Abstract
Objectives
To (1) describe the relationship between symptom scores and mobility function measures, (2) assess whether symptom scores and disease scores are similarly associated with mobility function, and (3) identify clusters of symptoms that are most strongly associated with functional status in older adults
Design
Secondary analysis of cross-sectional data from three cohorts
Setting
Academic medical center
Participants
195 community-dwelling subjects with poor flexibility or cardiorespiratory fitness (fitness cohort), 211 female retirement community residents with vertebral fractures (VF cohort), and 61 subjects with Parkinson's disease (PD cohort)
Measurements
20-item self-reported symptom scale, 17-item self-reported disease scale, Short Form 36 (SF-36) Physical Functioning Scale, 5-item Nagi Disability scale, 10-meter walk time, supine to stand time
Results
Symptom scores correlated with mobility function measures (Spearman correlation coefficients range from 0.222 to 0.509) at least as strongly as, if not more strongly than did disease scores. Symptom scores remained associated with functional outcomes after controlling for disease score and demographic variables. Adding symptom scores to models that contained disease scores significantly increased the association with functional outcomes. In the fitness cohort, muscle weakness was the most explanatory single symptom, associated with an average decrease of 17.8 points on the Physical Functioning Scale. A model that included only muscle weakness, pain, and shortness of breath accounted for 21.2% of the variability in the Physical Functioning Score.
Conclusion
Symptoms represent useful indicators of disability burden in older adults and are promising targets for interventions to improve function in complex patients.
Keywords: symptom, function, disability, comorbidity
Introduction
As the population ages, the number of individuals living with multiple, co-existing conditions (comorbidity) continues to rise, with the prevalence of comorbidity in the United States expected to reach 81 million by 20201. Comorbidity heightens the risk of healthcare utilization, mortality, and disability – over and above the risk from individual diseases1-5. One challenge that clinicians face in addressing the problem of comorbidity is that the role of independent diseases – much less combinations of diseases – in the disablement process is not fully understood. Previous work has suggested that disability typically results from multiple pathological insults, but most patients identify a single symptom which causes their inability to perform a task6-8. For decades, the Nagi model of disability has provided a conceptual framework that distinguishes pathological insults (such as disease) from impairments (or symptoms) in the disablement process9, 10. In this model, symptoms are a means by which disease produces functional limitations. Because they are common across multiple diseases and are correlated to disease, symptoms may represent clinically relevant and easily accessible indicators of disability burden.
Thus, it is important to understand how symptoms are correlated with function and whether specific clusters of symptoms are associated with poor function. This knowledge could provide a rationale for the development of symptom-based screening tools to assess disability risk and might suggest particular symptoms (or groups of symptoms) to target for intervention. Moreover, it could support a paradigm shift from a disease-based to a symptom-based approach in comorbidity research as well as clinical care of complex patients. The primary aim of this study was to describe the correlation between symptom scores and mobility function scales in three cohorts of older adults with high rates of comorbidity. An additional aim was to assess whether symptom scores are associated as strongly with mobility function as are disease scores. A third aim of this study was to conduct an exploratory analysis to identify symptoms that are particularly associated with poor function among older adults.
Methods
Participants
Data were obtained from baseline assessments of participants enrolled in three randomized controlled trials developed concurrently and conducted from 1992-1997 11-13. The participants in each trial received similar baseline assessment batteries. The original studies investigated physical activity interventions in groups of older adults at risk for functional decline. The present analysis uses baseline data, collected prior to randomization and intervention. The first cohort, hereafter referred to as the fitness cohort, consists of 195 community-dwelling older men and women with impaired spinal flexibility (combined left and right axial rotation < 120 degrees) or impaired cardio-respiratory fitness (determined by peak oxygen uptake)11. The second cohort, hereafter referred to as the VF cohort, includes 211 female residents of continuing care retirement communities with prevalent vertebral fractures by radiograph13. The third cohort, hereafter referred to as the PD cohort, includes 61 men and women with early- to mid-stage Parkinson's Disease (stages 2 and 3 of Hoehn and Yahr14)12. All studies were approved by the Institutional Review Boards of Duke University Medical Center and/or the Durham VA Medical Center.
Symptom and Disease Scores
The symptom and disease scores were derived from participant responses to interviewer-administered questionnaires. The 17-item disease index included the following questions “Since you were 55, has a doctor ever told you that you have or had [angina, congestive heart failure, heart attack, stroke, Parkinson's Disease, lung disease/emphysema/asthma/bronchitis, arthritis, osteoporosis, broken bones, depression/anxiety/emotional problems, sleep problems, chronic pain syndrome, cancer, diabetes, glaucoma, cataracts, amputation]?” The number of conditions endorsed was summed so that possible disease scores range from 0 to 17. The 20-item symptom index included the following questions, “Within the last month, have you had [chest pain or pressure at rest, chest pain or pressure with exertion, shortness of breath at rest, shortness of breath with exertion, muscle weakness, feeling tired much of the time, dizziness or light-headedness, nausea, shakiness or trembling, balance problems, feeling sad/depressed/blue, anxiety/worry/tension, problems with your eyesight, pain, muscle cramps, numbness/tingling, incontinence, confused thinking, memory loss, difficulty sleeping at night]?” Possible scores on the symptom index range from 0 to 20.
Mobility Function Measures
Mobility function was assessed with two self-reported scales: 1) the Medical Outcomes Study Short Form 36 (SF-36) Physical Functioning Scale15 and 2) the 5-item Nagi Disability Scale16. The SF-36 Physical Functioning Scale is derived from 10 items of the SF-36 which assess whether the participant's health limits ability to perform vigorous activities such as running, moderate activities such as golf or vacuuming, lifting or carrying groceries, climbing several sets of stairs, climbing one set of stairs, bending/kneeling/stooping, walking more than a mile, walking several blocks, walking one block, and bathing. Each item is scored according to whether health limits the function not at all (10 points), a little (5 points), or a lot (0 points). Possible scores range from 0 to 100 with higher scores indicating better function. The Nagi Disability Scale assesses whether the participant is able to independently perform the following tasks: pulling or pushing large objects, stooping/crouching/kneeling, lifting or carrying weights over 10 pounds, reaching or extending arms above shoulder level, and writing or handling small objects. Possible scores range from 0 to 5 with higher scores indicating more disability.
Mobility function was also assessed with performance measures recorded using standardized protocols. To determine supine-to-stand time, subjects were instructed to move at their normal pace from a supine position on a low treatment table to standing. For 10-meter walk time, participants were instructed to walk at a “comfortable pace” across a 10-meter distance marked on the floor. A stop watch was used to record the times. One practice and two test trials were conducted for each variable, and the values of the test trials were averaged.
Statistical Analysis
All analyses were performed with SAS V.9.1 and SAS EGuide V 4.0 (SAS Institute, Inc., Cary, NC). Descriptive statistics were used to characterize each cohort at baseline. Significant skew existed among the functional outcome data; thus, Spearman correlation coefficients are reported for all correlation analyses. The three cohorts represent different study populations with different average values for the variables of interest. Therefore, correlations were assessed separately in each cohort (rather than combining data from the three populations). Heterogeneity testing with Cochrane's Q statistic suggested sufficient homogeneity in the correlations across cohorts to pursue a fixed effects meta-analytic technique to obtain an overall effect for each pair of variables examined 17. Fisher's Z transformation statistics were used in the meta analytic model and the overall effect was then converted back to a Spearman correlation18-20.
A second aim of this analysis was to test whether symptom and disease scores were similarly associated with mobility function. Therefore, for each of the four mobility function variables, the following null hypothesis (H0) was tested: ρ(mobility function, symptom score)=ρ(mobility function, disease score). To test this null hypotheses, Z statistics were calculated as described by Kleinbaum and colleagues 20. The Z statistics were first calculated in each cohort and then combined using the Inverse Normal Method21. To further compare the association between symptom scores and mobility function and disease scores and mobility function, multivariable models were constructed that included symptom score and/or disease score, age, sex, education (years in school), and study population as predictors of the mobility function outcomes. Ten meter walk time, supine to stand time, and Physical Functioning score were modeled with linear regression; Nagi Disability scale score was modeled with Poisson regression.
A third aim of the study was to explore which particular symptoms are most strongly associated with function. In this exploratory analysis, linear regression models were constructed using data from the fitness cohort. We restricted this analysis to the fitness cohort because it includes both sexes and is not defined by an index disease (such as osteoporosis or Parkinson's) and was thus more representative of a general population of older adults at risk for functional decline. The models included individual symptoms as independent variables and SF-36 Physical Functioning score as the dependent variable. The SF-36 Physical Functioning score was chosen because it was the most strongly correlated to symptom scores and the data included a large range of values on this 100-point scale. In the exploratory analyses, all possible combinations of the 20 symptoms were considered to determine the R2 and adjusted R2 of each model. The R2 was used to determine the most explanatory 1-symptom model, the most explanatory 2-symptom model, most explanatory 3-symptom model, and so on. Adjusted R2's were compared to determine the point at which adding an additional variable to a model does not produce a significant, independent increase in explanatory power.
Results
The baseline characteristics of each cohort are summarized in Table 1. Although the demographics of these three older cohorts differ, a high prevalence of comorbidity (as determined by self-reported diseases) was common to each group. Some predictable discrepancies were noted in the frequency of individual symptoms; for example, the Parkinson's cohort was most likely to report shakiness and trembling, whereas pain was most often reported in the vertebral fracture cohort.
Table 1.
Characteristics of Cohort Participants
| Characteristic | Fitness Cohort N=195 |
Vertebral Fractures Cohort N=211 |
Parkinson's Disease Cohort N=61 |
|
|---|---|---|---|---|
| Age in years, mean ± SD | 71.4±5.0 | 80.8±5.5 | 70.9±7.4 | |
| Sex, % Female | 66.1 | 100 | 25.4 | |
| Race (Self-report) | ||||
| % White | 75.4 | 96.8 | 98.3 | |
| % Black | 24.6 | 0 | 1.7 | |
| % Native American | 0 | 3.2 | 0 | |
| Education | ||||
| % High school or less | 54.9 | 35.4 | 33.9 | |
| %College/Associate's | 28.7 | 42.6 | 28.8 | |
| % Graduate Degree | 16.4 | 22.0 | 37.3 | |
| Work status | ||||
| % retired | 76.4 | 89.6 | 79.7 | |
| % working | 6.7 | 0.5 | 13.6 | |
| % unemployed or homemaking |
16.9 | 9.9 | 6.7 | |
| Comorbidity, % with = ≥2 diagnoses |
61.0 | 95.3 | 91.8 | |
| Symptoms (in past month) | ||||
| Chest pain or pressure with exertion |
12.4 | 10.9 | 13.1 | |
| Shortness of breath at rest |
7.2 | 10.4 | 3.3 | |
| Shortness of breath with exertion |
50.0 | 53.6 | 50.8 | |
| Muscle weakness | 22.6 | 45.5 | 51.7 | |
| Feeling tired much of time |
29.7 | 49.8 | 59.0 | |
| Dizziness or lightheaded |
21.0 | 20.9 | 32.8 | |
| Nausea | 5.1 | 13.7 | 11.5 | |
| Shakiness or trembling | 10.3 | 17.5 | 83.6 | |
| Balance problems | 19.5 | 49.8 | 52.4 | |
| Feeling sad, depressed, or blue |
19.5 | 33.2 | 31.1 | |
| Anxiety, worry, or tension |
31.8 | 45.7 | 42.6 | |
| Problems with eyesight | 25.6 | 43.1 | 36.1 | |
| Pain | 44.6 | 64.9 | 42.6 | |
| Muscle cramps | 36.9 | 44.6 | 41.0 | |
| Numbness or tingling anywhere |
25.1 | 30.3 | 36.1 | |
| Incontinence | 22.1 | 35.6 | 24.6 | |
| Confused thinking | 8.2 | 23.2 | 26.2 | |
| Memory loss | 34.4 | 54.0 | 57.4 | |
| Difficulty sleeping at night |
32.3 | 38.4 | 27.9 | |
| Symptom Score | ||||
| Mean ± SD | 4.6 ± 3.6 | 6.9 ± 3.9 | 7.3 ± 3.3 | |
| Range of scores (maximum 20) |
0 to 20 | 0 to 18 | 0 to 14 | |
| Diseases (since age 55) | ||||
| Angina | 8.7 | 11.4 | 13.1 | |
| Congestive heart failure |
1.5 | 4.7 | 3.3 | |
| Heart attack | 7.7 | 5.7 | 9.8 | |
| Stroke | 5.1 | 6.7 | 4.9 | |
| Parkinson's Disease | 1.0 | 1.4 | 98.4 | |
| Lung disease | 14.9 | 15.2 | 11.5 | |
| Arthritis | 48.7 | 71.0 | 45.9 | |
| Osteoporosis | 9.3 | 61.0 | 3.3 | |
| Broken bone(s) | 22.6 | 61.1 | 14.8 | |
| Depression/anxiety | 14.4 | 21.8 | 27.9 | |
| Sleep problems (insomnia, narcolepsy) |
10.8 | 12.8 | 24.6 | |
| Chronic pain syndrome | 4.1 | 6.6 | 3.3 | |
| Cancer | 19.0 | 26.1 | 21.3 | |
| Diabetes | 12.3 | 0.5 | 8.2 | |
| Glaucoma | 8.7 | 15.2 | 6.6 | |
| Cataracts | 37.4 | 74.9 | 37.7 | |
| Amputation | 1.5 | 0.5 | 3.3 | |
| Disease Score | ||||
| Mean ± SD | 2.3 ± 1.7 | 4.0 ± 1.7 | 3.4 ± 1.5 | |
| Range of scores (maximum 17) |
0 to 7 | 0 to 10 | 1 to 8 | |
| Physical Function Scale, Median (IQR) |
80 (65 to 90) | 65 (40 to 80) | 60 (45 to 85) | |
| Nagi Disability Scale Median (IQR) |
2 (1 to 3) | 3 (2 to 4) | 3 (2 to 5) | |
| Ten Meter Walk Time, Mean ± SD (seconds) |
8.6 (7.9 to 9.7) | 10.1 (8.7 to 11.5) | 9.6 (8.0 to 11.0) | |
| Supine to Stand Time Mean ± SD (seconds) |
4.7 (4.0 to 5.8) | 4.7 (3.7 to 6.5) | 5.6 (4.5 to 8.8) | |
SD = standard deviation, IQR = inter-quartile range
Moderate correlation existed between self-reported symptom scores and disease scores in the fitness cohort (Spearman CC 0.435, p<0.0001) and VF cohorts (Spearman CC 0.330, p<0.0001), but symptom and disease scores were not significantly correlated in the PD cohort (Spearman CC 0.203, p=0.12). Symptom scores correlated with functional measures, as did disease scores. In general, the correlation between symptom scores and functional status measures was stronger than the correlation between disease scores and functional status measures (Figure 1). The difference was statistically significant only for supine to stand time (p = 0.01); the difference was borderline significant for Nagi Disability Scale (p = 0.07) and Physical Functioning Scale (p = 0.10).
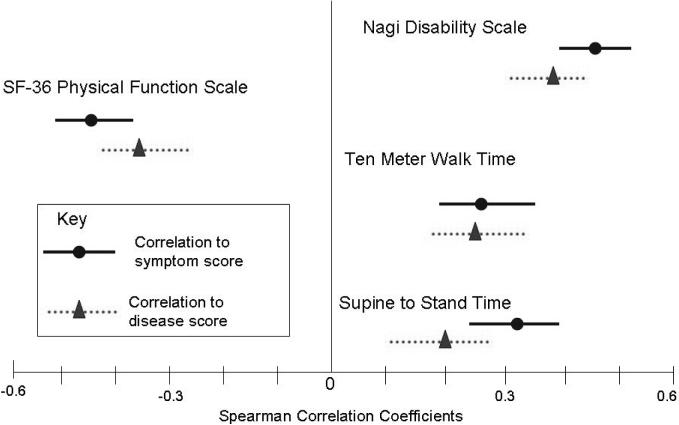
Figure 1. Correlation of mobility function measures to symptom scores and disease scores.
This figure provides a graphical comparison of the strength of correlation between measures of mobility function and symptom scores verses measures of mobility function and disease scores. The center line indicates a correlation coefficient of zero; points further from the center line indicate stronger correlation between two variables. Correlations between mobility function measures and symptom scores are represented by circles (Spearman correlation coefficient [CC]) and solid lines (95% confidence intervals); correlations between mobility function measures and disease scores are represented by triangles (Spearman CC) and dotted lines (95% confidence intervals). The Spearman CC for Nagi Disability Scale to symptom score is 0.435 (0.355 to 0.509) and the Spearman CC for Nagi Disability Scale to disease score is 0.349 (0.262 to 0.429). The Spearman CC for Ten Meter Walk time to symptom score is 0.273 (0.186 to 0.356) and the Spearman CC for Ten Meter Walk time to disease score is 0.260 (0.173 to 0.344) The Spearman CC for Supine to Stand time to symptom score is 0.311 (0.225 to 0.392) and the Spearman CC for Supine to Stand time to disease score is 0.201 (0.111 to 0.288) The Spearman CC for SF-36 Physical Functioning Scale to symptom score is −0.442 (−0.512 to −0.365) and the Spearman CC for SF-36 Physical Functioning Scale to disease score is −0.349 (−0.427 to −0.266). NB: Higher scores on the SF-36 Physical Functioning Scale indicate higher functioning, whereas higher scores on the other three mobility function measures indicate lower functioning.
In multivariable regression models, symptom score remained a significantly associated with mobility function outcomes after inclusion of disease score, age, sex, study group, and education (Table 2). Models that include symptom score (but not disease score) explain more variability in the outcomes than do models that contain disease score (but not symptom score). When full models containing symptom score, disease score and covariates are compared to reduced models containing only disease score and covariates, the full models are significantly better at explaining the mobility function outcomes (p<0.001, data not shown).
Table 2.
Symptom scores and disease scores as predictors of mobility function outcomes in multivariable models
| Ten Meter Walk Time |
Supine to Stand Time |
Physical Functioning Scale |
Nagi Disability Scale |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent Variables | Estimate (95% CI) |
P value |
Estimate (95% CI) |
P value |
Estimate (95% CI) |
P value |
Estimate (95% CI) |
P value |
|
| Model 1 | |||||||||
| Disease score | 0.240 (0.097 to 0.382) |
0.001 | 0.331 (0.134 to 0.527) |
0.001 | −4.166 (−5.355 to −2.977) |
<0.001 | 0.107 (0.072 to 0.142) |
<0.001 | |
| Model 2 | |||||||||
| Symptom Score | 0.150 (0.084 to 0.216) |
<0.001 | 0.223 (0.132 to 0.314) |
<0.001 | −2.559 (−3.096 to −2.023) |
<0.001 | 0.063 (0.047 to 0.079) |
<0.001 | |
| Model 3 | |||||||||
| Disease Score | 0.146 (−0.004 to 0.296) |
0.06 | 0.189 (−0.017 to 0.395) |
0.07 | −2.599 (−3.794 to −1.404) |
<0.001 | 0.068 (0.032 to 0.105) |
<0.001 | |
| Symptom Score | 0.126 (0.056 to 0.196) |
<0.001 | 0.193 (0.096 to 0.289) |
<0.001 | −2.141 (−2.701 to −1.580) |
<0.001 | 0.052 (0.035 to 0.069) |
<0.001 | |
| Model R2 | Model R2 | Model R2 | Log Likelihood | ||||||
| Model 1 | 0.246 | 0.077 | 0.229 | −48.6 | |||||
| Model 2 | 0.261 | 0.102 | 0.287 | −37.3 | |||||
| Model 3 | 0.267 | 0.108 | 0.315 | −30.7 | |||||
CI = confidence interval
Model 1 – independent variables include disease score, age, sex, study group, education
Model 2 - independent variables include symptom score, age, sex, study group, education
Model 3 - independent variables include disease score, symptom score, age, sex, study group, education
Linear regression was used to model 10 meter walk time, supine to stand time, and score on the Physical functioning scale; Poisson regression was used to model score on the Nagi Disability scale.
Finally, exploratory analyses were conducted using data from the fitness cohort to identify particular symptoms that were most strongly associated with functional status as determined by the 100-point SF-36 Physical Functioning score. The full model, containing all 20 symptoms, accounts for 28.7% of the variability in the outcome. The most explanatory single symptom was muscle weakness, which accounted for 13.3% of the variability in the SF-36 Physical Functioning Scale. The presence of muscle weakness was associated with, on average, a 17.8 point decrease in the 100-point SF-36 Physical Functioning Scale. Even after controlling for age, race, and sex, muscle weakness remained associated with a 17.0 point decrease in the SF-36 Physical Functioning Scale. The most explanatory 2-symptom model included muscle weakness and pain, which accounted for 19.2% of the variability in the outcome. The most explanatory 3-symptom model included muscle weakness, pain, and shortness of breath at rest and accounted for 21.2% of the variability in the outcome. Pain and shortness of breath at rest were associated with average Physical Functioning score decreases of 13.9 and 20.8, respectively. The highest adjusted R2 was achieved with an 11-symptom model which included muscle weakness, pain, shortness of breath at rest, shortness of breath with exertion, chest pain at rest, balance problems, dizziness, shakiness or trembling, pain, numbness or tingling, nausea, and anxiety.
Discussion
In three cohorts of older adults with high rates of comorbid diagnoses, the correlation between symptoms and mobility function measures was at least as strong as, if not stronger than the correlation between self-reported diseases and mobility function measures. In multivariable analyses that controlled for disease scores, symptom scores remained associated with mobility function. The inclusion of symptom scores in these models added significant explanatory value over and above the contribution from disease scores alone. Furthermore, preliminary data presented here suggest that a relatively short list of symptoms contribute importantly to the disablement process.
These findings are clinically useful because a symptom-oriented approach has some practical advantages over a disease-oriented approach to maximizing function in older adults with significant comorbidity. Symptom scores may be useful in identifying medically complicated older adults who are at highest risk for adverse functional outcomes. Symptoms provide easily accessible information that can be obtained reliably through self-report. By contrast, older adults may be unaware of some of their co-existing diseases, either because a disease has not been formally diagnosed or because the patient is not aware of the diagnosis. This is evidenced by the fact that 39% of the women in the vertebral fractures cohort did not endorse osteoporosis as a diagnosis.
Even when accurate information on underlying disease is available, symptoms may represent the best target for interventions to improve function in patients with comorbidity. First, chronic diseases are often less modifiable than the symptoms they produce. Second, whereas the list of potential disease combinations is overwhelming, the list of disabling symptoms is more manageable. Thus, one promising strategy to maximize function in patients with comorbidity is to focus on pairs and clusters of particularly disabling symptoms, rather than combinations of diseases.
One important goal, therefore, is to understand which particular symptoms -- or combinations of symptoms -- are most strongly associated with poor function. Exploratory analyses presented here suggest that, of 20 symptoms considered, muscle weakness, pain, and shortness of breath were especially important contributors to poor functional status. The average decreases in SF-36 Physical Functioning score associated with muscle weakness, pain, and shortness of breath at rest were impressive: 17.8, 13.9, and 20.8 points, respectively. In comparison, the average decreases in SF-36 Physical Functioning score associated with congestive heart failure, chronic lung disease, and arthritis are 9.8, 9.4, and 7.4, repsectively22. A change in the SF-36 Physical Functioning score of 6.5 points is considered clinically significant and correlates with health outcomes and mortality23.
The finding that muscle weakness, pain, and shortness of breath are particularly important contributors to disability is consistent with previous work. Over 90% of participants in the Women's Health and Aging Study (WHAS) with activities of daily living (ADL) disability attributed their disability to weakness, pain, endurance problems (defined as shortness of breath or fatigue), and balance problems6. The etiology of these symptoms is often multi-factorial in older patients, arising from more than a single condition. It is possible that understanding important symptom interfaces will yield targeted treatments and management strategies to improve function in complex patients.
One interesting observation was that symptoms and diseases correlated better to self-reported measures of function (Nagi Disability Scale and SF-36 Physical Function scale) than to performance measures. One potential explanation is that a subjective reporting bias existed, such that those participants who were more likely to endorse the presence of many symptoms (or diseases) were also more likely to report difficulty with functional tasks. However, previous groups have found that self-reported function generally correlates well to similar measured functions24. An alternate explanation is that the performance measures assess discrete functions (speed of walking or standing), whereas the scales provide a more comprehensive assessment of functional capacity and are therefore more likely to reflect differences produced by a wide range of symptoms.
Several limitations of these analyses should be noted. First, the data were obtained from baseline assessments in trials which assessed exercise interventions; thus, the participants are likely to be healthier than many older adults with comorbidity. For example, subjects with active cardiac disease were excluded from the fitness and VF trials, so the functional impact of chest pain may be under-represented. Second, although the primary correlations all achieved statistical significance, it is important to note that the magnitude of correlation with symptom or disease scores was moderate for functional scales (correlation coefficients 0.3 to 0.5) and small for performance measures (correlation coefficients generally <0.3). Third, the symptom and disease indices have not been tested for reliability and validity and due to the inherent overlap in the domains of diseases and symptoms, the indices contain several redundant items. Fourth, to examine which symptoms and symptom combinations were particularly associated with functional status, an exploratory analysis was conducted with multiple comparisons. Given the paucity of data on this subject, an exploratory approach to generate hypotheses is reasonable, but the findings require confirmation.
The study makes several important contributions. It highlights the importance of the accumulation of symptoms in the disablement process by demonstrating in three distinct populations of older adults that a count of symptoms correlated with mobility function at least as strongly as, if not more strongly than a count of diseases. The findings support the hypothesis that symptoms are a promising target for interventions aimed at modifying the pathway that leads from comorbidity to disability. Finally, in an exploratory analysis, a relatively small group of symptoms were identified that contributed importantly to functional status in a group of older adults at risk for functional decline. Future efforts should seek to further describe the functional impact of these particular symptoms and symptom clusters as well as the potential benefits of therapies that target these symptoms in older adults with comorbidity.
Acknowledgments
Sponsor's Role This work was supported by the Duke Claude D. Pepper Older American Independence Center (5P30AG028716-02) and the John A. Hartford Foundation. The sponsors did not have a role in the design or conduct of the study, analysis of data, or preparation of the manuscript.
Additional Contributions We are grateful to Dr. Margaret Schenkman, now of the University of Colorado Health Sciences Center, who was the principal investigator for the Parkinson's Disease study.
Funding
The work was supported by the Duke Claude D. Pepper Older American Independence Center (5P30AG028716-02) and the John A. Hartford Foundation
Footnotes
Meeting
Some results were presented at the Gerontological Society of America (GSA) meeting, Late-breaker Poster session, San Francisco, CA, November 17, 2007
Conflicts of interest: The authors have no conflicts of interest to report.
References
- 1.Anderson G, H J. Chronic Conditions: Making the case for ongoing care. September 2004 update. Robert Wood Johnson Foundation's Partnership for Solutions; Princeton, NJ: 2004. [Google Scholar]
- 2.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 3.Fillenbaum GG, Pieper CF, Cohen HJ, et al. Comorbidity of five chronic health conditions in elderly community residents: determinants and impact on mortality. J Gerontol A Biol Sci Med Sci. 2000;55:M84–89. doi: 10.1093/gerona/55.2.m84. [DOI] [PubMed] [Google Scholar]
- 4.Ettinger WH, Davis MA, Neuhaus JM, Mallon KP. Long-term physical functioning in persons with knee osteoarthritis from NHANES. I: Effects of comorbid medical conditions. J Clin Epidemiol. 1994;47:809–815. doi: 10.1016/0895-4356(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 5.Verbrugge LM, Lepkowski JM, Imanaka Y. Comorbidity and its impact on disability. Milbank Q. 1989;67:450–484. [PubMed] [Google Scholar]
- 6.Leveille SG, Fried LP, McMullen W, Guralnik JM. Advancing the taxonomy of disability in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:86–93. doi: 10.1093/gerona/59.1.m86. [DOI] [PubMed] [Google Scholar]
- 7.Leveille SG, Fried L, Guralnik JM. Disabling symptoms: what do older women report? J Gen Intern Med. 2002;17:766–773. doi: 10.1046/j.1525-1497.2002.20229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ettinger WH, Jr., Fried LP, Harris T, et al. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. CHS Collaborative Research Group. J Am Geriatr Soc. 1994;42:1035–1044. doi: 10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 9.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–467. [PubMed] [Google Scholar]
- 10.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 11.Morey MC, Schenkman M, Studenski SA, et al. Spinal-flexibility-plus-aerobic versus aerobic-only training: effect of a randomized clinical trial on function in at-risk older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M335–342. doi: 10.1093/gerona/54.7.m335. [DOI] [PubMed] [Google Scholar]
- 12.Schenkman M, Cutson TM, Kuchibhatla M, et al. Exercise to improve spinal flexibility and function for people with Parkinson's disease: a randomized, controlled trial. J Am Geriatr Soc. 1998;46:1207–1216. doi: 10.1111/j.1532-5415.1998.tb04535.x. [DOI] [PubMed] [Google Scholar]
- 13.Gold DT, Shipp KM, Pieper CF, et al. Group treatment improves trunk strength and psychological status in older women with vertebral fractures: results of a randomized, clinical trial. J Am Geriatr Soc. 2004;52:1471–1478. doi: 10.1111/j.1532-5415.2004.52409.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 16.Nagi S. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q. 1976;54:439–468. [PubMed] [Google Scholar]
- 17.Cochran W. Problems arising in the analysis of a series of similar experiments. Journal of the Royal Statistical Society. 1937;4:102–118. [Google Scholar]
- 18.Brockwell SE, I G. A comparison of statistical methods for meta-analysis. Statistics in Medicine. 2001;20:825–840. doi: 10.1002/sim.650. [DOI] [PubMed] [Google Scholar]
- 19.Hedges LV, O I. Statistical methods for meta-analysis. Academic Press; New York: 1985. [Google Scholar]
- 20.Kleinbaum DG, K L, Muller KE, Nizam A. Applied Regression Analysis and Other Multivariable Methods. Third edition Duxbury Press; California: 1998. [Google Scholar]
- 21.Stouffer SA, Suchman EA, DeVinney LC, Star SA, Williams RM., Jr . The American soldier: Adjustment during army life. Vol. 1. Princeton University Press; Princeton NJ: 1949. [Google Scholar]
- 22.Alonso J, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res. 2004;13:283–298. doi: 10.1023/b:qure.0000018472.46236.05. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr., Bayliss MS, Rogers WH, et al. Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems. Results from the Medical Outcomes Study. Jama. 1996;276:1039–1047. [PubMed] [Google Scholar]
- 24.Coman L, Richardson J. Relationship between Self-Report and Performance Measures of Function: A Systematic Review. Can J Aging. 2006;25:253–270. doi: 10.1353/cja.2007.0001. [DOI] [PubMed] [Google Scholar]