Abstract
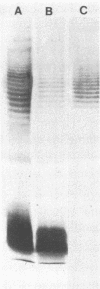
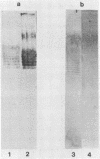
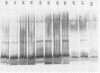
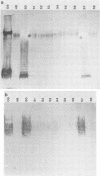
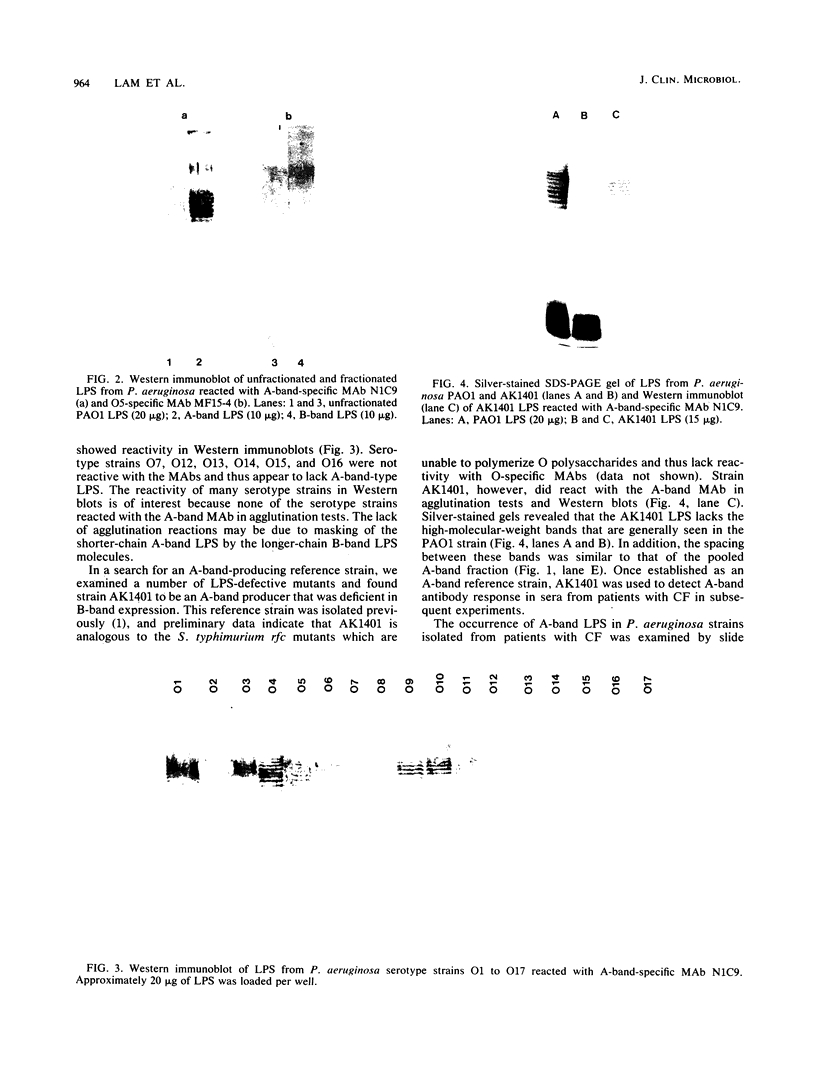
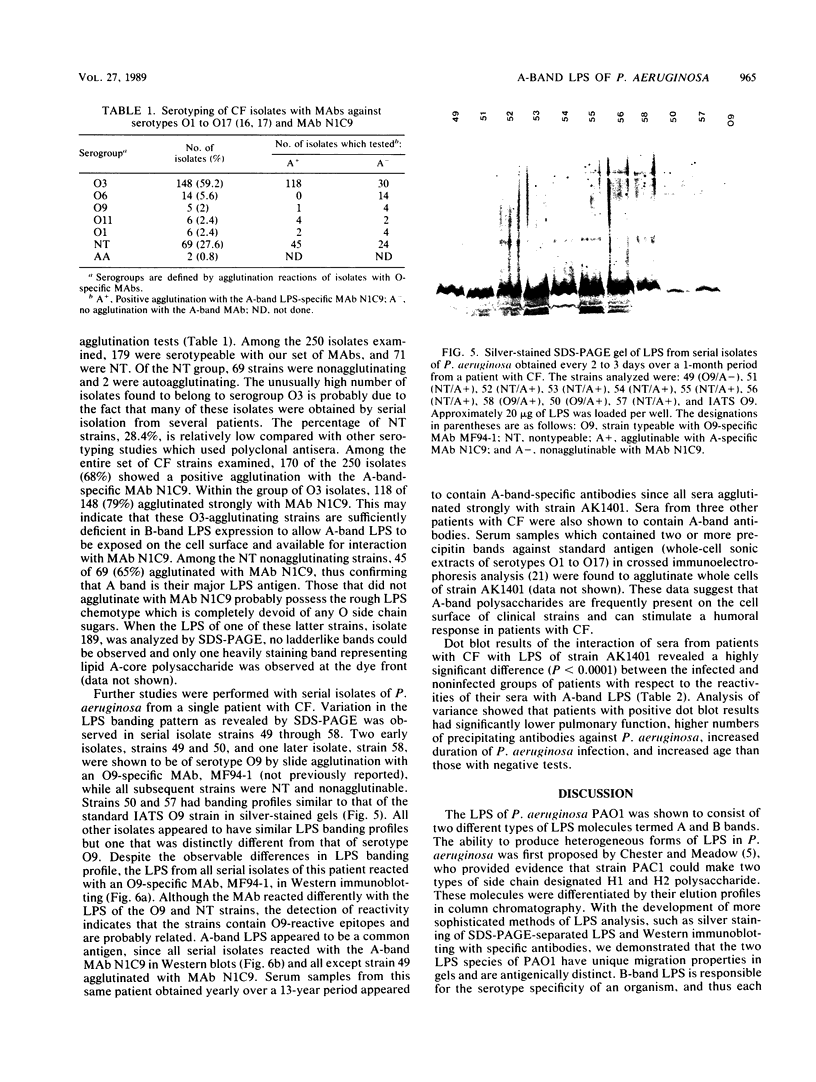
The lipopolysaccharide (LPS) of Pseudomonas aeruginosa PAO1 contains two species of O polysaccharide termed A and B bands. The high-molecular-weight B-band LPS determines the O specificity of the bacterium, while the antigenically distinct A-band LPS consists of only shorter-chain polysaccharides. Seven hybridomas secreting A-band-specific monoclonal antibodies were produced and used to study the LPS of standard and clinical strains. Although A-band antibodies did not agglutinate any of the serotype strains presently in the International Antigenic Typing Scheme, Western immunoblots revealed that 11 of the 17 serotype strains possessed A-band LPS. In a group of 250 clinical isolates from patients with cystic fibrosis, 170 (68%) had A-band LPS on the basis of agglutination tests, but in silver-stained gels all were shown to be deficient in O-antigen-containing B band. Investigation of serial isolates from a single patient revealed a pattern of antigenic variation. During the course of the infection, serotypeable isolates became nontypeable, and the O antigen was replaced with A band as the major LPS antigen. These results suggest that A-band LPS may be the major LPS antigen in nontypeable clinical isolates and a common antigen among other P. aeruginosa strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry D., Kropinski A. M. Effect of lipopolysaccharide mutations and temperature on plasmid transformation efficiency in Pseudomonas aeruginosa. Can J Microbiol. 1986 May;32(5):436–438. doi: 10.1139/m86-082. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carof M., Lebbar S., Szabó L. Detection of 3-deoxy-2-octulosonic acid in thiobarbiturate-negative endotoxins. Carbohydr Res. 1987 Mar 15;161(1):c4–c7. doi: 10.1016/0008-6215(87)84019-3. [DOI] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M. Heterogeneity of the lipopolysaccharide from Pseudomonas aeruginosa. Eur J Biochem. 1975 Oct 15;58(2):273–282. doi: 10.1111/j.1432-1033.1975.tb02373.x. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Pitt T. L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFFMANN F. A new antigen of Salmonella paratyphi B and Salmonella typhi murium. Acta Pathol Microbiol Scand. 1956;39(4):299–304. doi: 10.1111/j.1699-0463.1956.tb03405.x. [DOI] [PubMed] [Google Scholar]
- Koval S. F., Meadow P. M. The isolation and characterization of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa PAC1. J Gen Microbiol. 1977 Feb;98(2):387–398. doi: 10.1099/00221287-98-2-387. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Jewell B., Kuzio J., Milazzo F., Berry D. Structure and functions of Pseudomonas aeruginosa lipopolysaccharide. Antibiot Chemother (1971) 1985;36:58–73. doi: 10.1159/000410472. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Lewis V., Berry D. Effect of growth temperature on the lipids, outer membrane proteins, and lipopolysaccharides of Pseudomonas aeruginosa PAO. J Bacteriol. 1987 May;169(5):1960–1966. doi: 10.1128/jb.169.5.1960-1966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y., Duchesne L. G., Southam G. G. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 May;55(5):1051–1057. doi: 10.1128/iai.55.5.1051-1057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y. Production of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 Nov;55(11):2854–2856. doi: 10.1128/iai.55.11.2854-2856.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeniyi B., Baek L., Høiby N. Polyagglutinability due to loss of O-antigenic determinants in Pseudomonas aeruginosa strains isolated from cystic fibrosis patients. Acta Pathol Microbiol Immunol Scand B. 1985 Feb;93(1):7–13. doi: 10.1111/j.1699-0463.1985.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Pedersen S. S., Espersen F., Høiby N. Diagnosis of chronic Pseudomonas aeruginosa infection in cystic fibrosis by enzyme-linked immunosorbent assay. J Clin Microbiol. 1987 Oct;25(10):1830–1836. doi: 10.1128/jcm.25.10.1830-1836.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penketh A., Pitt T., Roberts D., Hodson M. E., Batten J. C. The relationship of phenotype changes in Pseudomonas aeruginosa to the clinical condition of patients with cystic fibrosis. Am Rev Respir Dis. 1983 May;127(5):605–608. doi: 10.1164/arrd.1983.127.5.605. [DOI] [PubMed] [Google Scholar]
- Pitt T. L., MacDougall J., Penketh A. R., Cooke E. M. Polyagglutinating and non-typable strains of Pseudomonas aeruginosa in cystic fibrosis. J Med Microbiol. 1986 Mar;21(2):179–186. doi: 10.1099/00222615-21-2-179. [DOI] [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S., Kawamura T., Masuho Y., Tomibe K. A new common polysaccharide antigen of strains of Pseudomonas aeruginosa detected with a monoclonal antibody. J Infect Dis. 1985 Dec;152(6):1290–1299. doi: 10.1093/infdis/152.6.1290. [DOI] [PubMed] [Google Scholar]