history of stroke. Exclusion criteria were (a) history of major head injury requiring visit to an emergency department; (b) history of seizure disorder requiring anticonvulsant therapy; (c) chronic transfusion therapy; (d) episode of SCD pain, acute chest syndrome, or other important medical problem in the period between laboratory blood testing and US; (e) history of prenatal or perinatal hypoxic-ischemic brain injury; and (f) evidence of human immunodeficiency virus infection.
Measurements of hematocrit value and hemoglobin concentration were obtained from a well-visit closest in time to the US study (mean, 21 days ± 16; range, 0–62 days). For eight children without recent laboratory values, we used the average hemoglobin and hematocrit values from the last three visits (mean, 140 days ± 70; range, 73–386 days).
Study Procedures
A pediatric neurologist assessed each child, including language, cranial nerves, sensorimotor ability, and coordination. Children without abnormalities were scheduled for transcranial color-coded duplex US, carotid US, and MR angiography. MR angiographic studies were nondiagnostic in three children because of motion artifacts. Six children examined with US did not show up for MR angiography. No child was excluded on the basis of neurologic examination findings.
Transcranial Color-coded Duplex US and Carotid US
Transcranial color-coded duplex US and carotid US examinations (HDI 5000; Philips, Eindhoven, Netherlands), with 1.8–3.6-MHz probe for transcranial Doppler US and linear-array 11-MHz probe for carotid US, were performed by one of three sonographers with more than 3 years of experience. Children were not permitted to sleep during the examinations and were not sedated. The tICA and M-1 segment of the MCA were identified by means of temporal acoustic windows by using published standards (18). To determine the angle of insonation, a sample volume adjusted to the size of the insonated artery and a linear marker provided by the scanner software were placed with visual guidance on the color image of the artery being insonated, on the point of the highest velocity acceleration as determined by the color aliasing artifact, if present. The direction of the linear marker was fitted to the long axis of the vessel (Fig 11c). This allowed the angle-corrected flow velocities to be measured. The angle was recorded in each artery. The mean time-averaged maximum flow velocity, peak systolic velocity, and end-diastolic velocity were calculated by automatic tracing or, in case of a weak signal, manual tracing of the Doppler waveform. The tracing was performed by the sonographers during the examination. Since many institutions use uncorrected flow velocities from “blind” or transcranial color-coded duplex US to estimate the risk of stroke, we also calculated uncorrected velocities for each artery as a product of angle-corrected velocities and the cosine of the recorded angle based on the Doppler equation.
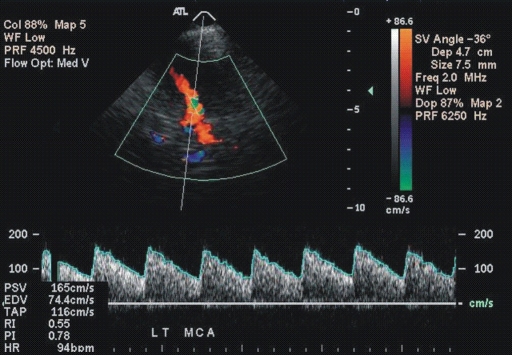
Figure 1a:
(a, b) Transcranial color-coded duplex US scans with typical velocity waveform in a patient with SCD depict (a) left and (b) right MCA in red. (c) Carotid US scan of the left ICA (red). Note that sample volumes are placed on sites of the highest velocity acceleration indicated by aliasing artifacts (green). The angle between the course of an artery and the ultrasound beam indicated by dashed lines is more than 20° (left MCA, 36°; right MCA, 28°), as assumed in the conventional transcranial Doppler US.
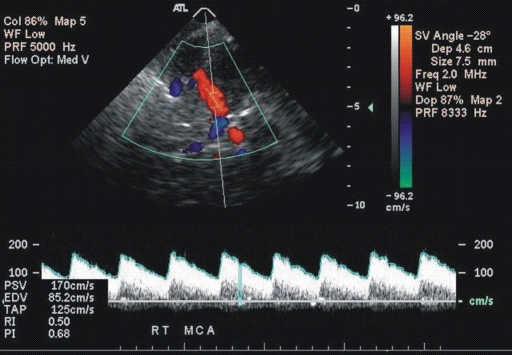
Figure 1b:
(a, b) Transcranial color-coded duplex US scans with typical velocity waveform in a patient with SCD depict (a) left and (b) right MCA in red. (c) Carotid US scan of the left ICA (red). Note that sample volumes are placed on sites of the highest velocity acceleration indicated by aliasing artifacts (green). The angle between the course of an artery and the ultrasound beam indicated by dashed lines is more than 20° (left MCA, 36°; right MCA, 28°), as assumed in the conventional transcranial Doppler US.
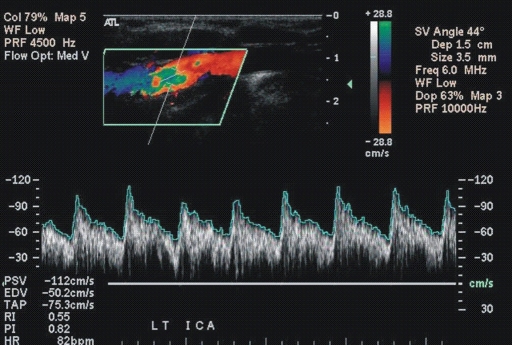
Figure 1c:
(a, b) Transcranial color-coded duplex US scans with typical velocity waveform in a patient with SCD depict (a) left and (b) right MCA in red. (c) Carotid US scan of the left ICA (red). Note that sample volumes are placed on sites of the highest velocity acceleration indicated by aliasing artifacts (green). The angle between the course of an artery and the ultrasound beam indicated by dashed lines is more than 20° (left MCA, 36°; right MCA, 28°), as assumed in the conventional transcranial Doppler US.
After completion of the transcranial color-coded duplex US examination, ICAs were examined by using carotid US (19). Sample volume adjustment for the diameter was placed 15–20 mm distal to the carotid bifurcation, and angle-corrected velocities were obtained. In cases with two systolic peaks, we measured the highest velocity. Both US studies in each child were performed by the same sonographer during a single session. Sonographers were blinded to the results of MR angiography. The VMCA/VICA and VtICA/VICA ratios were calculated by referencing MCA and tICA velocities to corresponding velocities in the ipsilateral ICA.
Velocity data from left MCA and ICA in one patient were mistakenly deleted from the US unit. Thus, the numbers of velocity measurements obtained from individual arteries were as follows: 55 from left MCA and tICA; 56 from left ICA; and 56 from right MCA, tICA, and ICA. The following angle-corrected measurements were considered as outliers: end-diastolic velocity of 16 cm/sec for right MCA, 139 cm/sec for right tICA, and 100 cm/sec for right ICA; measurements from one left ICA were a peak systolic velocity of 246 cm/sec, end-diastolic velocity of 120 cm/sec, and mean time-averaged maximum flow velocity of 136 cm/sec. An artery with an outlier was removed from further analysis. Consequently, we calculated velocity ratios from the 53 arteries on the right side and from 52 arteries on the left side.
MR Angiography
To exclude children with arterial narrowing, we performed time-of-flight three-dimensional gradient-echo imaging (repetition time msec/echo time msec, 28/3.28; flip angle, 25°; matrix, 512 × 448) using a 3-T imager (Siemens Trio, Erlangen, Germany) covering the extracranial and intracranial arteries in the axial plane. Raw data from time-of-flight MR angiography were transferred to an online workstation for the generation of segmented two-dimensional arterial reprojections by using a commercially available maximum intensity projection ray trace and multiplanar reconstruction algorithms. The segmented two-dimensional reprojections and raw data of the ICAs and arteries of the circle of Willis were displayed on a 1024 × 1024-pixel workstation and were evaluated independently by two pediatric neuroradiologists (E.R.M., with 15 and another one with 25 years of experience in angiography) unaware of the US findings for potential segmental narrowing and flow restriction. Each evaluated all studies, and discrepancies were resolved by consensus.
Statistical Analysis
We used statistical software (SYSTAT 10; SPSS Science, Chicago, Ill) and free software (GraphPad software, La Jolla, Calif; http://www.graphpad.com). Doppler data were treated for outliers by using Grubbs T statistic at α level of less than .05 (20). The velocity ratios were calculated for angle-corrected and uncorrected velocities. Outliers were not considered while we were checking for normality using the Lilliefors test (21) provided by the SYSTAT. Since data were normally distributed, the values of flow velocities and velocity ratios from both hemispheres and corrected versus uncorrected values were compared by using the paired two-sided t test, which also was used to compare the mean values in the angle between left- and right-sided MCAs and tICAs. Pearson correlation coefficient (r) was used to quantify bilateral relationship between the Doppler parameters. Bartlett χ2 test provided by SYSTAT was used to determine significant correlations. Nonpaired two-sided t test was used to compare velocities between sex.
To determine the normal ranges of Doppler parameters, we used estimates of the Gaussian tolerance interval, which has 0.90 probability of containing 95% of the population (22). If L1 and L2 are the lower and upper limits of the interval, then L1 = μ − ks and L2 = μ + ks, where the k values are those obtained from an article by Weissberg-Beatty (23), μ is the mean and s is the standard deviation. Multivariable linear regression analysis was used to quantify associations of Doppler parameters with hemoglobin and hematocrit after adjustment for age. Left and right parameters were treated separately. A P value of less than .05 was considered to indicate a significant difference.
To better illustrate associations between age and velocities in healthy children and those with SCD, we estimated the relationship in healthy children on the basis of published data (24,25). General trends of Doppler parameters with age were illustrated by line-fitting linear regression equation (y = a + bx). Curves showing the relationship of Doppler parameters, hematocrit, and hemoglobin with age in children with SCD were created by using distance-weighed, least-squares smoothing method provided by SYSTAT, with tension set at 0.5.
RESULTS
MR angiography
MR angiography depicted narrowing in the left ICA and ACA in a 6-year-old girl, in the left MCA in an 8-year-old boy, and in the right ACA in a 12-year-old girl. These patients were excluded from further analyses, which included 56 children (mean age, 8.0 years ± 3.3 [97.4 months ± 38]; median age, 9 years [108 months]. No extracranial arterial narrowing was identified.
Angle-corrected and Uncorrected Blood Flow Velocities
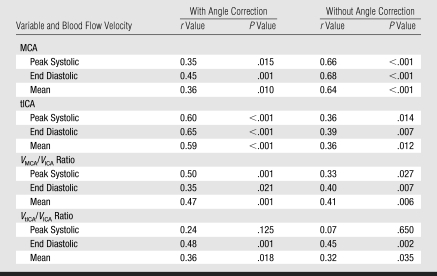
The angle-corrected velocity values were higher than respective uncorrected values in all examined arteries (Tables 1–3). Angle of insonation for the right MCA (29° ± 15) and tICA (19° ± 22) was not significantly different from the angle for the left MCA (31° ± 13) and tICA (21° ± 23). There were no statistically significant sex differences in flow velocities in any artery. Similarly, no significance side difference was noted except for the higher angle-corrected end-diastolic velocities in the left tICA (75 cm/sec ± 25) compared with the right tICA (68 cm/sec ± 19, P = .017). Such difference was absent in the proximal ICA, where mean values and their standard deviations of peak systolic velocity, time-averaged maximum flow velocity, and end-diastolic velocity on the left side were 113 cm/sec ± 34 (tolerance interval, 37–190), 67 cm/sec ± 22 (tolerance interval, 18–116), and 47 cm/sec ± 17 (tolerance interval, 8–86), respectively, while respective velocities on the right side were 121 cm/sec ± 38 (tolerance interval, 35–207), 70 cm/sec ± 24 (tolerance interval, 15–95), and 47 cm/sec ± 15 (tolerance interval, 13–62).
Table 1.
Blood Flow Velocities in MCA and tICA Obtained with Imaging Transcranial Doppler US with and without Correction for the Angle of Insonation in Children with SCD
Note.—Data are means ± standard deviation. Data in parentheses are tolerance intervals. n = Number of examined arteries.
Table 2.
Pearson Correlations Coefficients for US Doppler Parameters from Right-sided Arteries with Corresponding Parameters from Left-sided Arteries in Children with SCD
Table 3.
Associations of Peak Systolic, Mean, and End-diastolic Velocities in Proximal ICA with Age-determined Linear Regression Analysis after Adjustment for Hemoglobin and Hematocrit in Children with SCD
Note.—β = partial regression coefficient, F = F Snedecor ratio.
Correlation coefficients between velocities of left- versus right-sided intracranial arteries were higher for angle-corrected than for uncorrected values (Table 2). Between-sides coefficients for ICA velocities were highest for time-averaged maximum flow velocity (0.56, P < .001), moderate for end-diastolic velocity (0.45, P = .001), and lowest for peak systolic velocity (0.35, P = .015).
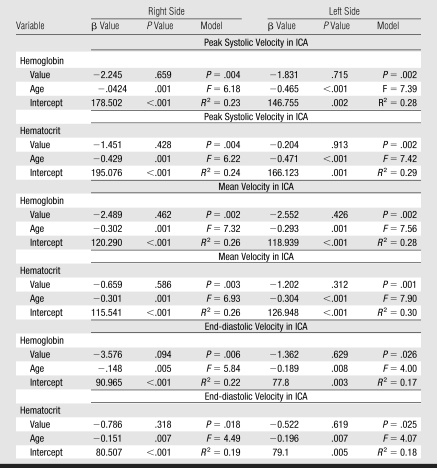
Multivariable regression analysis showed significant age dependence of flow velocity in the ICA after controlling for either hemoglobin or hematocrit (Table 3). Intracranial flow velocities on both sides were independent of age (Fig 2). Comparison of linear relationships between age and flow velocity in the MCA and ICA in our group with the published relationships (24,25) for healthy children showed that in healthy children MCA velocity is lower than in those who are older, while the age-velocity dependency line for the ICA is less steep than it is in our group (Fig 3).
Figure 2a:
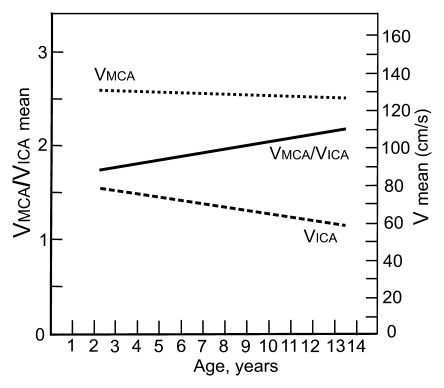
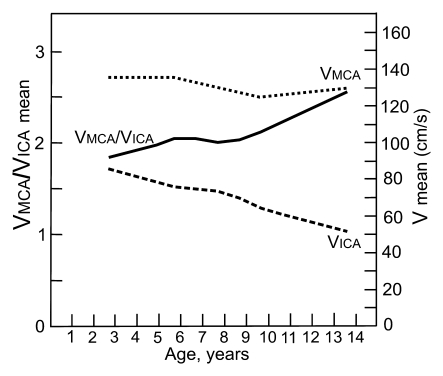
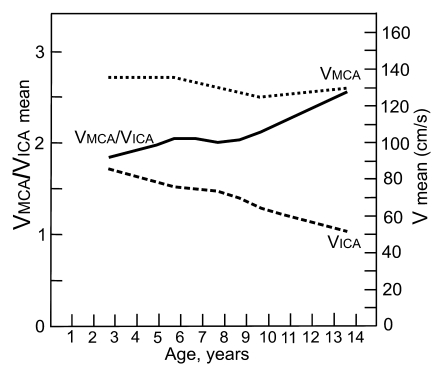
Graphs depict lines that show the associations of mean blood flow velocities (V) and VMCA/VICA ratio with age in (a) children with SCD and (b) healthy children. Association of Doppler parameters with age in healthy children was estimated on the basis of two published studies (24,25).
Figure 2b:
Graphs depict lines that show the associations of mean blood flow velocities (V) and VMCA/VICA ratio with age in (a) children with SCD and (b) healthy children. Association of Doppler parameters with age in healthy children was estimated on the basis of two published studies (24,25).
Figure 3a:
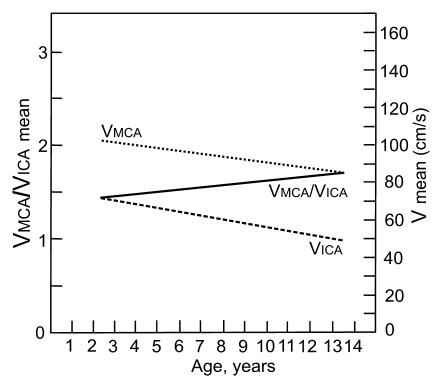
Graphs depict the curves of age dependency of the mean blood flow velocity in MCA and ICA and VMCA/VICA ratio on the (a) right and (b) left side in children with SCD. Lines were obtained from distance-weighed, least-squares smoothing of individual corresponding values.
Figure 3b:
Graphs depict the curves of age dependency of the mean blood flow velocity in MCA and ICA and VMCA/VICA ratio on the (a) right and (b) left side in children with SCD. Lines were obtained from distance-weighed, least-squares smoothing of individual corresponding values.
VMCA/VICA and VtICA/VICA Ratios
There were no significant sex and side differences in velocity ratios. Correlation coefficients between left and right sides for ratios were in general higher for angle-corrected than for uncorrected flow velocities, yet for uncorrected MCA velocities they were higher than for angle-corrected ones (Table 2).
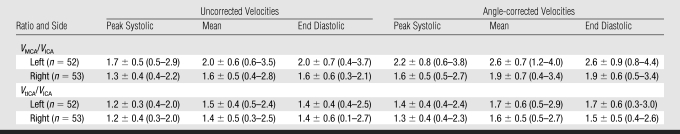
Velocity ratios calculated by using angle-corrected velocities were higher (13%–21%) than the ratios calculated on the basis of uncorrected velocities (Table 4). The tolerance intervals for ratios calculated from angle-corrected velocities were in general wider than the intervals for uncorrected velocity ratios (Table 4).
Table 4.
Mean Values and Gaussian Tolerance Intervals of Ratios of Peak Systolic, Mean, and End-diastolic Velocities in MCA and tICA to Respective Velocities in the Extracranial ICA at Transcranial Doppler US with and without Correction for the Angle of Insonation in Children with SCD
Note.—Data are means ± standard deviation. Data in parentheses are tolerance intervals. n = Number of children included in statistical analysis.
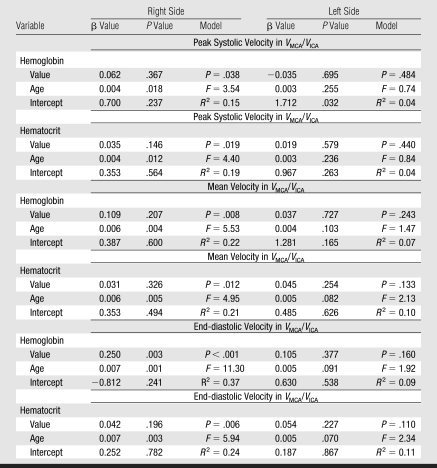
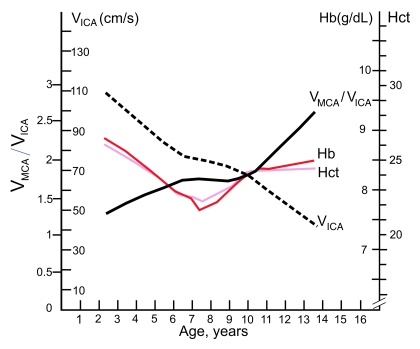
The mean VMCA/VICA ratio was lowest in children younger than 6 years of age and highest in children older than 10 years of age (Fig 2). The VtICA/VICA ratio showed similar trend, but the age-dependent changes were less prominent. Multivariable regression analysis showed significant association of the VMCA/VICA ratio with age after controlling for hemoglobin or hematocrit on the right side, whereas no such associations were found on the left side and between the VtICA/VICA ratio and age (Table 5). A linear relationship of the VMCA/VICA ratio with age in our group was compared with the respective relationship in healthy children (Fig 3). Although age-dependency ratios were higher in older children with SCD, linear regression showed that neither flow velocities nor velocity ratios are associated with hemoglobin or hematocrit values (Tables 3, 5). Relationships between ICA velocity, VMCA/VICA ratio, hemoglobin, and hematocrit with age are, however, more complex and nonlinear (Fig 4). To the age of about 8–10 years, age-dependency curve for VICA is almost parallel with the age-dependency curve for hematocrit and hemoglobin, while age-dependency for VMCA/VICA ratio shows an opposite trend (Fig 4).
Table 5.
Associations of VMCA/VICA Ratio with Age-determined Linear Regression Analysis after Adjustment for Hemoglobin and Hematocrit in Children with SCD
Note.—β = Partial regression coefficient, F = F Snedecor ratio.
Figure 4:
Graph depicts age-dependence curves for mean blood flow velocities in extracranial portion of the right ICAs, mean VMCA/VICA ratio on the right side, hemoglobin concentration (Hb), and hematocrit value (Hct) in children with SCD. The velocity changes with age in the ICA are very similar to age-related changes in hemoglobin and hematocrit up to the age of 10 years, while the ratio shows opposite trend. Lines were obtained from distance-weighed, least-squares smoothing of individual corresponding values.
DISCUSSION
Our study provides reference limits for VMCA/VICA and VtICA/VICA ratios obtained from children with SCD without neurologic deficits and intracranial arterial narrowing on MR angiograms. In situations of increased MCA or tICA velocity, low ratios suggest hyperemia, while high ratios suggest narrowing (26). Such differentiation is frequently impossible on the basis of only intracranial velocity measurements because of common side-to-side velocity differences (24,25).
Intracranial Flow Velocities
We also provided reference velocity values for MCAs, tICAs, and ICAs. Though we excluded children with narrowing at MR angiography, we identified several velocity outliers, which could have been recognized as abnormal transcranial Doppler US findings. Large variability in the angle of insonation indicates that the trajectory of an individual artery is unpredictable; hence, interindividual discrepancies in angle-corrected velocities are higher than those in uncorrected velocities. Subsequently, the reference ranges for angle-corrected velocities are wider. Additionally, angle correction itself increases the variability of flow velocity measurements because determination of the angle is operator dependent (18). The exact contribution of this variability needs to be estimated. The angle-corrected velocities are closer to true values, however, because arterial course is accounted in velocity measurements (12,18,19). Lack of angle correction can result in an underestimation of velocity values (12), but a trial is required to determine whether angle-corrected velocities are better in estimating stroke risk than is the currently used protocol (1). Upper limits of uncorrected mean velocities are only slightly lower than the commonly used threshold of 170 cm/sec, which indicates probably abnormal findings, whereas upper limits of angle-corrected velocities are closer to the threshold of 200 cm/sec, which indicates definitely abnormal transcranial Doppler US findings (1).
Interhemispheric velocity difference as a marker of arterial narrowing may not work because we found substantial differences in children without narrowing. Such finding supports a notion that arterial trajectory and its caliber, instant blood flow, metabolic activity, distribution of microvascular changes, and the circle of Willis are not perfectly symmetrical as it was assumed by inventors of the transcranial Doppler US (27–31).
Extracranial Flow Velocities
As expected, flow velocities in the ICAs were on average about 10% (peak systolic velocity) to 22% (end-diastolic velocity) higher than respective values reported for healthy children (25). Also, variability in velocities in our group was substantially higher. Our findings confirm, however, absence of side-to-side velocity differences and a lack of sex and age dependency, though the age-dependency curve is rather steeper in our group.
Flow Velocity Ratios
Those who use transcranial color-coded duplex US and uncorrected velocities can find reference limits for uncorrected velocity ratios useful. The ratios are lower than angle-corrected velocity ratios, which, however, vary more among children presumably due to differences in the ICA course. It is interesting that the upper limit of the mean VMCA/VICA ratio is lower only by 0.1–0.2 than the threshold of 3.6 used to detect mild vasospasm in adult patients with subarachnoid hemorrhage (17).
Relatively modest but significant higher right-to-left correlations of angle-corrected ratios than correlations for corresponding velocities increase the credibility of ratios, because not only unavoidable errors from two velocity measurements contributed to the ratio's variability but also individual differences in the ICAs course. Better right-to-left correlation for angle-corrected ratios than “uncorrected” ratios further indicate that the course of intracranial arteries is an important source of variability in flow velocity measurements.
Association of Doppler Parameters with Age and Sex
Significantly higher VMCA/VICA ratios in older children are the result of lower corresponding ICA flow velocities, because velocities in the MCA were similar across the entire age span. In healthy children, in contrast, flow velocities in the MCA and ICA were lower in older children (24,25), probably as a result of proportional growth of both arteries along with body development (32). The dissociation between MCA and ICA velocity in children with SCD can be explained by the effect of hyperdynamic circulation, which apparently dilates the elastic and very compliant ICA to a greater extent than the stiffer MCA (3,6,33). The right-side predominance of findings is similar to the right-side effects observed in elderly people and in those with hypertension (34,35).
As in healthy children, sex differences in Doppler parameters were not observed in our group (24,25). Two other reports, however, did show sex differences (36,37), which were explained by sex differences in cerebrovascular resistance and vascular reactivity. In children with SCD, altered resistance and reactivity may counterbalance sex differences in Doppler parameters (13,38,39).
Hemoglobin Concentration, Hematocrit Value, and Doppler Parameters
Low hematocrit and hemoglobin in children with SCD can lead to hyperemia and abnormally high flow velocity (6–8), which can be mistakenly interpreted as resulting from narrowing. We did not find associations between Doppler and hematologic parameters, what can be expected in a selected homogeneous group with low variability in hematologic parameters. The “resistance” of velocity ratios to hemoglobin and hematocrit changes is important in discriminating hyperemia from arterial narrowing.
Limitations of the Study
Our group was relatively small compared with studied groups of healthy children. The National Committee for Clinical Laboratory Standards recommends that the sample size should consists of al least 120 values (40,41). The committee recognizes, however, that in special category of individuals, 39 observations is a required minimum to compute a 95% reference interval at 2.5% and 97.5% points of the distribution (40,41). Lack of effects of hematologic variables on velocity ratios could have been affected by a small sample size, thus a larger study is needed to further assess this result. We believe that the use of average values of hematologic parameters from a 1-year interval in eight children did not affect our results as such approach was used in many other studies.
Uncorrected flow velocities obtained with transcranial color-coded duplex US are not necessarily the same as velocities obtained with “blind” transcranial Doppler US (2,11,12), thus generalization of our results to measurements obtained with transcranial Doppler US may be limited.
In conclusion, our study provides reference ranges of VMCA/VICA and VtICA/VICA ratios, which can help to differentiate high hyperemic blood flow velocity from that resulting from narrowing of the MCA or tICA in children with SCD. We also provide reference ranges for flow velocities in the MCA, tICA, and ICA. These ratios may be helpful in identifying children with SCD who are at a high risk of stroke.
ADVANCES IN KNOWLEDGE
Reference ranges of the ratio of flow velocity in the middle cerebral artery (VMCA) to that in the extracranial portion of internal carotid artery (VICA) and the ratio of flow velocity in the terminal portion of the ICA (VtICA) to VICA, as well as blood flow velocities in the MCA, tICA, and ICA are provided for children with sickle cell disease (SCD) without neurologic deficits and no intracranial narrowing at MR angiography.
No significant association of blood flow velocities in the MCAs and tICAs with age was found.
There is a significant association of blood flow velocities in ICAs with respect to age, after adjusting for hemoglobin or hematocrit in children with SCD who have no neurologic deficits and no intracranial narrowing at MR angiography.
Our study showed a significant association of VMCA/VICA ratio on the right side with age in children with SCD and a trend toward significance on the left side.
Blood flow velocities in tICAs, ICAs, and MCAs and the ratios are not influenced by changes in hemoglobin and hematocrit in children with SCD without neurologic deficits and intracranial narrowing at MR angiography, although sample size is small.
IMPLICATIONS FOR PATIENT CARE
Established reference limits of VMCA/VICA and VtICA/VICA ratios and MCA, tICA, and ICA velocities have potential to improve stroke risk assessment in patients with questionable transcranial Doppler US results.
Acknowledgments
The authors thank the Comprehensive Sickle Cell Center at the Children's Hospital of Philadelphia for facilitating this research project.
Abbreviations
ICA = internal carotid artery
MCA = middle cerebral artery
SCD = sickle cell disease
tICA = terminal portion of the ICA
VICA = flow velocity in the extracranial portion of ICA
VMCA = flow velocity in the MCA
VtICA = flow velocity in the tICA
Author contributions: Guarantors of integrity of entire study, J.K., W.R., E.R.M.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; literature research, M.A.P., J.K., W.R., J.L.K., A.F.J., M.T.; clinical studies, M.A.P., W.R., J.L.K., R.I., A.F.J., E.R.M.; experimental studies, M.A.P., A.F.J.; statistical analysis, J.K., W.R., A.F.J.; and manuscript editing, M.A.P., J.K., W.R., J.L.K., A.F.J., E.R.M.
Funding: This research was supported by the National Institutes of Health (grant 5-R01 NS-046717).
References
- 1.Adams RJ, Mckie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle, cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 1998;339:5–11. [DOI] [PubMed] [Google Scholar]
- 2.Neish AS, Blews DE, Simms CA, Merritt RK, Spinks AJ. Screening for stroke in sickle cell anemia: comparison of transcranial Doppler imaging and nonimaging US techniques. Radiology 2002;222:709–714. [DOI] [PubMed] [Google Scholar]
- 3.Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006;108:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talano JA, Hillery CA, Gottschall JL, Baylerian DM, Scott JP. Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease. Pediatrics 2003;111(6 pt 1):e661–e665. [DOI] [PubMed] [Google Scholar]
- 5.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease: the cooperative study of sickle cell disease. Blood 1990;76:1431–1437. [PubMed] [Google Scholar]
- 6.Prohovnik I, Pavlakis SG, Piomelli S, et al. Cerebral hyperemia, stroke, and transfusion in sickle cell disease. Neurology 1989;39:344–348. [DOI] [PubMed] [Google Scholar]
- 7.Ausavarungnirun P, Sabio H, Kim J, Tegeler CH. Dynamic vascular analysis shows a hyperemic flow pattern in sickle cell disease. J Neuroimaging 2006;16:311–317. [DOI] [PubMed] [Google Scholar]
- 8.Venketasubramanian N, Prohovnik I, Hurlet A, Mohr JP, Piomelli S. Middle cerebral artery velocity changes during transfusion in sickle cell anemia. Stroke 1994;25:2153–2158. [DOI] [PubMed] [Google Scholar]
- 9.Belhassen L, Pelle G, Sediame S, et al. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood 2001;97:1584–1589. [DOI] [PubMed] [Google Scholar]
- 10.Mikkonen RH, Kreula JM, Virkkunen PJ. Reliability of Doppler ultrasound in follow-up studies. Acta Radiol 1998;39:193–199. [DOI] [PubMed] [Google Scholar]
- 11.McCarville MB, Li C, Xiong X, Wang W. Comparison of transcranial Doppler sonography with and without imaging in the evaluation of children with sickle cell anemia. AJR Am J Roentgenol 2004;183:1117–1122. [DOI] [PubMed] [Google Scholar]
- 12.Krejza J, Rudzinski W, Pawlak MA, et al. Imaging of major brain arteries in children with sickle cell disease: transcranial color-coded duplex sonography versus conventional transcranial Doppler sonography. AJNR Am J Neuroradiol 2007;28:1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown MM, Wade JP, Marshall J. Fundamental importance of arterial oxygen content in the regulation of cerebral blood flow in man. Brain 1985;108:81–93. [DOI] [PubMed] [Google Scholar]
- 14.Merrill EW, Gilliland ER, Cokelet G, Shin H, Britten A, Wells RE Jr. Rheology of human blood, near and at zero flow: effects of temperature and hematocrit level. Biophys J 1963;3:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cinar Y, Demir G, Pac M, Cinar AB. Effect of hematocrit on blood pressure via hyperviscosity. Am J Hypertens 1999;12:739–743. [DOI] [PubMed] [Google Scholar]
- 16.McDonald DA. Arterial impedance. In: McDonald DA, ed. Blood flow in arteries. Baltimore, Md: Williams & Wilkins, 1977; 351–389.
- 17.Krejza J, Kochanowicz J, Mariak Z, Lewko J, Melhem ER. Middle cerebral artery spasm after subarachnoid hemorrhage: detection with transcranial color-coded duplex US. Radiology 2005;236:621–629. [DOI] [PubMed] [Google Scholar]
- 18.Krejza J, Mariak Z, Melhem ER, Bert RJ. A guide to the identification of major cerebral arteries with transcranial color Doppler sonography. AJR Am J Roentgenol 2000;174:1297–1303. [DOI] [PubMed] [Google Scholar]
- 19.Forteza AM, Krejza J, Koch S, Babikian VL. Ultrasound imaging of cerebrovascular disease. In: Babikian VL, Wechsler L, Higashida RT, eds. Imaging cerebrovascular disease. Philadelphia, Pa: Butterworth-Heinemann, 2003; 3–35.
- 20.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics 1969;11:1–21. [Google Scholar]
- 21.Lilliefors HW. On the Kolmogorov-Smirnov test for normality with mean and variances unknown. J Am Stat Assoc 1967;62:399–402. [Google Scholar]
- 22.Lumsden JH, Mullen K. On establishing reference values. Can J Comp Med 1978;42:293–301. [PMC free article] [PubMed] [Google Scholar]
- 23.Weissberg A, Beaty GH. Tables of tolerance limit factors for the normal distribution. Technometrics 1960;2:483–500. [Google Scholar]
- 24.Schöning M, Staab M, Walter J, Niemann G. Transcranial color duplex sonography in childhood and adolescence: age dependence of flow velocities and waveform parameters. Stroke 1993;24:1305–1309. [DOI] [PubMed] [Google Scholar]
- 25.Schöning M, Hartig B. The development of hemodynamics in the extracranial carotid and vertebral arteries. Ultrasound Med Biol 1998;24:655–662. [DOI] [PubMed] [Google Scholar]
- 26.Romner B, Bellner J, Kongstad P, Sjöholm H. Elevated transcranial Doppler flow velocities after severe head injury: cerebral vasospasm or hyperemia? J Neurosurg 1996;85:90–97. [DOI] [PubMed] [Google Scholar]
- 27.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982;57:769–774. [DOI] [PubMed] [Google Scholar]
- 28.Hendrikse J, van Raamt AF, van der Graaf Y, Mali WP, van der Grond J. Distribution of cerebral blood flow in the circle of Willis. Radiology 2005;235:184–189. [DOI] [PubMed] [Google Scholar]
- 29.Orlandini GE, Ruggiero C, Orlandini SZ, Gulisano M. Blood vessel size of circulus arteriosus cerebri (circle of Willis): a statistical research on 100 human subjects. Acta Anat (Basel) 1985;123:72–76. [DOI] [PubMed] [Google Scholar]
- 30.Kamath S. Observations on the length and diameter of vessels forming the circle of Willis. J Anat 1981;133:419–423. [PMC free article] [PubMed] [Google Scholar]
- 31.Van Bogaert P, Wikler D, Damhaut P, Szliwowski HB, Goldman S. Regional changes in glucose metabolism during brain development from the age of 6 years. Neuroimage 1998;8:62–68. [DOI] [PubMed] [Google Scholar]
- 32.Seong J, Lieber BB, Wakhloo AK. Morphological age-dependent development of the human carotid bifurcation. J Biomech 2005;38:453–465. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech 1980;13:175–184. [DOI] [PubMed] [Google Scholar]
- 34.Krejza J, Arkuszewski M, Kasner SE, et al. Carotid artery diameter in men and women and the relation to body and neck size. Stroke 2006;37:1103–1105. [DOI] [PubMed] [Google Scholar]
- 35.Zbornikova V, Lassvik C. Duplex scanning in presumably normal persons of different ages. Ultrasound Med Biol 1986;12:371–378. [DOI] [PubMed] [Google Scholar]
- 36.Vavilala MS, Kincaid MS, Muangman SL, Suz P, Rozet I, Lam AM. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res 2005;58:574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tontisirin N, Muangman SL, Suz P, et al. Early childhood gender differences in anterior and posterior cerebral blood flow velocity and autoregulation. Pediatrics 2007;119:e610–e615. [DOI] [PubMed] [Google Scholar]
- 38.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 2002;8:1383–1389. [DOI] [PubMed] [Google Scholar]
- 39.Van Mil AH, Spilt A, Van Buchem MA, et al. Nitric oxide mediates hypoxia-induced cerebral vasodilation in humans. J Appl Physiol 2002;92:962–966. [DOI] [PubMed] [Google Scholar]
- 40.Horn PS, Pesce AJ. Reference intervals: an update. Clin Chim Acta 2003;334:5–23. [DOI] [PubMed] [Google Scholar]
- 41.National Committee for Clinical Laboratory Standards. How to define and determine reference intervals in the clinical laboratory: approved guideline, NCCLS document C28-A and C28–A2. Villanova, Pa: National Committee for Clinical Laboratory Standards, 1995, 2001.