Abstract
Nearest-neighbor recognition experiments have been carried out in fluid liposomal membranes made from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and cholesterol, using exchangeable dimers derived from 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine and cholesterol. In cholesterol-rich bilayers, the association between these two exchangeable lipids was reduced as the curvature of the membrane increases; that is, when the diameter of the liposomes was below ca. 200 nm. In sharp contrast, the mixing of these exchangeable lipids was close to random in the cholesterol-poor membranes, regardless of their curvature. The biological implications of these findings are briefly discussed.
Introduction
One of the most important problems that remains to be solved in modern cell biology is the determination of the two-dimensional structures of biological membranes; that is, the time-averaged lateral distribution of the component lipids and proteins. Because these natural enclosures are rich in complexity, most researchers have used simple model systems to gain insight into membrane organization. Thus, based on lipid mixing experiments that have been carried out in monolayers at the air/water interface and in liposomes, it is now clear that cholesterol can favor high-melting lipids as nearest-neighbors in the physiologically-relevant fluid phase.1–7 Although it has been postulated that a similar association occurs between cholesterol and high-melting sphingolipids in cell membranes to produce “lipid rafts”, this hypothesis remains controversial.8–14
In the work reported herein, we sought to answer one fundamental question regarding sterol-phospholipid association that, to our knowledge, has not previously been addressed: Can membrane curvature influence such association in fluid bilayers? Given (i) the prevalence of curved bilayers in living organisms (e.g., endocytotic vesicles, enveloped viruses, membrane fusion processes, etc.), (ii) the strong condensing and fluidizing effects that cholesterol is known to have on neighboring lipids, and (iii) the possible existence of lipid rafts, this question has considerable importance.15 Thus, any redistribution of cholesterol within biological membranes that results from the introduction of curvature has the potential for altering the microenvironment, the structure and the functioning of active components; e.g., signaling proteins.
Experimental Section
Nearest-Neighbor Recognition Analysis
In a typical extruded liposome preparation, a thin film of lipid was prepared by evaporating a chloroform solution containing 0.30 μmol AB (or 0.15 μmol AA plus 0.15 μmol BB) and either 6.9 μmol of DPPC plus 4.5 μmol of cholesterol or 11.4 μmol of DPPC under a stream of argon. After drying the thin film overnight under reduced pressure (0.1 mm Hg), 2.0 mL of a 10 mM Tris-HCl buffer (10 mM Tris, 150 mM NaCl, 2 mM NaN3, 1 mM EDTA, pH = 7.4) was added to the dried film. The mixture was vortex mixed for 30 s, incubated for 5 min at 60 °C, vortex mixed for an additional 30 s, and incubated for an additional 25 min at 60°C. The dispersion was then subjected to five freeze/thaw cycles (liquid nitrogen/60°C water bath), and extruded through a given polycarbonate (Nuclepore) filter having pore diameters of 400 (12 times), 200 (12 times), 100 (7 times after first extruding 12 times through 200 nm filter) or 50 nm (7 times after first extruding 12 times through 200 nm filter).
For the formation of analogous liposomes using the reverse-phase evaporation method, the same amounts of lipids as shown above were dissolved in 100 μL of chloroform, 270 μL of diisopropyl ether and 35 μL of Tris buffer (3.3 mM Tris, 50 mM NaCl, 0.7 mM NaN3, 0.3 mM EDTA, pH 7.4). The solution was then mixed by vortexing and sonicated for 3 min in a bath sonicator. After removal of solvent by passage of a stream of argon flow, 2 mL of 10 mM Tris buffer was added, followed by vortex mixing, and the dispersion allowed to incubate at 60 °C for 30 min. The resulting reverse phase evaporation vesicles (REVs) were dialyzed against 500 mL of buffer overnight. The REVs were harvested by centrifugation and redispersed in 1.9 mL of 10 mM Tris buffer.
After the removal of oxygen from the system at 60°C by purging with argon, 10.5 μL of a 1.0 M NaOH aqueous solution was added to 1900 μL of the dispersion to adjust its pH to ca. 7.4. Thiolate-disulfide interchange was then initiated by adding 100 nmol (0.4 eq) of threo-dithiothreitol into 1650 μL of the dispersion. The liposomal dispersions were maintained under an argon atmosphere throughout the course of the interchange reactions. Aliquots (250 μL) were withdrawn as a function of time and the exchange reactions quenched by adding them to 7.3 μL of 10 M acetic acid at room temperature. The lipids were then quickly extracted into 1 mL of CHCl3/MeOH (2/1, v/v) and stored at −20°C. Just prior to analysis, a given sample was lyophilized and the lipids dissolved in 100 μL of solvent [i.e., HPLC mobile phase/CHCl3 (80/20, v/v)]. This solution was then immediately analyzed by HPLC using a C18 reverse phase column and a mobile phase that was composed of 10 mM tetrabutylammonium acetate in ethanol/water/hexane (76/13/10, v/v/v) using a flow rate of 0.9 mL/min. The column was maintained at 31°C and the components were monitored at 203 nm using a Waters 996-photodiode-array detector.
Results and Discussion
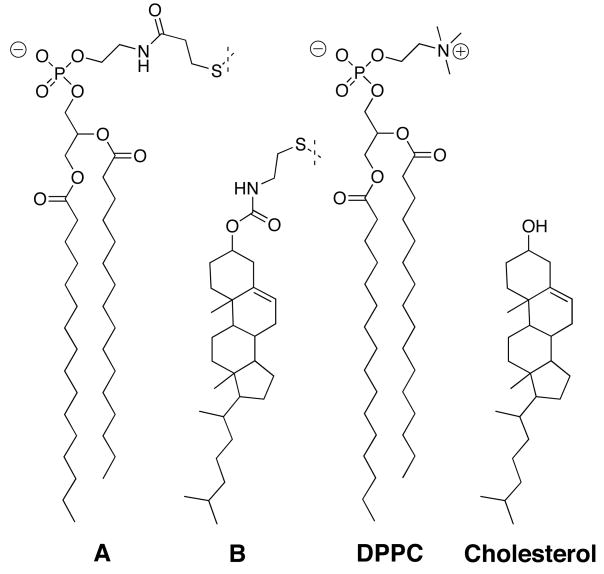
To probe the effects of curvature on sterol-phospholipid association, we employed the nearest-neighbor recognition (NNR) method.5–7 Specifically, we determined the affinity that the exchangeable lipids A and B have toward each other in model membranes made from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and cholesterol (Chart 1).
Chart 1.
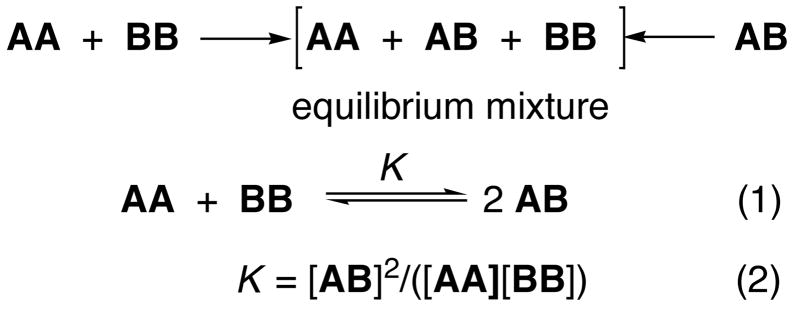
As discussed elsewhere, NNR experiments take molecular-level snapshots of bilayer organization by detecting and quantifying the thermodynamic tendency of exchangeable monomers to become nearest-neighbors of one another.5–7 Typically, two lipids of interest (A and B) are converted into exchangeable dimers (AA, AB and BB), which are then allowed to undergo monomer interchange via thiolate-disulfide interchange. The resulting equilibrium that is established, whereby one molecule of AA reacts with one molecule of BB to give two molecules of AB, is then governed by an equilibrium constant, K (Scheme 1). When monomers A and B mix ideally, this is reflected by an equilibrium constant that equals 4.0. When homo-associations are favored, the equilibrium constant is less than 4.0; favored hetero-associations are indicated by a value that is greater than 4.0.
Scheme 1.
Exchangeable phospholipid dimers AA, BB and AB were synthesized using established procedures.6 As we have shown previously, the gel to liquid-crystalline phase transition temperature (Tm) of AA (41.9.°C) is nearly identical to that of DPPC (41.5°C).16 In addition, the condensing properties of B have also been shown to be strikingly similar to that of cholesterol.17a This fact, together with previous NNR-evidence that hydrophobic forces dominate cholesterol—phospholipid interactions, provide a strong argument that cholesterol’s hydroxyl group, and the polar, exchangeable group attached to cholesterol, play a minor role in this sterol’s functioning as a condensing agent.17b
The mixing behavior of A with B in the fluid bilayer state was investigated by carrying out thiolate-disulfide interchange reactions in liposomes containing 5 mol% of an equimolar mixture of A and B (i.e., 2.5 mol% AB) and 95 mol% of a mixture of DPPC/cholesterol (57.5/37.5, mol/mol) or pure DPPC. At the temperature used for the exchange reactions (60°C), these membranes exist in the liquid-ordered (lo) and liquid-disordered (ld) phases, respectively.18 The curvature of the liposomes was controlled by using different polycarbonate (Nuclepore) membranes for extrusion or by preparing reverse-phase evaporation vesicles (REVs). Actual sizes of the resulting liposomes were determined by dynamic light scattering. Dimer compositions were monitored as a function of time by HPLC, and quantified by use of appropriate calibration curves. Values of K were calculated from equation 2.
Figure 1 summarizes our principal results. In brief, for the cholesterol-rich membranes, increased curvature (i.e., smaller diameter liposomes) led to an overall reduction in the association between A and B. It should be noted that this effect becomes more evident when the diameter of the liposomes is reduced below ca. 200 nm, as the packing of the lipids becomes less efficient due to increased curvature. In sharp contrast, the mixing of A with B was close to random in the cholesterol-poor membranes, regardless of their curvature. Similar results were obtained from NNR experiments starting with equimolar mixtures of AA and BB (see Supporting Information). In this case, however, K value were slightly lower. This difference is presumed to be due to an uneven distribution of A and B between the inner and outer leaflets; that is, a preference for A and B to be in different leaflets would result in higher quantities of homodimers.
Figure 1.
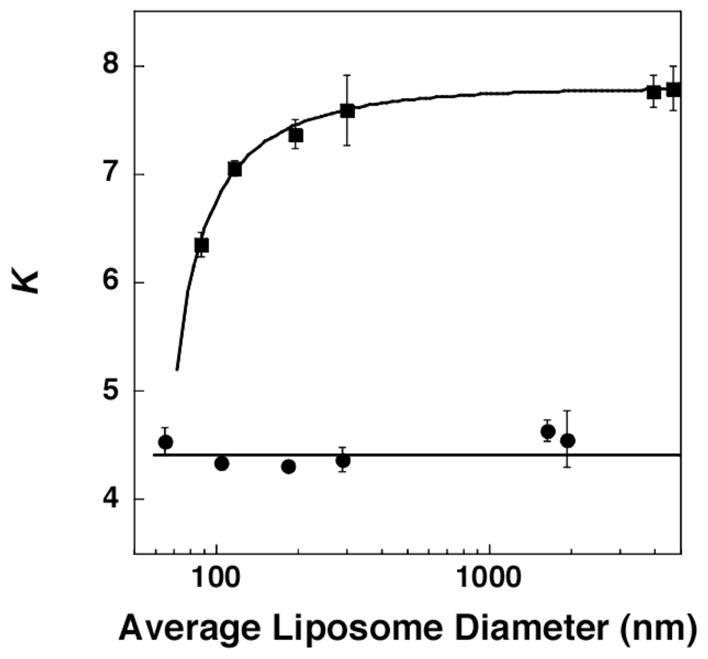
Plot of K versus the averge diameter of liposomes that were cholesterol-rich (40 mol%; ■) and cholesterol-poor (2.5 mol%; ●). All dispersions showed gaussian distributions with standard deviations that were typically 30–40% via extrusion and 50–60% for REVs (having diameters >1000 nm). Particle sizes before and after NNR reactions were essentially unchanged.19
As a further confirmation that increased curvature leads to a reduction in sterol-phospholipid recognition, and that this reduction is not the result of an asymmetric distribution of cholesterol between the inner and outer leaflet of the bilayers, liposomes were prepared exclusively from AB.20 In this case, all monolayers were composed, entirely, of an equimolar quantity of A and B. The fact that 178 ± 41 nm and 85 ± 34 nm liposomes (measured after the NNR reaction) yielded K values of 8.22 ± 0.16 and 7.31 ± 0.20, respectively, lends further support that increased curvature reduces sterol-phospholipid recognition.21
It is important to note that while the NNR experiments reported herein provide strong evidence for to an overall reduction in the association between A and B, they do not allow us to judge differences in nearest-neighbor interactions between the inner-concave and outer-convex leaflets. Thus, it is possible that sterol-phospholipid association might be enhanced in one of the curved monolayers, which partially compensates for a relatively strong reduction in sterol-phospholipid association in the adjoining leaflet. It is also possible that sterol-phospholipid association is being reduced in both leaflets. Regardless of this uncertainty, that curvature leads to a net reduction in sterol-phospholipid association and hence a change in the two-dimensional structure of cholesterol-rich, fluid phospholipid bilayers, is clear. Given the fact that enveloped virus and endocytotic vesicles can have even greater curvature (e.g., the average size of an HIV particle is ca. 100 nm), it is now reasonable to expect that their two-dimensional structure may be different from mammalian membranes from which they are derived.
Finally, additional evidence for less efficient lipid packing in curved phospholipid bilayers was obtained by measuring the melting behavior of DPPC using high sensitivity differential scanning calorimetry (hs-DSC). Here, 2.5 mol% of 1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DPPG) was included in the membrane to match the percentage of negatively charged A that was used in our NNR experiments. As seen in Figure 2, incremental increases in curvature results in substantial broadening of the gel to liquid-crystalline phase transition and the disappearance of the pre-transition at 35°C.22 Broad gel to liquid-crystalline phase transitions are known to reflect melting processes that are low in cooperativity and bilayers that are not well-packed.23
Figure 2.
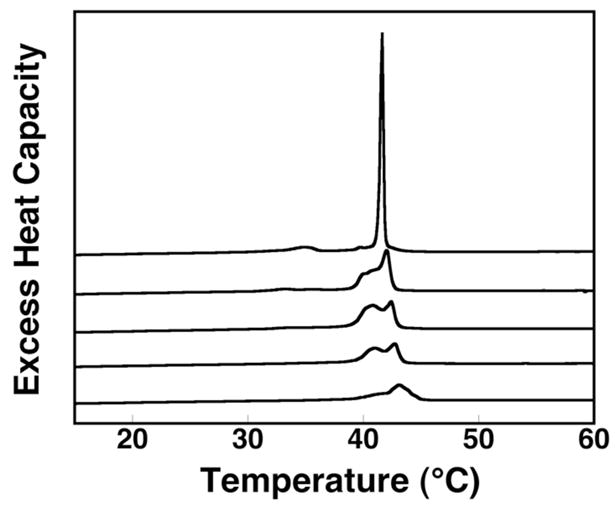
High sensitivity excess heat capacity profiles of DPPC/DPPG (97.5/2.5, mol/mol) in the form of (top to bottom): multilamellar liposomes and liposomes that were formed via extrusion through 400, 200, 100, and 50 nm diameter Nuclepore membranes.
The NNR results reported herein provide new insight into the effects of curvature on lipid packing and lipid mixing. Specifically, they show that in cholesterol-poor membranes, where lipid interactions are relatively weak, curvature has little influence on sterol—phospholipid association. In sharp contrast, increased curvature significantly reduces the preference of sterols and high-melting phospholipids to become nearest neighbors in cholesterol-rich membranes, where lipid interactions are stronger.
Conclusions
The results of this study have shown that increased membrane curvature can lead to a reduction in sterol-phospholipid association in cholesterol-rich membranes. Based on these findings, it is now reasonable to expect that curvature should also change cholesterol’s nearest-neighbor interactions with lipids and proteins in biological membranes. Such changes would also be expected to alter their two-dimensional organization. In principle, exploiting differences in membrane structure and function that result from differences in curvature could provide new opportunities for drug design; e.g., the targeting of enveloped viruses such as HIV.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (PHS GM56149).
Footnotes
SUPPORTING INFORMATION. Data obtained from NNR experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES and NOTES
- 1.Radhakrishnan A, McConnell HM. J Am Chem Soc. 1999;121:486–487. [Google Scholar]
- 2.Veatch SS, Keller SL. Biophys J. 2003;84:725–726. doi: 10.1016/S0006-3495(03)74891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McConnell HM, Radhakrishnan A. Biochim Biophys Acta. 2003;1610:159–173. doi: 10.1016/s0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 4.Veatch SL, Keller SL. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Cao H, Tokutake N, Regen SL. J Am Chem Soc. 2003;125:16182–16183. doi: 10.1021/ja039172x. [DOI] [PubMed] [Google Scholar]
- 6.Cao H, Zhang J, Jing B, Regen SL. J Am Chem Soc. 2005;127:8813–8816. doi: 10.1021/ja0513988. [DOI] [PubMed] [Google Scholar]
- 7.Sugahara M, Uragami M, Regen SL. J Am Chem Soc. 2003;125:13040–13041. doi: 10.1021/ja038102n. [DOI] [PubMed] [Google Scholar]
- 8.McIntosh TJ, editor. Lipid Rafts. Springer-Verlage; New York, N. Y: 2007. [Google Scholar]
- 9.Simons K, Ikonen E. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 10.Simons K, Vaz WLC. Annu Rev Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 11.Edidin M. Annu Rev Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 12.Munro S. Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 13.Nichols B. Nature. 2005;436:638–639. doi: 10.1038/436638a. [DOI] [PubMed] [Google Scholar]
- 14.Heerklotz H. Biophys J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gennis RB. Biomembranes: Molecular Structure and Function. Springer-Verlag; New York: 1989. [Google Scholar]
- 16.Krisovitch SM, Regen SL. J Am Chem Soc. 1992;114:9828–9835. [Google Scholar]
- 17.(a) Sugahara M, Uragami M, Yan X, Regen SLJ. Am Chem Soc. 2001;123:7939–7940. doi: 10.1021/ja016199c. [DOI] [PubMed] [Google Scholar]; (b) Cao H, Tokutake N, Regen SL. J Am Chem Soc. 2003;125:16182–16183. doi: 10.1021/ja039172x. [DOI] [PubMed] [Google Scholar]
- 18.Chiang YW, Costa-Filho J, Freed JH. J Phys Chem B. 2007;111:11260–11270. doi: 10.1021/jp0732110. [DOI] [PubMed] [Google Scholar]
- 19.Particle sizes measured before (and after) NNR reactions confirmed that the liposomes were stable under the conditions used: 2.5% sterol: 184 ± 64 nm (180 ± 54 nm), 65 ± 23 nm (66± 21 nm); 40% sterol: 200 ± 64 nm (198 ± 59 nm), 88 ± 34 nm (93 ± 38 nm).
- 20.Roy MT, Gallardo M, Estelrich J. Bioconjugate Chem. 1997;8:941–945. doi: 10.1021/bc9701050. [DOI] [PubMed] [Google Scholar]
- 21.The fact that K values were consistently lower with liposomes made from AA and BB, as compared with ones made from AB, implies that transbilayer “flip-flop” of A and B is negligible during NNR measurements. If flip-flop were significant, then heterodimer and homodimer experiments should yield the same K values. This fact also implies that liposomes made from the heterodimer maintain an equimolar mixture of A and B in each leaflet.
- 22.Similar broadening has previously been reported in single component systems: Brocca P, Cantu L, Corti M, DelFavero E, Motta S, Nodari MC. Colloids and Surfaces A: Physicochem Eng Aspects. 2006;291:63–68.
- 23.Mabrey-Gaud, S. in “Liposomes: From Physical Structure to Therapeutic Applications”, C. G. Knight, Ed., Chpt. 5, Elsevier, New York, 1981.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.