Abstract
In obese humans, metabolism of glucocorticoids by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) and A-ring reduction (by 5α- and 5β-reductases) is dysregulated in a tissue specific manner. These changes have been recapitulated in leptin resistant obese Zucker rats but were not observed in high-fat fed Wistar rats. Recent data from mouse models suggest that such discrepancies may reflect differences in leptin signalling. We therefore compared glucocorticoid metabolism in murine models of leptin deficiency and resistance. Male ob/ob and db/db mice and their respective littermate controls (n=10–12/group) were studied at the age of 12 weeks. Enzyme activities and mRNA expression were quantified in snap-frozen tissues. The patterns of altered pathways of steroid metabolism in obesity were similar in ob/ob and db/db mice. In liver, 5β-reductase activity and mRNA were increased and 11β-HSD1 decreased in obese mice, whereas 5α-reductase 1 (5αR1) mRNA was not altered. In visceral adipose depots, 5β-reductase was not expressed, 11β-HSD1 activity was increased and 5αR1 mRNA was not altered in obesity. By contrast, in subcutaneous adipose tissue 11β-HSD1 and 5αR1 mRNA were decreased. Systematic differences were not found between ob/ob and db/db murine models of obesity, suggesting that variations in leptin signalling through the short splice variant of the Ob receptor do not contribute to dysregulation of glucocorticoid metabolism.
Introduction
Tissue-specific dysregulation of the glucocorticoid-generating enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in rodent (Livingstone et al. 2000, Liu et al. 2003) and human obesity (Bujalska et al. 1997, Fraser et al. 1999, Rask et al. 2001) and the effects of 11β-HSD1 deficiency (Morton et al. 2004) or inhibition (Alberts et al. 2002, Hermanowski-Vosatka et al. 2005) to protect against obesity-associated metabolic dysfunction, has supported the hypothesis that variations in glucocorticoid metabolism within target tissues play an important role in the pathophysiology of Metabolic Syndrome.
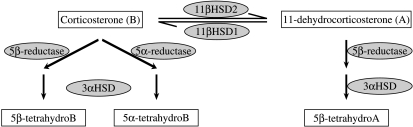
In addition to 11β-HSD1, glucocorticoids are metabolised by several other enzymes (Fig. 1). In liver, glucocorticoids are inactivated by A-ring reductases (5α- and 5β-reductases and 3α-HSD) and regenerated by 11β-HSD1 (Andrew & Walker 2002). The pattern of metabolism in adipose tissue is similar, with 5α-reductase 1 (5αR1), 3α-HSD and 11β-HSD1 being expressed (Barat et al. 2007, Wake et al. 2007b). A-ring reductases are also dysregulated in obesity. In human obesity, whole body A-ring reduction is enhanced, as judged by urinary steroid excretion, increasing peripheral clearance of cortisol and (Andrew et al. 1998, Tomlinson et al. 2008) potentially inducing compensatory activation of the hypothalamic–pituitary–adrenal axis (Andrew et al. 1998, Rask et al. 2001, 2002). In leptin-resistant obese Zucker rats increased urinary 5α-reduced metabolites can be accounted for by up-regulation of expression and activities of hepatic A-ring reductases (Livingstone et al. 2005). 5αR1 is also expressed in adipose tissue but is not dysregulated in subcutaneous (s.c.) adipose in obese humans or rats (Barat et al. 2007, Wake et al. 2007b).
Figure 1.
Glucocorticoid metabolic pathways. A is 11-dehydrocorticosterone; B is corticosterone and HSD is hydroxysteroid dehydrogenase.
In studies of 11β-HSD1, up-regulation of enzyme expression in adipose tissue and down-regulation in liver has not been a universal finding in obesity. For example, in diet-induced obesity in mice and rats adipose 11β-HSD1 is down-regulated (Morton et al. 2004, Drake et al. 2005). Furthermore, leptin resistant and deficient mice have been shown by Liu et al. to have divergent changes in hepatic 11β-HSD1 activity, with expression and activity being decreased in leptin deficient ob/ob mice (Liu et al. 2003) but paradoxically increased in leptin resistant db/db mice (Liu et al. 2005). These differences may reflect the distinctions between the defects in leptin signalling in these models.
Leptin signals through several splice variants of the leptin receptor (Ob-R; Lee et al. 1996). The long-form of the receptor (Ob-Rb) has an intracellular domain crucial to its signalling properties via Stat3, and is predominantly expressed in the hypothalamus, where it controls appetite regulation; this cytosolic region is truncated in the db/db mouse (Lee et al. 1996, Sahai et al. 2004). A short form of the receptor lacking the intracellular domain (Ob-Ra) is expressed in the liver (Hoggard et al. 1997), where it can activate the inositol trisphosphate kinase cascade, thus potentially modulating insulin signalling pathways (Cohen et al. 1996, Zhao et al. 2000). Stimulation of Ob-Ra may occur in db/db mice, but not in ob/ob mice that lack circulating leptin. Indeed, Ob-Ra activation may be critical in the development of hepatic insulin resistance and non-alcoholic steatohepatitis (Sahai et al. 2004). It is possible that activation of Ob-Ra may be responsible for leptin-induced up-regulation of 11β-HSD1 expression in hepatocytes (which lack Ob-Rb; Liu et al. 2005), and this mechanism may mediate up-regulation of liver 11β-HSD1 in db/db mice. This capacity for residual leptin signalling in db/db mice is not predicted in Zucker obese rats, since the fa mutation in the leptin receptor affects the extracellular domains of both Ob-Ra and b (Chua et al. 1996, Da Silva et al. 1998). We therefore hypothesised that deficient leptin signalling underlies dysregulation of hepatic glucocorticoid metabolism by 11β-HSD1 and A-ring reductases.
The aims of the present study were to explore glucocorticoid metabolism – not only by 11β-HSD1 but also by A-ring reductases – in murine models of obesity and, by comparing findings in ob/ob and db/db mice, to dissect the potential role of Ob-Ra in mediating dysregulation of hepatic steroid metabolism in obesity.
Materials and Methods
Materials
All chemicals were obtained from Sigma unless otherwise stated. Solvents were glass distilled HPLC grade from Fisher Scientific (Loughborough, UK). Steroid standards were obtained from Steraloids (Newport, RI, USA). Radiolabelled-steroids were from GE Healthcare (Buckinghamshire, UK).
Animals
Male obese (LeprDB/LeprDB (db/db) and Lepob/Lepob (ob/ob)) mice and their respective lean heterozygote or wild-type littermates (Db/? and Ob/?; C57BL background; Harlan, Bicester, UK) were characterised by phenotype, maintained under controlled conditions of light (on 0700–1900 h) and temperature (21 °C), and allowed free access to standard chow (Special Diet Services, Witham, UK) and drinking water. At 12 weeks of age, they were decapitated at 0800–1100 h and tissues dissected and snap frozen on dry ice. All animal experiments were carried out under UK Home Office guidelines.
Biochemical assays
Glucose and insulin were measured by hexokinase (Thermo Electron, Melbourne, Australia) and ELISA (Crystal Chem Inc., Downers Grove, IL, USA) respectively. Hepatic triglycerides were measured spectrophotometrically (Microgenics, Passau, Germany) as previously reported (Raubenheimer et al. 2006). Corticosterone was quantified in plasma by in-house RIA (Holmes et al. 1995).
11β-HSD1 activity assay
11β-HSD1 is a reductase in vivo, converting inactive 11-dehydrocorticosterone to corticosterone. However, in vitro dehydrogenase activity predominates and measurements of reductase activity are confounded by competition with other enzymes. Therefore, to estimate 11β-HSD1 protein, we measured enzyme activity as conversion of corticosterone to 11-dehydrocorticosterone in the presence of an excess of cofactor NADP+. Aliquots of tissues were homogenised in Krebs Ringer buffer as previously described (Livingstone et al. 2000), and protein concentrations determined colorimetrically using a Bradford kit (Bio-Rad). Standardised amounts of protein for each tissue were incubated in duplicate at 37 °C in Krebs Ringer buffer containing 0·2% glucose, NADP+ (2 mmol/l), [3H]4-corticosterone (10 nmol/l) and unlabelled corticosterone (1·99 μmol/l). Protein concentrations and incubation times were optimised for each tissue to ensure first order kinetics (liver, 25 μg/ml per h; adipose tissue, 100–200 μg/ml per h). After incubation, steroids were extracted with ethyl acetate, the organic phase evaporated under nitrogen and extracts re-solubilised in mobile phase (water:acetonitrile:methanol; 60:15:25, 1·5 ml/min). Steroids were separated by HPLC using a C18 reverse phase Symmetry column (4·6 mm, 15 cm, 5 μm; Waters, Elstree, UK) at 35 °C and quantified by on-line liquid scintillation counting.
Owing to the paucity of intra-abdominal adipose tissue in lean mice, omental adipose tissue was used to quantify enzyme activity, whereas mesenteric adipose was used to quantify transcript abundance.
5β-Reductase activity assay
Hepatic 5β-reductase (5βR) activity was assessed by the conversion of [3H]4-corticosterone to [3H]4-5β-tetrahydrocorticosterone in hepatic cytosol (Livingstone et al. 2005). Enzyme velocity was measured by incubating cytosol in duplicate at 37 °C, in sodium phosphate buffer (40 mmol/l Na2PO4, 320 mmol/l sucrose, 1 mmol/l dithiothreitol, pH 7·5) containing NADPH (1 mmol/l), glucose-6-phosphate (5 mmol/l M), glucose-6-phosphate dehydrogenase (1 unit/ml), [3H]4-corticosterone (10 nmol/l) and unlabelled corticosterone (1·99 μmol/l; Livingstone et al. 2005). Protein concentration and incubation period (0·5 mg/ml for 24 h) were optimised to ensure first order kinetics. Steroids were extracted with ethyl acetate, the organic phase was evaporated under nitrogen and extracts re-solubilised in mobile phase and analysed by HPLC as above.
Quantification of mRNA by real-time quantitative PCR
Total RNA was extracted from snap-frozen tissue samples, and 500 ng reverse transcribed into cDNA with random primers using the QuantiTect DNase/reverse transcription kit (Qiagen Ltd). cDNA (equivalent to 10 ng total RNA) was incubated in triplicate with 1× gene specific assay mix (Applied Biosystems, Warrington, UK) in 1× LightCycler480 Probes mastermix (Roche Diagnostics Ltd). PCR cycling and detection of fluorescent signal was carried out using a Roche LightCycler480. A standard curve was constructed for each primer probe set using a serial dilution of cDNA pooled from all samples. For liver and adipose, results were corrected for 18S and cyclophilin A RNA respectively, which were not different between groups. Assays used were: 11β-HSD1, Mm00476182_m1; 5αR1, Mm00614213_m1; 5βR, Mm00520266_m1; 18S, Hs99999901_s1 and Cyclophilin A, Mm02342430_g1.
Statistical analysis
Data are mean±s.e.m. and groups (n=10–12 unless otherwise stated) were compared by Student's t-test.
Results
Both db/db mice and ob/ob mice were heavier than their respective control groups at the time of cull (Table 1), and had increased liver weight. Both db/db and ob/ob mice had higher circulating glucose, insulin and corticosterone and hepatic triglycerides than lean controls.
Table 1.
Body and liver weights and plasma biochemistry of mice
| Db/? control | db/db | Ob/? control | ob/ob | |
|---|---|---|---|---|
| Weight at cull (g) | 25·0±0·54 | 34·5±0·92* | 25·7±0·46 | 42·1±0·55† |
| Liver (g) | 1·31±0·02 | 2·14±0·14* | 1·41±0·08 | 3·79±0·04† |
| Glucose (mg/dl) | 223±14 | 550±68* | 207±109 | 367±42† |
| Insulin (pg/ml) | 1·2±0·2 | 5·5±0·9* | 0·5±0·1 | >12·8† |
| Liver TAG (μmol/g) | 34·7±3·5 | 728±178* | 36·3±5·3 | 2287±130† |
| Corticosterone (nM) | 7·2±1·4 | 60·3±19·5** | 17·9±4·7 | 77·4±16·0† |
Data are mean±s.e.m., compared by Student's t-test. *P<0·005, **P<0·01 for db/db mice versus Db/? control. †P<0·005 versus ob/ob mice versus Ob/? control. N=10–13/group. NB insulin concentrations in all ob/ob mice exceeded the maximum point of the assay. TAG, triglycerides.
Hepatic glucocorticoid metabolism
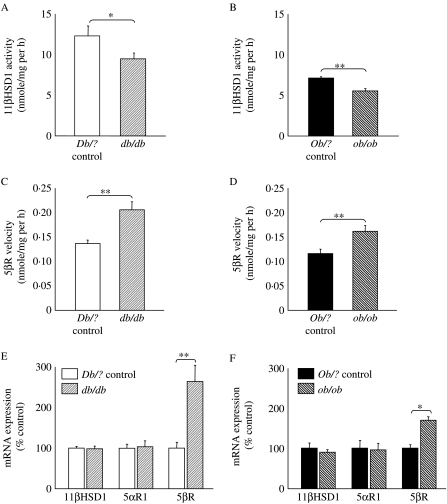
Hepatic 11β-HSD1 activity was lower in both db/db and ob/ob mice compared with lean controls (Fig. 2A and B), although mRNA for 11β-HSD1 was not different in either model (Fig. 2E and F). 5βR activity and transcript abundance were higher in both db/db and ob/ob mice compared with their controls (Fig. 2C–F). There was no difference in abundance of 5αR1 mRNA between lean and obese animals of either strain (Fig. 2E and F). Activity of 5αR1 was not measured due to instability of the protein (Eicheler et al. 1995).
Figure 2.
Hepatic glucocorticoid metabolism. 11β-HSD1 activity measured as velocity of formation of product following incubation of [3H]4-corticosterone with hepatic homogenate from (A) Db/? control (open) or db/db mice (light striped); (B) Ob/? control (black) or ob/ob mice (dark striped). 5β-Reductase activity measured as velocity of formation of product following incubation of [3H]4-corticosterone with hepatic cytosol from (C) Db/? control or db/db mice; (D) Ob/? control or ob/ob mice. Abundance of mRNAs for hepatic enzymes measured by real-time PCR (corrected for 18S as a housekeeping gene and presented as a percentage of respective control group) in (E) Db/? control or db/db mice; (F) Ob/? control mice or ob/ob mice. Data are mean±s.e.m.; n=10–12/group; *P<0·05; **P<0·01.
Glucocorticoid metabolism in adipose tissue
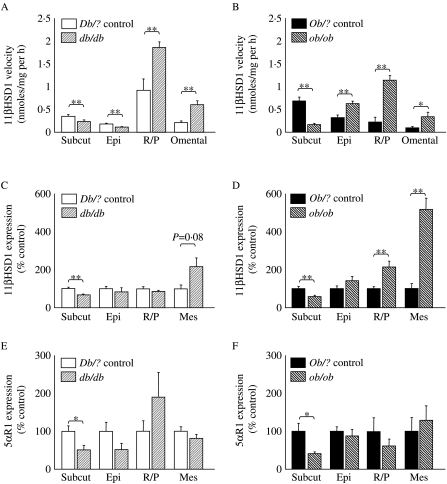
In db/db mice, activity of 11β-HSD1 was higher in retro-peritoneal and omental adipose but lower in s.c. and epidydimal adipose compared with controls (Fig. 3A). In ob/ob mice, 11β-HSD1 activity was higher in epididymal, retro-peritoneal and omental adipose but lower in s.c. adipose tissue compared with controls (Fig. 3B). Expression of 11β-HSD1 mRNA followed a similar pattern, in the main (Fig. 3C and D), although dysregulation of 11β-HSD1 mRNA was not observed in epididymal adipose tissue. Note that in mesenteric adipose tissue, limited amounts of tissue in lean mice resulted in analysis of only n=6 samples, and hence borderline statistical significance for the up-regulation of abundance of 11β-HSD1 mRNA in db/db mice.
Figure 3.
Glucocorticoid metabolism in adipose tissues. 11β-HSD1 activity measured as velocity of formation of product following incubation of [3H]4-corticosterone with homogenates of adipose tissues from (A) Db/? control (open) or db/db mice (light striped); (B) Ob/? control (black) or ob/ob mice (dark striped). Abundance of mRNA for 11β-HSD1 in adipose tissue depots measured by real-time PCR (corrected for cyclophilin A as a housekeeping gene and presented as a percentage of respective control group) in (C) Db/? control or db/db mice; (D) Ob/? control or ob/ob mice. Abundance of mRNAs for 5α-R1 in adipose beds measured by real-time PCR (corrected for cyclophilin A as a housekeeping gene and presented as a percentage of respective control group) in (E) Db/? control or db/db mice; (F) Ob/? control or ob/ob mice. Subcut is s.c. adipose, Epi is epididymal, R/P is retro-peritoneal and Mes is mesenteric. Data are mean±s.e.m.; n=6–12/group; *P<0·05; **P<0·01.
Abundance of mRNA for 5αR1 was lower in s.c. adipose tissue from obese mice of both strains compared with their respective controls, but was not altered in mesenteric, epididymal or retro-peritoneal adipose tissue in either obese strain (Fig. 3E and F).
Neither 5β- nor 5α-reductase 2 mRNAs were detected in adipose tissue.
Discussion
These studies demonstrate that mice with genetic obesity due to either defective leptin secretion (ob/ob) or sensitivity (db/db) have similar alterations in 11β-HSD1 and 5βR as Zucker obese rats (Livingstone et al. 2005). This includes down-regulation of 11β-HSD1 in liver and up-regulation in visceral adipose tissue depots, although in obese mice 11β-HSD1 was lower in s.c. adipose depots. By contrast with Zucker rats (Livingstone et al. 2005), however, 5αR1 expression was not increased in liver of obese mice and was decreased in s.c. adipose tissue. Strikingly, we did not find systematic differences between glucocorticoid metabolism in leptin deficient ob/ob mice and leptin-resistant db/db mice. This contrasts with the previous reports suggesting up-regulation of 11β-HSD1 in the liver of db/db mice (Liu et al. 2005, Nakano et al. 2007), and suggests that enhanced signalling through the short Ob-Ra splice variant does not contribute to the regulation of hepatic glucocorticoid metabolism in vivo. Previously observed effects of leptin administration in vivo to reverse changes in 11β-HSD1 in ob/ob mice (Liu et al. 2003) may have been mediated indirectly through weight loss and reversal of the metabolic phenotype, which inevitably follow leptin replacement.
The pattern of dysregulation of glucocorticoid metabolism in obese rodents differs in some respects from that in obese humans. In human adipose tissue, up-regulation of s.c. 11β-HSD1 is widely reported but 5αR1 is not altered (Wake et al. 2007b) and alterations in visceral adipose 11β-HSD1 are inconsistent (Walker & Andrew 2006). In human liver, down-regulation of 11β-HSD1 and up-regulation of both 5αR1 and 5βR activity have been reported consistently (Andrew et al. 1998, Fraser et al. 1999, Rask et al. 2001, Tomlinson et al. 2008), and some of these hepatic changes are paralleled here in mice and in our studies of obese Zucker rats (Livingstone et al. 2000, 2005, Barat et al. 2007).
This species specificity provides a potential opportunity to dissect mechanisms determining dysregulation of glucocorticoid metabolism in humans using comparative studies in rodents. However, given limited knowledge of regulation of expression of A-ring reductases, the mechanism of altered A-ring reductase activity in obesity remains uncertain. 5βR is transiently up-regulated in rats fed a high-fat diet (Drake et al. 2005), and in humans is selectively up-regulated in insulin resistance associated with fatty liver (Westerbacka et al. 2003). The murine models reported here had markedly fatty liver, moreso than that induced with diet-induced obesity. However, the severity of steatosis was more marked in the ob/ob mice, whereas the activity of 5β-reductase was increased to a greater extent in db/db mice. The db/db mice demonstrated partial insulin deficiency, and progression towards hyperglycaemia, whereas the ob/ob mice maintained near to normal glucose concentrations, albeit with higher insulin. This perhaps implicates the elevated insulin concentrations themselves in the dysregulation of steroid metabolism. The other potent regulators of 5βR identified to date are androgens, which imprint permanent down-regulation of 5βR in liver following in utero exposure (Einarsson & Gustafsson 1973, Gustafsson & Stenberg 1974, Jansson et al. 1985). In addition, withdrawal of androgens increases 5βR (Barat et al. 2007). Hence, the observed up-regulation may reflect the characteristic lowering of circulating androgens in obesity (Whitaker et al. 1983, Zumoff et al. 1990).
Regarding the regulation of 5αR1, these data suggest that the mechanism of dysregulation in human obesity is context and/or species-specific and does not operate in ob/ob or db/db mice. A caveat, however, is that protein levels or activity of 5αR1 might vary in the absence of changes in mRNA, but this cannot be readily tested given the instability of the hepatic 5αR1 protein ex vivo (Eicheler et al. 1995). Previous reports in both humans and rats support the notion that up-regulation of 5αR1 is secondary to the development of insulin resistance/hyperinsulinaemia and is reversible on treatment (Tsilchorozidou et al. 2003, Livingstone et al. 2005, Tomlinson et al. 2008). IGF-1 has been suggested as the principle candidate for dysregulation of hepatic 5αR1 (Horton et al. 1993). However, both mouse strains studied exhibit profound insulin resistance and therefore this explanation may be overly simplistic. Studies of 5αRs in mouse reproductive physiology have highlighted a possible redundancy between the two isozymes compared with other species (Mahendroo et al. 1996, 2001), but this is unlikely to explain the differences in 5αR1 dysregulation in obese mice, since the expression of 5αR2 was not detected in either liver or adipose tissue.
The few reports to date examining 5αR1 in adipose tissue in humans and Zucker rats suggest that the abundance of transcript is not altered by obesity in s.c. depots (Barat et al. 2007, Wake et al. 2007b). However, in both ob/ob and db/db mice, 5αR1 mRNA was down-regulated selectively in s.c. adipose, again highlighting the differences in regulation in this enzyme between species. In contrast to Zucker rats, in which 5αR1 expression was increased in omental adipose tissue (Barat et al. 2007), changes in mRNA expression were not observed in the murine mesenteric depot, although the greater omental depot was not studied as a direct comparison due to a paucity of tissue in lean mice.
Regulation of 11β-HSD1 transcription has been studied extensively but the basis for tissues-specific dysregulation in obesity remains elusive. Species differences in regulation of 11β-HSD1 have been demonstrated, most recently in relation to PPAR agonists (Hermanowski-Vosatka et al. 2000, Wake et al. 2007a). Elevated circulating glucocorticoid levels, that are much more striking in rodent than in human obesity, may contribute since 11β-HSD1 is a glucocorticoid-responsive gene (Low et al. 1994, Jamieson et al. 1995, Voice et al. 1996). The striking observation in murine obesity in the present data is the down-regulation of 11β-HSD1 in s.c. adipose tissue. This has also been observed in diet-induced and in polygenic obesity in mice (Morton et al. 2004, 2005). However in humans, inhibition of 11β-HSD1 in s.c. adipose tissue has become an attractive target for restricting glucocorticoid action, with most groups agreeing, that in humans, there is up-regulation of the enzyme in this depot (Paulmyer-Lacroix et al. 2002, Lindsay et al. 2003, Wake et al. 2003). Of interest is the observation that changes in 11β-HSD1 activity in both obese models are more marked than changes in mRNA, indeed in epididymal fat mRNA was not altered. This discrepancy has been reported by ourselves (Morton et al. 2004) and others (Bujalska et al. 2005, Jang et al. 2006) previously. The relationship between activity and abundance of transcript appears most robust in s.c. adipose in humans (Wake et al. 2003, Goedecke et al. 2006); discrepancies existing at other sites and observed here may reflect an additional level of control of 11β-HSD1 protein by post-translational modification, e.g. glycosylation (Opperman et al. 1995). Another source of variation between species and depots is the mixture of cell types. 11β-HSD1 is expressed in macrophages as well as adipocytes (Gilmour et al. 2006). There is emerging evidence that some depots, and some animal models are more susceptible to macrophage infiltration in the adipose tissue in obesity (Surmi & Hasty 2008).
In conclusion, murine obesity is characterised by some but not all of the changes in steroid metabolism that are observed in human obesity. The consequences of disrupted glucocorticoid metabolism in rodents may differ from those in humans, since rodents also exhibit substantially elevated circulating concentrations of corticosterone, contrasting with low to normal circulating cortisol in human obesity (Phillips et al. 2000). Nevertheless, mice may provide useful models in which to investigate dysregulation of 5βR and 11β-HSD1 but not 5αR1 in liver. None of these changes differ substantially in mice with or without leptin signalling through Ob-Ra. The pattern of dysregulation of metabolism in adipose tissue is, however, subtly different between species, offering the possibility that further comparative biology studies may elucidate relevant mechanisms.
Declaration of interest
D E W L, S L G, G L C and R A have no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported. Within the past 2 years, B R W has consulted for Astra-Zeneca, Dainippon Sumitomo, Merck, Johnson & Johnson, Incyte, Ipsen, Roche, Vitae, Wyeth, Zydus Research Centre, received lecture fees from Abbott and Bristol Myers Squibb, and received research funding from Wyeth. B R W is an inventor on relevant patents held by University of Edinburgh.
Funding
This work was supported by the Wellcome Trust (060707 and VS/06/UED/A8).
Author contribution statement
D E W L, S L G, G L C contributed to the execution and analysis of the studies. D E W L, B R W and R A contributed to study design, data analysis and interpretation and preparation of the manuscript.
Acknowledgements
We are grateful to Mrs Carolynn Cairns and Mrs Rachel McDonnell for their excellent technical support.
References
- Alberts P, Engblom L, Edling N, Forsgren M, Klingstrom G, Larsson C, Rönquist-Nii Y, Öhman B, Abrahmsén L. Selective inhibition of 11β-hydroxysteroid dehydrogenase type 1 decreases blood glucose concentrations in hyperglycaemic mice. Diabetologia. 2002;45:1528–1532. doi: 10.1007/s00125-002-0959-6. [DOI] [PubMed] [Google Scholar]
- Andrew R, Walker BR. Glucocorticoid metabolism in health and disease. Recent Research Developments in Endocrinology. 2002;3:425–449. [Google Scholar]
- Andrew R, Phillips DIW, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. Journal of Clinical Endocrinology and Metabolism. 1998;83:1806–1809. doi: 10.1210/jcem.83.5.4951. [DOI] [PubMed] [Google Scholar]
- Barat P, Livingstone DEW, Elferink C, MacDonnell R, Walker BR, Andrew R. Effects of gonadectomy on glucocorticoid metabolism in obese Zucker rats. Endocrinology. 2007;148:4836–4843. doi: 10.1210/en.2007-0597. [DOI] [PubMed] [Google Scholar]
- Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect ‘Cushing's disease of the omentum’? Lancet. 1997;349:1210–1213. doi: 10.1016/S0140-6736(96)11222-8. [DOI] [PubMed] [Google Scholar]
- Bujalska IJ, Draper N, Michailidou Z, Tomlinson JW, White PC, Chapman KE, Walker EA, Stewart PM. Hexose-6-phosphate dehydrogenase confers oxo-reductase activity upon 11β-hydroxysteroid dehydrogenase type 1. Journal of Molecular Endocrinology. 2005;34:675–684. doi: 10.1677/jme.1.01718. [DOI] [PubMed] [Google Scholar]
- Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu S-M, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science. 1996;274:1185–1188. doi: 10.1126/science.274.5290.1185. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Livingstone DEW, Morton NM, Andrew R, Seckl JR, Walker BR. Reduced adipose glucocorticoid reactivation and increased hepatic glucocorticoid clearance as an early adaptation to high fat feeding in rats. Endocrinology. 2005;146:913–919. doi: 10.1210/en.2004-1063. [DOI] [PubMed] [Google Scholar]
- Eicheler W, Seitz J, Steinhoff M, Forssmann WG, Adermann K, Aumuller G. Distribution of rat hepatic steroid 5alpha-reductase 1 as shown by immunohistochemistry. Experimental and Clinical Endocrinology and Diabetes. 1995;103:105–112. doi: 10.1055/s-0029-1211337. [DOI] [PubMed] [Google Scholar]
- Einarsson K, Gustafsson J-A. Neonatal imprinting of liver microsomal hydroxylation and reduction of steroids. Journal of Biological Chemistry. 1973;248:4987–4997. [PubMed] [Google Scholar]
- Fraser R, Ingram MC, Anderson NH, Morrison C, Davies E, Connell JMC. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. American Journal of Physiology. Endocrinology and Metabolism. 1999;33:1364–1368. doi: 10.1161/01.hyp.33.6.1364. [DOI] [PubMed] [Google Scholar]
- Gilmour JS, Coutinho AE, Cailhier J.-F, Man TY, Clay M, Thomas G, Harris HG, Mullins JJ, Seckl JR, Savill JS, et al. Local amplification of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1 promotes macrophage phagocytosis of apoptotic leukocytes. Journal of Immunology. 2006;176:7605–7611. doi: 10.4049/jimmunol.176.12.7605. [DOI] [PubMed] [Google Scholar]
- Goedecke JH, Wake DJ, Levitt NS, Lambert EV, Collins MR, Morton NM, Andrew R, Walker BR, Seckl JR. Glucocorticoid metabolism within superficial subcutaneous rather than visceral adipose tissue is associated with features of the metabolic syndrome. Clinical Endocrinology. 2006;65:81–87. doi: 10.1111/j.1365-2265.2006.02552.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson J-A, Stenberg A. Irreversible androgenic programming at birth of microsomal and soluble rat liver steroid metabolism by neonatal testosterone. Journal of Biological Chemistry. 1974;249:711–718. [PubMed] [Google Scholar]
- Hermanowski-Vosatka A, Gerhold D, Mundt SS, Loving VA, Lu M, Chen Y, Elbrecht A, Wu M, Doebber T, Kelly L, et al. PPARalpha agonists reduce 11beta-hydroxysteroid dehydrogenase type 1 in the liver. Biochemical and Biophysical Research Communications. 2000;279:330–336. doi: 10.1006/bbrc.2000.3966. [DOI] [PubMed] [Google Scholar]
- Hermanowski-Vosatka A, Balkovec JM, Cheng K, Chen HY, Hernandez M, Koo GC, Le Grand CB, Li Z, Metzger JM, Mundt SS, et al. 11β-HSD1 inhibition ameliorates metabolic syndrome and prevents progression of atherosclerosis in mice. Journal of Experimental Medicine. 2005;202:517–527. doi: 10.1084/jem.20050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard N, Mercer JG, Rayner DV, Moar K, Trayhurn P, Williams LM. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochemical and Biophysical Research Communications. 1997;232:383–387. doi: 10.1006/bbrc.1997.6245. [DOI] [PubMed] [Google Scholar]
- Holmes MC, French KL, Seckl JR. Modulation of serotonin and corticosteroid receptor gene expression in the rat hippocampus with circadian rhythm and stress. Molecular Brain Research. 1995;28:186–192. doi: 10.1016/0169-328x(94)00207-u. [DOI] [PubMed] [Google Scholar]
- Horton R, Pasupuletti V, Antonipillai I. Androgen induction of steroid 5α-reductase may be mediated via insulin-like growth factor-1. Endocrinology. 1993;133:447–451. doi: 10.1210/endo.133.2.8344190. [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Chapman KE, Edwards CRW, Seckl JR. 11β-Hydroxysteroid dehydrogenase is an exclusive 11β-reductase in primary cultures of rat hepatocytes: effect of physicochemical and hormonal manipulations. Endocrinology. 1995;136:4754–4761. doi: 10.1210/endo.136.11.7588203. [DOI] [PubMed] [Google Scholar]
- Jang C, Obeyesekere VR, Dilley RJ, Alford FP, Inder WJ. 11β-Hydroxysteroid dehydrogenase type 1 is expressed and is biologically active in human skeletal muscle. Clinical Endocrinology. 2006;65:800–805. doi: 10.1111/j.1365-2265.2006.02669.x. [DOI] [PubMed] [Google Scholar]
- Jansson JO, Ekberg S, Isaksson O. Imprinting of growth hormone secretion, body growth, and hepatic steroid metabolism by neonatal testosterone. Endocrinology. 1985;117:1881–1889. doi: 10.1210/endo-117-5-1881. [DOI] [PubMed] [Google Scholar]
- Lee G-H, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Tataranni A, Permana P, Livingstone DEW, Wake DJ, Walker BR. Subcutaneous adipose 11β-hydroxysteroid dehydrogenase type 1 activity and mRNA levels are associated with adiposity and insulinaemia in Pima Indians and Caucasians. Journal of Clinical Endocrinology and Metabolism. 2003;88:2738–2744. doi: 10.1210/jc.2002-030017. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nakagawa Y, Wang Y, Li R, Li X, Ohzeki T, Friedman TC. Leptin activation of corticosterone production in hepatocytes may contribute to the reversal of obesity and hyperglycaemia in leptin deficient ob/ob mice. Diabetes. 2003;52:1409–1416. doi: 10.2337/diabetes.52.6.1409. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nakagawa Y, Wang Y, Sakurai R, Tripathi PV, Lutfy K, Friedman TC. Increased glucocorticoid receptor and 11β-hydroxysteroid dehydrogenase type 1 expression in hepatocytes may contribute to the phenotype of type 2 diabetes in db/db mice. Diabetes. 2005;54:32–40. doi: 10.2337/diabetes.54.1.32. [DOI] [PubMed] [Google Scholar]
- Livingstone DEW, Jones GC, Smith K, Andrew R, Kenyon CJ, Walker BR. Understanding the role of glucocorticoids in obesity: tissue-specific alterations of corticosterone metabolism in obese Zucker rats. Endocrinology. 2000;141:560–563. doi: 10.1210/endo.141.2.7297. [DOI] [PubMed] [Google Scholar]
- Livingstone DEW, McInnes KJ, Walker BR, Andrew R. Increased A-ring reduction of glucocorticoids in obese rats: attenuation by insulin sensitisation. Obesity Research. 2005;13:1523–1526. doi: 10.1038/oby.2005.186. [DOI] [PubMed] [Google Scholar]
- Low SC, Moisan M-P, Edwards CRW, Seckl JR. Glucocorticoids and chronic stress up-regulate 11b-hydroxysteroid dehydrogenase activity and gene expression in the hippocampus. Journal of Neuroendocrinology. 1994;6:285–290. doi: 10.1111/j.1365-2826.1994.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Russell DW. 5α-Reduced androgens play a key role in murine parturition. Molecular Endocrinology. 1996;10:380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Hess DL, Russell DW. Unexpected virilization in male mice lacking steroid 5α-reductase enzymes. Endocrinology. 2001;142:4652–4662. doi: 10.1210/endo.142.11.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton NM, Paterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C, Walker BR, Flier JS, Mullins JJ, Seckl JR. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2004;53:931–938. doi: 10.2337/diabetes.53.4.931. [DOI] [PubMed] [Google Scholar]
- Morton NM, Densmore V, Wamil M, Ramage L, Nichol K, Bunger L, Seckl JR, Kenyon CJ. A polygenic model of the Metabolic Syndrome with reduced circulating and intra-adipose glucocorticoid action. Diabetes. 2005;54:3371–3378. doi: 10.2337/diabetes.54.12.3371. [DOI] [PubMed] [Google Scholar]
- Nakano K, Inada Y, Masuzaki H, Tanaka T, Yasue S, Ishii T, Arai N, Ebihara K, Hosada K, Maruyama K, et al. Bezafibrate regulated the expression and enzyme activity of 11β-hydroxysteroid dehydrogenase type 1 in murine adipose tissue and 3T3-L1 adipocytes. American Journal of Physiology. Endocrinology and Metabolism. 2007;292:E1213–E1222. doi: 10.1152/ajpendo.00340.2006. [DOI] [PubMed] [Google Scholar]
- Opperman UC, Netter KJ, Maser E. Cloning and primary structure of murine 11β-hydroxysteroid microsomal carbonyl reductase. European Journal of Biochemistry. 1995;227:202–208. doi: 10.1111/j.1432-1033.1995.tb20377.x. [DOI] [PubMed] [Google Scholar]
- Paulmyer-Lacroix O, Boullu S, Oliver C, Alessi MC, Grino M. Expression of the mRNA coding for 11β-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: an in situ hybridisation study. Journal of Clinical Endocrinology and Metabolism. 2002;87:2701–2705. doi: 10.1210/jcem.87.6.8614. [DOI] [PubMed] [Google Scholar]
- Phillips DIW, Walker BR, Reynolds RM, Flanagan DEH, Wood PJ, Osmond C, Barker DJ, Whorwood CB. Low birthweight predicts elevated plasma cortisol concentrations in adults from three populations. Journal of Internal Medicine. 2000;35:1301–1306. doi: 10.1161/01.hyp.35.6.1301. [DOI] [PubMed] [Google Scholar]
- Rask E, Olsson T, Söderberg S, Andrew R, Livingstone DEW, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. Journal of Clinical Endocrinology and Metabolism. 2001;86:1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- Rask E, Walker BR, Söderberg S, Livingstone DEW, Eliasson M, Johnson O, Andrew R, Olsson T. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11β-hydroxysteroid dehydrogenase type 1 activity. Journal of Clinical Endocrinology and Metabolism. 2002;87:3330–3336. doi: 10.1210/jcem.87.7.8661. [DOI] [PubMed] [Google Scholar]
- Raubenheimer PJ, Nyirenda MJ, Walker BR. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes. 2006;55:2015–2020. doi: 10.2337/db06-0097. [DOI] [PubMed] [Google Scholar]
- Sahai M, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whittington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2004;287:G1035–G1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- Da Silva BA, Bjorbaek C, Uotani S, Flier JS. Functional properties of leptin receptor isoforms containing the Gln-Pro extracellular domain mutation of the fatty rat. Endocrinology. 1998;139:3681–3690. doi: 10.1210/endo.139.9.6168. [DOI] [PubMed] [Google Scholar]
- Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidology. 2008;3:545–556. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson JW, Finney J, Hughes BA, Hughes S, Stewart PM. Reduced glucocorticoid production rate, decreased 5α-reductase activity and adipose tissue insulin sensitization following weight loss. Diabetes. 2008;57:1536–1543. doi: 10.2337/db08-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilchorozidou T, Honour JW, Conway GS. Altered cortisol metabolism in Polycystic Ovary Syndrome: insulin enhances 5α-reduction but not the elevated adrenal steroid production rates. Journal of Clinical Endocrinology and Metabolism. 2003;88:5907–5913. doi: 10.1210/jc.2003-030240. [DOI] [PubMed] [Google Scholar]
- Voice MW, Seckl JR, Edwards CRW, Chapman KE. 11beta-Hydroxysteroid dehydrogenase type 1 expression in 2S FAZA hepatoma cells is hormonally regulated: a model system for the study of hepatic glucocorticoid metabolism. Biochemical Journal. 1996;317:621–625. doi: 10.1042/bj3170621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake DJ, Rask E, Livingstone DEW, Soderberg S, Olsson T, Walker BR. Local and systemic impact of transcriptional upregulation of 11β-hydroxysteroid dehydrogenase type 1 in human adipose tissues in obesity. Journal of Clinical Endocrinology and Metabolism. 2003;88:3983–3988. doi: 10.1210/jc.2003-030286. [DOI] [PubMed] [Google Scholar]
- Wake DJ, Stimson RH, Tan GD, Andrew R, Homer NZM, Karpe F, Walker BR. Influence of peroxisome proliferator-activated receptor (PPAR) α and γ agonists on 11β-hydroxysteroid dehydrogenase type 1 in vivo in humans. Journal of Clinical Endocrinology and Metabolism. 2007a;92:1848–1856. doi: 10.1210/jc.2006-2713. [DOI] [PubMed] [Google Scholar]
- Wake DJ, Strand M, Rask E, Westerbacka J, Livingstone DEW, Soderberg S, Andrew R, Olsson T, Yki-Jarvinen H, Walker BR. The influence of intra-adipose enzymes generating estrogens and androgens on body fat distribution in idiopathic human obesity. Clinical Endocrinology. 2007b;66:440–446. doi: 10.1111/j.1365-2265.2007.02755.x. [DOI] [PubMed] [Google Scholar]
- Walker BR, Andrew R. Tissue production of cortisol by 11beta-hydroxysteroid dehydrogenase type 1 and metabolic disease. Annals of the New York Academy of Sciences. 2006;1083:165–184. doi: 10.1196/annals.1367.012. [DOI] [PubMed] [Google Scholar]
- Westerbacka J, Yki-Jarvinen H, Vehkavaara S, Häkkinen A-M, Andrew R, Wake DJ, Seckl JR, Walker BR. Body fat distribution and cortisol metabolism in healthy men: enhanced 5β-reductase and lower cortisol/cortisone metabolite ratios in men with fatty liver. Journal of Clinical Endocrinology and Metabolism. 2003;88:4924–4931. doi: 10.1210/jc.2003-030596. [DOI] [PubMed] [Google Scholar]
- Whitaker EM, Shaw MA, Hervey GR. Plasma oestradiol-17beta and testosterone concentrations as possible causes of the infertility of congenitally obese Zucker rats. Journal of Endocrinology. 1983;99:485–490. doi: 10.1677/joe.0.0990485. [DOI] [PubMed] [Google Scholar]
- Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, Beavo JA, Bornfeldt KE. Leptin induces insulin-like signalling that antagonizes cAMP elevation by glucagon in hepatocytes. Journal of Biological Chemistry. 2000;275:11348–11354. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]
- Zumoff B, Strain GW, Miller LK, Rosner W, Senie R, Seres D. Plasma free and nonsex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. Journal of Clinical Endocrinology and Metabolism. 1990;71:929–931. doi: 10.1210/jcem-71-4-929. [DOI] [PubMed] [Google Scholar]