Abstract
Purpose
Tissue inhibitor of metalloproteinases-3 (TIMP-3) is one of four members of a family of proteins that were originally classified according to their ability to inhibit matrix metalloproteinases (MMP). We analyzed TIMP-3 methylation in 175 urine sediment DNA samples from bladder cancer patients with well characterized clinicopathological parameters including patient outcome.
Materials and methods
We examined urine sediment DNA for aberrant methylation of 9 genes including TIMP-3 by quantitative fluorogenic real-time PCR.
Results
Using an optimal cutoff value by Taqman quantitation, we found that the risk of death was statistically significantly higher in patients with higher TIMP-3 and ARF methylation (hazard ratio [HR] =1.99, 95% confidence interval [CI] =1.12 to 3.27; p= 0.01 and HR=1.66, 95% CI=1.00 to 2.76; p=0.05 respectively) than in patients without/lower TIMP3 and ARF methylation in urine. A significant correlation was also seen between risk of death and stage 3 tumor (HR=2.73, 95% CI=1.58 to 4.72; p=0.003 and the presence of metastasis (HR=3.32, 95% CI=1.98 to 5.57; p=0.0001). Multivariate analysis subsequently revealed that TIMP-3 methylation was an independent prognostic factor for bladder cancer survival with stage and metastasis (p=0.001 and 0.02 respectively).
Conclusion
These results suggest that TIMP-3 promoter methylation could be a clinically applicable marker for bladder cancer progression.
INTRODUCTION
High grade, invasive bladder cancer is potentially a lethal disease. Definitive therapy includes radical cystectomy and long-term outcome depends in part on pathological stage, grade and lymph node involvement 1. Evaluating various tumor markers in an attempt better to define and stratify bladder tumors and patient clinical outcome has become an important and evolving field 2.
A paramount goal of the management of high grade, invasive transitional cell carcinoma (TCC) of the bladder is to identify patients with malignant tumors that will behave more aggressively. Histological evaluation of tumor grade and stage has long served as the most important clinical prognostic indicators that determine intervention and adjuvant treatment. However, these conventional histopathological factors lack the ability to define accurately the true biological or malignant behavior of each individual tumor. Accurate, reliable prediction of the biological potential of a neoplasm may help direct the proper treatment plan and identify patients who would benefit from adjuvant therapies and others who would require less aggressive treatments.
Hypermethylation of gene promoters has been explored as both a mechanism and marker of tumorigenesis 3, 4. TIMP-3 is a secreted 24-kDa protein that, unlike other TIMP family members, binds to the extracellular matrix. TIMP-3 overexpression in tumor cells has been shown to induce apoptosis 5, 6, as well as to suppress primary tumor growth and angiogenesis 7, indicating that TIMP-3 may suppress various aspects of tumor development. Furthermore, decreased TIMP-3 expression at the invasive front of human colon carcinomas suggests that a regional loss of TIMP-3 may facilitate tumor invasion and metastasis 8. Progressive neoplastic variants of the mouse JB6 model of tumor progression lack TIMP-3 expression, whereas the pre-neoplastic variants retain TIMP-3 expression. As neoplastic cells from bladder cancers are shed into the urine, we tested various methylation markers in urine DNA and correlated the results with clinical outcome. We found that TIMP-3 promoter methylation correlated strongly with tumor invasion and metastasis. By multivariate analysis, we show that TIMP-3 promoter methylation is an independent prognostic indicator of bladder cancer survival.
MATERIALS AND METHODS
Sample collection
We examined the urine sediment of 175 patients with bladder cancer (total=175). Detailed information on these patients is summarized in Table 1. The Institutional Review Board of the Johns Hopkins Hospital approved the study. Fifty milliliters of voided urine were collected from all cases prior to definite surgery. Urine samples were spun at 3000 × g for 10 min and washed twice with phosphate-buffered saline. All samples were stored at −80°C.
Table 1.
Demographic and clinical information of bladder cancer patients
| Histological Cell Type | Grade ‡ | ||
|---|---|---|---|
| TCC | 157 | Lower grade (grade 1 and 2) | 30 |
| Adeno | 4 | Higher grade (grade 3) | 137 |
| Squamous | 2 | Unknown | 8 |
| Mixed | 1 | Metastasis | |
| Other | 2 | Yes | 39 |
| Large cell | 3 | No | 128 |
| Small cell | 2 | unknown | 8 |
| Carcinosarcoma | 4 | Alcohol | |
| Drinker | 50 | ||
| Race | Not drinker or rarely drinker | 51 | |
| White | 126 | Unknown | 74 |
| Hispanic | 1 | Stage † | |
| African American | 11 | pTa | 32 |
| Other | 6 | pTis | 16 |
| unknown | 31 | pT1 | 26 |
| pT2a, pT2b | 31 | ||
| Smoking | pT3a, PT3b | 54 | |
| Yes | 93 | pT4 | 16 |
| No | 25 | Noninvasive (Ta-T1) | 74 |
| unknown | 57 | Muscle invasive (≥pT2) | 101 |
| Recurrence | |||
| Sex | Yes | 29 | |
| Female | 47 | No | 128 |
| Male | 128 | unknown | 18 |
American Joint Committee on Cancer staging
American Joint Committee on Cancer
DNA extraction
The frozen urine cell pellet was digested with 1% SDS and 50μg/ml proteinase K (Boehringer Mannheim, Germany) at 48°C overnight, followed by phenol/chloroform extraction and ethanol precipitation of DNA as previously described 9.
Bisulfite treatment
DNA from urine sediment was subjected to bisulfite treatment, as described previously with little modification 10. Briefly, 2 μg of genomic DNA was denatured in 0.2 M NaOH for 20 min at 50°C. The denatured DNA was diluted in 500 μl of freshly prepared solution of 10 mM hydroquinone and 3 M sodium bisulfite, and incubated for 3 hours at 70° C. After incubation, the DNA sample was desalted through a column (Wizard DNA Clean-Up System, Promega), treated with 0.3 M NaOH for 10 min at room temperature, and precipitated with ethanol. The bisulfite-modified genomic DNA was resuspended in 120 μl of LoTE (2.5 mM EDTA, 10mM Tris-HCL) and stored at −80°C.
Methylation analysis
Gene promoters were amplified by a fluorescence based-real-time PCR (Taqman) as previously described 11. In brief, primers and probes were designed to specifically amplify the bisulfite-converted promoter of the gene of interest and are reported previously12. The ratios between the values of the gene of interest and the internal reference gene, β-actin, obtained by Taqman analysis were used as a measure for representing the relative level of methylation in the particular sample (Target gene/β –actin × 1000). Fluorogenic PCRs were carried out in a reaction volume of 20 μl consisting of 600 nM of each primer, 200 nM of probe, 5 units of Taq Polymerase, 200 μM each of dATP, dCTP, and dGTP; 200 of μM dTTP; and 5.5 mM MgCl2. Three microliters of treated DNA solution were used in each real-time MSP reaction. Amplifications were carried out in 384-well plates in a 7900 Sequence detector (Perkin-Elmer Applied Biosystems). Each plate consisted of patient samples and multiple water blanks, as well as positive and negative controls. Leukocytes from a healthy individual were methylated in vitro with excess SssI methyltransferase (New England Biolabs Inc., Beverly, MA) to generate completely methylated DNA and serial dilutions of this DNA were used for constructing the calibration curves on each plate.
Statistical analysis
Tumor stage (pTa, pT1, pT2≥) and grade (higher grade and lower grade) were recorded when reported. Life-table estimation was performed according to the method of Kaplan and Meier. Univariate comparison of survival curves was performed with the use of the log-rank and Wilcoxon tests. The multivariable Cox proportional-hazards model was used to estimate the hazard ratios and 95 percent confidence intervals. Variables in the model included tumor grade, stage, invasiveness, metastasis and methylation status of all nine gene markers at the time of diagnosis. The nine markers were tested as continuous variables (with logarithmic transformation when appropriate) and as binary variables using the same cutpoints determined for distinguishing bladder cancer cases from controls detection 13. Statistical computations were performed using the SAS system and all p values reported are two sided.
RESULTS
The demographic and clinical characteristics of 175 primary bladder cancer patients included in this study are summarized in Table 1. The study population was predominantly male (73%), with a median age of 67 years (interquartile range 29–90 years). Bladder cancer cases were finally confirmed by standard pathology. Most of the tumors were transitional cell carcinomas of all stages and grades (Table 1). We performed QMSP on nine gene promoters (APC, ARF, CDH1, GSTP1, MGMT, p16, RAR-β2, Rassf1A and TIMP3) in urine DNA from each patient.
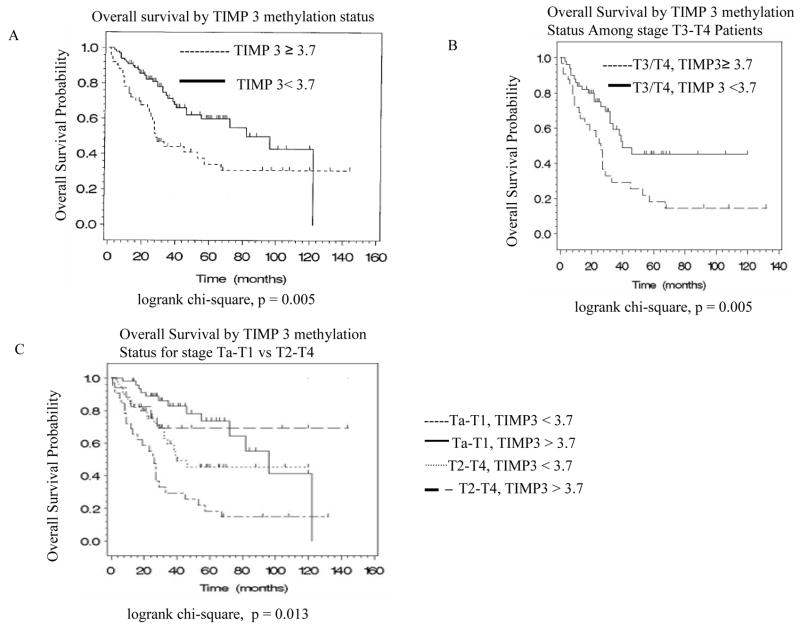
Out of 175 total cases, 149 were available for follow up. Among these 149 patients, 85 have follow-up period for more than 12 months. Overall median follow up of for these 85 patients was 36 months (range, 12–144 months). Overall survival was calculated from the first day of diagnosis until death or the last follow-up visit. Median survival for unmethylated TIMP3 (<3.7) was 82 months while median survival for methylated TIMP3 (≥3.7) was only 28 months. Univariate Cox regression analysis indicated that among the clinical parameters, invasiveness, tumor stage and metastasis were the critical factors predicting survival (Table 2A). Among the 9 methylation markers in urine, only TIMP3 and ARF methylation state correlated significantly with patient survival (HR 1.99, 95% CI 1.22–3.27, P=0.01 and HR 1.66, 95% CI 1.00–2.76, p=0.05 respectively) (Table 2, Figure 1A). These findings remained statistically significant in multivariate analysis for TIMP3 after adjusting for the stage (Table 2B, Figure 1B, 1C; HR 1.83, 95% CI 1.11–3.01, p=0.02). In a multivariable model, TIMP3 methylation was associated with an almost two and a half fold increase in risk of death even after adjusting for the presence of metastasis (odd ratio=2.45, 95% CI 1.14–5.26) (Table 2B). However, ARF was not remained statistically significant in multivariate model (data not shown).
Table 2.
Variables that predict decreased overall survival of patients with statistical significance in Cox proportional hazards models
| Hazard ratio | 95% Confidence interval | P | |
|---|---|---|---|
| A. Univariate Cox proportional hazards model results | |||
| Variable | |||
| Invasive | 1.91 | 1.09–3.34 | 0.02 |
| Stage 3 | 2.73 | 1.58–4.72 | 0.0003 |
| Metastasis | 3.32 | 1.98–5.57 | 0.0001 |
| Smoke | 2.51 | 0.98–6.39 | 0.05 |
| TIMP3(≥3.7 vs <3.7) | 1.99 | 1.22–3.27 | 0.01 |
| ARF(0 vs >0) | 1.66 | 1.00–2.76 | 0.05 |
| B. Multivariate Cox proportional hazards results | |||
| Covariate | |||
| TIMP3(≥3.7 vs <3.7) | 1.83 | 1.11–3.01 | 0.02 |
| Stage 3 | 2.54 | 1.46–4.40 | 0.001 |
| Covariate | |||
| TIMP3(≥3.7 vs <3.7) | 1.7 | 0.97–2.96 | 0.063 |
| Metastasis(present vs absent) | 2.45 | 1.14–5.26 | 0.02 |
Figure 1.
Kaplan-Meier curve for the study population stratified for the various prognosis factors. (A) Overall survival and TIMP3 methylation in 149 (available for follow up) patients with bladder cancer who exhibited or did not exhibit TIMP3 methylation in urine (p=0.005). (B) Overall survival was significantly lower in patients with stage T3/T4 whose tumors contained methylated TIMP3 than in those whose tumor without TIMP-3 methylation (p=0.005). (C) Patients with low stage (Ta-T1) disease have similarly good survival regardless of whether TIMP-3 is methylated or unmethylated. Among patients with high stage (T2–T4) (muscle invasive) disease, those with methylated TIMP3 have significantly worse survival than those with unmethylated TIMP-3 (p=0.0001).
When analysis of TIMP3 methylation and recurrence-free survival was performed, no survival differences were observed, nor were the relative risks of recurrence-free survival appreciably different or statistically significant (data not shown). Aberrant methylation of TIMP3 in urine sediment had no correlation with patient demographic data, including age and gender, histological subtype, and grade of the tumor (data not shown).
DISCUSSION
In this study, we found that TIMP3 methylation in urinary sediment of bladder cancer patients significantly correlated with metastasis and patient prognosis by QMSP. This suggests that within the shed cells from primary bladder tumor, cancer cells with TIMP3 promoter methylation are readily detectable and reflect tumor behavior. Metastasis is a powerful independent prognostic factor for bladder cancer survival. Of the 149 cases where status of metastasis is available, only 39 cases had proven metastasis. As a consequence of this patient distribution, any marker found to be significantly correlated with bladder cancer prognosis in our selected patients must be relatively independent of metastasis status. In this study, TIMP3 was statistically significant independent prognostic factor by multivariate analysis [HR, 2.45 (95% CI, 1.14–5.26; p=0.02)] even after accounting for the powerful prognostic effect of metastasis.
An independent prognostic factor’s clinical utility is its ability to more accurately predict patient survival when used with other known prognostic factors. Such a marker would be invaluable to surgeons and patients selecting treatment options. Our data showed that TIMP3 promoter methylation, in combination with pathological stage (muscle invasive) more accurately predicted patient prognosis (Figure 1B and Figure 1C) than stage alone. However, we did not found any additive effect based on TIMP3 methylation status in non-muscle invasive tumors (Figure 1C). A further increase in sample size is needed to explore the feasibility of urine TIMP3 methylation as a clinical tool for non-muscle invasive tumors. Furthermore, perhaps other yet to be identified prognostic factors could be combined with TIMP3 methylation status to better predict patient prognosis. A combination of several methylation markers was reported to be significantly correlated with patient prognosis in esophageal and prostate cancer 14, 15. However, in this study TIMP-3 methylation was found to be an independent prognostic factor as a single DNA marker. We anticipate that some samples could fail clinically, however in our cohort of samples we able to get sufficient amount of DNA from 50 ml of urine samples for our QMSP assay. Another potential problem is whether promoter methylation in urine sediment DNA reflected the methylation status of primary tumor DNA. We previously measured the levels of promoter methylation for several genes including TIMP3 in paired primary bladder tumor and urine sediment DNA samples 12. In paired samples, methylation in urine sediment DNA was always accompanied by methylation of tumor DNA, whereas methylation of tumor DNA was not always accompanied by methylation of urine sediment DNA. We detected no aberrant methylation (i.e., hypermethylation) in the urine of bladder cancer patients who did not also have aberrant methylation of the same promoter in the corresponding tumor sample.
Specific genetic and epigenetic abnormalities will lead to the identification of patient subgroups benefiting from certain therapies. The use of genetic and epigenetic markers for cancer diagnosis and management will become increasingly important for patient care. After radical cystectomy for clinically localized bladder cancer the risk of recurrence and death is still high. The recurrence is predominantly due to distant metastases from occult micrometastases present at the time of the radical cystectomy. For this reason, there is growing interest in combining systemic chemotherapy in an attempt to eradicate these occult metastases and as a result improving survival rates. This approach was shown to be effective in breast and colon cancer and recently the results of a large randomized trial demonstrated improvement in survival for patients receiving neo-adjuvant chemotherapy for locally advanced bladder cancer 16. Systemic treatment is offered today to high-risk patients based predominantly on stage of disease. Our study suggests that the methylation status of TIMP-3 might provide additional information to the clinician beyond stage or metastasis for the risk assessment of patients following cystectomy. The tissue inhibitors of metalloproteinases (TIMPs) control the activities of matrix metalloproteinases (MMPs) and as such, they have been recognized as potential suppressors of angiogenesis and tumor invasion and metastasis. MMP inhibitors have been tried as therapeutic agents. Recent data suggest that high levels of TIMP-3 mRNAs in human breast tumors are predictor of response to tamoxifen therapy, Kaplan-Meier analysis showed a significant association between high expression of TIMP-3 and longer relapse free survival(RFS) 17. Bachman et al 18 have demonstrated a correlation between promoter methylation and reduction of TIMP-3 expression by IHC in cell lines of various cancers(lung, breast and colon) and in vivo in renal cell carcinoma. Thus, our TIMP3 methylation data in urine suggest that promoter methylation well lead to low expression in primary bladder tumors.
Determining the functional relevance of TIMP-3 with respect to tumor’s metastatic ability is crucial to legitimizing its use as a prognostic marker. There is increasing evidence to suggest that TIMP-3 protects against tumor development by suppressing tumor growth, metastasis and angiogenesis, and inducing apoptosis 6, 7. TIMP-3 is the only member of the TIMPs family that associate directly with the ECM (extracellular matrix) through interaction with heparin sulphate 19. In addition, TIMP-3 shows a selective ability to inhibit several ADAMS (adamalysin metalloproteinase) as well as MMPs. Its anti-tumor activity further strengthen by the recently reported anti-angiogenic action via blockade of the binding of vascular endothelial growth factor (VEGF) to its signaling receptor, VEGFR2 20.
Longer follow-up, still in progress for the current cohort will clarify the relevance of these and other markers for survival and other clinical outcomes. Testing for relevant epigenetic markers in voided urine holds promise for detection 14 and more individualized therapeutic strategies. Restoring the function of TIMP-3, when lost, might be a useful intervention for gene therapy of bladder cancer, including treatment by demethylating agents.
Acknowledgments
This work was supported by National Cancer Institute Grant U01-CA84986 & Oncomethylome Sciences, SA
The abbreviations used are
- TCC
Transitional cell carcinoma
- PCR
Polymerase Chain Reaction
- TUR
Transurethral Resection
- QMSP
Quantitative Methylation Specific Polymerase Chain Reaction
Footnotes
Under a licensing agreement between Oncomethylome Sciences, SA and the Johns Hopkins University, D. Sidransky is entitled to a share of royalty received by the University upon sales of products described in this article. D. Sidransky owns Oncomethylome Sciences, SA stock, which is subject to certain restrictions under University policy. Dr. Sidransky is a paid consultant to Oncomethylome Sciences, SA and is a paid member of the company’s Scientific Advisory Board. The Johns Hopkins University in accordance with its conflict of interest policies is managing the terms of this agreement. Dr. Hoque is supported by a Young Clinical Scientist Award from the Flight Attendant Medical Research Institute and the Young Investigator Award from the International Association for the Study of Lung Cancer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Grossfeld GD, Ginsberg DA, Esrig D, Freeman JA, Figueroa AJ, et al. Prognostic markers in bladder cancer: a contemporary review of the literature. J Urol. 1998;160:645. doi: 10.1016/S0022-5347(01)62747-2. [DOI] [PubMed] [Google Scholar]
- 3.Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2:210. doi: 10.1038/nrc755. [DOI] [PubMed] [Google Scholar]
- 4.Jones PA. DNA methylation and cancer. Oncogene. 2002;21:5358. doi: 10.1038/sj.onc.1205597. [DOI] [PubMed] [Google Scholar]
- 5.Airola K, Ahonen M, Johansson N, Heikkila P, Kere J, Kahari VM, et al. Human TIMP-3 is expressed during fetal development, hair growth cycle, and cancer progression. J Histochem Cytochem. 1998;46:437. doi: 10.1177/002215549804600403. [DOI] [PubMed] [Google Scholar]
- 6.Ahonen M, Baker AH, Kahari VM. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res. 1998;58:2310. [PubMed] [Google Scholar]
- 7.Bian J, Wang Y, Smith MR, Kim H, Jacobs C, Jackman J, et al. Suppression of in vivo tumor growth and induction of suspension cell death by tissue inhibitor of metalloproteinases (TIMP)-3. Carcinogenesis. 1996;17:1805. doi: 10.1093/carcin/17.9.1805. [DOI] [PubMed] [Google Scholar]
- 8.Powe DG, Brough JL, Carter GI, Bailey EM, Stetler-Stevenson WG, Turner DR, et al. TIMP-3 mRNA expression is regionally increased in moderately and poorly differentiated colorectal adenocarcinoma. Br J Cancer. 1997;75:1678. doi: 10.1038/bjc.1997.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoque MO, Lee CC, Cairns P, Schoenberg M, Sidransky D. Genome-wide genetic characterization of bladder cancer: a comparison of high-density single-nucleotide polymorphism arrays and PCR-based microsatellite analysis. Cancer Res. 2003;63:2216. [PubMed] [Google Scholar]
- 10.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harden SV, Tokumaru Y, Westra WH, Goodman S, Ahrendt SA, Yang SC, et al. Gene promoter hypermethylation in tumors and lymph nodes of stage I lung cancer patients. Clin Cancer Res. 2003;9:1370. [PubMed] [Google Scholar]
- 12.Hoque MO, Begum S, Topaloglu O, Chatterjee A, Rosenbaum E, Van Criekinge W, et al. Quantitation of promoter methylation of multiple genes in urine DNA and bladder cancer detection. J Natl Cancer Inst. 2006;98:996. doi: 10.1093/jnci/djj265. [DOI] [PubMed] [Google Scholar]
- 13.Hoque MO, Lee J, Begum S, Yamashita K, Engles JM, Schoenberg M, et al. High-throughput molecular analysis of urine sediment for the detection of bladder cancer by high-density single-nucleotide polymorphism array. Cancer Res. 2003;63:5723. [PubMed] [Google Scholar]
- 14.Brock MV, Gou M, Akiyama Y, Muller A, Wu TT, Montgomery E, et al. Prognostic importance of promoter hypermethylation of multiple genes in esophageal adenocarcinoma. Clin Cancer Res. 2003;9:2912. [PubMed] [Google Scholar]
- 15.Hoque MO, Begum S, Sommer M, Lee T, Trink B, Ratovitski E, et al. PUMA in head and neck cancer. Cancer Lett. 2003;199:75. doi: 10.1016/s0304-3835(03)00344-6. [DOI] [PubMed] [Google Scholar]
- 16.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 17.Span PN, Lindberg RL, Manders P, Tjan-Heijnen VC, Heuvel JJ, Beex LV, et al. Tissue inhibitors of metalloproteinase expression in human breast cancer: TIMP-3 is associated with adjuvant endocrine therapy success. J Pathol. 2004;202:395. doi: 10.1002/path.1528. [DOI] [PubMed] [Google Scholar]
- 18.Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, et al. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res. 1999;59:798. [PubMed] [Google Scholar]
- 19.Yu WH, Yu S, Meng Q, Brew K, Woessner JF. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- 20.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]