Abstract
This review focuses on the relationships among dietary fat type, plasma and liver lipid, and lipoprotein metabolism and atherosclerosis. Dietary polyunsaturated fatty acids are beneficial for the prevention of coronary artery atherosclerosis. By contrast, dietary monounsaturated fatty acids appear to alter hepatic lipoprotein metabolism, promote cholesteryl oleate accumulation, and confer atherogenic properties to lipoproteins as shown in data from experimental animal studies. Polyunsaturated fat appears to provide atheroprotection, at least in part, because it limits the accumulation of cholesteryl oleate in favor of cholesteryl linoleate in plasma lipoproteins.
Keywords: cholesteryl ester, lipoprotein metabolism, polyunsaturated fat, monounsaturated fat
Relationships between dietary fatty acids and blood cholesterol and between blood cholesterol and coronary heart disease (CHD) have been widely documented. Evidence of differential effects of dietary fatty acids on serum cholesterol concentrations was first provided by Kinsell et al. and Groen et al. (1, 2), who showed that feeding polyunsaturated fatty acids (PUFA) in the diet lowers total plasma cholesterol compared with saturated fatty acids (SFA). These observations were further supported by ecologic studies, such as the Seven Countries Study, in which strong correlations were found between intake of saturated fat and CHD prevalence (3). Comparison of selected cohorts indicated that the lower incidence of death from CHD was associated with the consumption of the so-called Mediterranean diet, which is enriched in monounsaturated fatty acids (MUFA) and poor in SFA. Subsequent epidemiologic studies supported the notion that monounsaturated fat was protective against CHD compared with saturated fat. MUFA effectively lowers LDL-cholesterol (LDL-C) while, in contrast to PUFA, provides no HDL-cholesterol (HDL-C) lowering (4–6).
Recommendations for a preference of MUFA over PUFA for replacing SFA in the diet initially stemmed from the metabolic study of Mattson and Grundy (7) in which plasma lipid and lipoprotein concentrations were measured in 20 hypertriglyceridemic patients consuming liquid diets enriched in SFA, MUFA, or PUFA. Dietary monounsaturated and polyunsaturated fat were equally effective in reducing plasma total cholesterol and LDL-C, while polyunsaturated fat lowered HDL-C compared with monounsaturated fat. Further, within the controversy over the recommendation for restriction of specific types of dietary fat in the prevention of CHD, Katan, Grundy, and Willett (8) suggested that a diet in which the total fat content is held constant, but is relatively enriched in MUFA, offers better protection against CHD than does a low-fat diet. However, as suggested by Brown, Shelness, and Rudel (9), dietary-fat-intervention studies have only been able to show statistical correlations between MUFA and CHD risk factors and have lacked direct measurements of associations with the extent of CHD. In this paper, we review pertinent literature addressing the influence of the various dietary fat-induced modifications of lipoprotein concentrations and compositions and their associations with atherosclerosis as the underlying cause of CHD.
CHOLESTERYL OLEATE ENRICHMENT OF LDL PREDICTS ATHEROSCLEROSIS IN ANIMAL MODELS
Early indications that an atherogenic LDL particle is characterized by a lipid core enriched in cholesteryl oleate were provided by Rudel, Pitts, and Nelson (10, 11). In three different species of monkeys, and in both sexes, changes in LDL molecular weight as a marker for particle size occurred in response to dietary cholesterol (12). Increases in particle size reflected a proportional increase in LDL particle cholesteryl ester content. Several studies indicated that LDL particles were enlarged most in individual primates that developed the most coronary artery atherosclerosis (CAA) and that increased size made a contribution to atherogenicity, which was in addition to LDL-C concentration (13, 14).
Dietary intervention studies in nonhuman primates helped establish the relationship between LDL particle composition and extent of CAA (12, 15). The extent of atherosclerosis was measured after 5 years of diets containing cholesterol and either SFA, MUFA, or n-6 PUFA enrichment. In agreement with the observations made by Mattson and Grundy (7) in humans, dietary MUFA reduced LDL-C without lowering HDL-C and, compared with dietary SFA and PUFA, provided the lowest LDL/HDL ratio. However, substitution of MUFA for some of the SFA promoted an even greater enrichment of LDL particles with cholesteryl oleate at the expense of cholesteryl linoleate. When atherosclerosis extent was directly measured, SFA and MUFA fed monkeys developed equivalent amounts of CAA as assessed histologically and by the similar cholesteryl ester content of the coronary arteries. The findings suggested that the shift in LDL cholesteryl ester composition is likely an important atherogenic factor that should be considered along with LDL-C and HDL-C concentration.
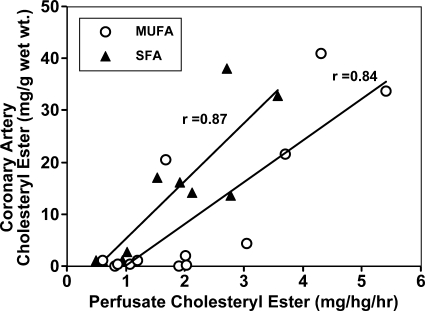
Two enzymes, hepatic acyl-CoA:cholesterol acyltransferase (ACAT) and plasma lecithin cholesterol-acyl-transferase contribute their products to the cholesteryl esters in LDL particles. The identification of hepatic ACAT [later shown to be ACAT2 (16)] as a source of cholesteryl oleate in plasma was achieved by taking advantage of isolated liver perfusion experiments. In monkeys fed diets rich in fat (lard, safflower oil, or fish oil) and cholesterol for 3–6 years before liver perfusion, hepatic ACAT activity was positively correlated with hepatic cholesteryl ester secretion, plasma cholesteryl ester concentration, and extent of CAA (17). Evidence was provided that accumulation of cholesteryl oleate in the liver is highest in monkeys fed MUFA-enriched diets and is associated with increased secretion of cholesteryl ester into apoB-containing lipoproteins. This in turn was highly correlated with atherosclerosis quantified by the accumulation of cholesteryl esters in the coronary arteries, as shown in the data provided by Rudel et al. (18) (Fig. 1).
Fig. 1.
In African green monkeys fed diets containing cholesterol and 35% calories as fat enriched in monounsaturated (MUFA) or saturated fatty acids (SFA), the relationship between cholesteryl ester secretion by the liver and the extent of coronary artery atherosclerosis (CAA) that developed during 5 years of diet induction was examined. In both dietary fat groups, the high correlation (r > 0.8, P < 0.01) between liver cholesteryl ester secretion and coronary artery cholesteryl ester concentration provided the first direct demonstration of the importance of hepatic cholesteryl ester secretion in promoting the development of atherosclerosis in the coronary arteries. Modified from Ref. 18.
The atherogenicity of cholesteryl oleate enrichment of LDL is not species specific and has been reproduced in mouse models of atherosclerosis. In mice with genetically engineered high levels of plasma LDL (LDL receptor-null, human apoB100 transgenic mice), dietary MUFA promoted accumulation of plasma LDL particles enriched in cholesteryl oleate and an accumulation of cholesteryl esters in the aortas that was positively correlated (r = 0.7) with total plasma cholesterol levels (19). The effects of diets enriched in SFA, MUFA, or PUFA on atherosclerotic lesion area and lipoprotein levels were also measured in LDL-receptor-deficient mice (20). In a fashion comparable to SFA feeding, dietary MUFA promoted significant atherosclerosis in both sexes, increased VLDL-cholesterol (VLDL-C) and LDL-C and the atherosclerotic lesion area, which correlated positively with VLDL-C levels (r = 0.47 and r = 0.53 for males and females, respectively).
ACAT2 has been identified as the isoform of ACAT responsible for the synthesis of cholesteryl esters incorporated into the apoB-containing lipoproteins secreted by the liver and intestine (16, 21). In a mouse model of atherosclerosis, Bell et al. (22) demonstrated that dietary monounsaturated fat is the fat type that promoted the highest accumulation in the liver of oleoyl-CoA, the preferred substrate for ACAT2. Such an increase in substrate availability from exogenous sources, together with that provided by the activity of hepatic SCD1, may be partly responsible for the accumulation and secretion of ACAT2-derived cholesteryl oleate. The ability of dietary monounsaturated fat to promote hepatic ACAT2-mediated cholesterol oleate secretion into lipoproteins, thereby enhancing atherosclerosis, was monitored in apoB100-only LDL-receptor-deficient mice fed diets enriched in several different fatty acids (23). Relative to other diets, wild-type mice receiving dietary MUFA displayed the greatest increase in cholesteryl ester content of VLDL and LDL, with the LDL particles becoming highly enriched in cholesteryl oleate. Atherosclerosis was also as high in the MUFA group as in any dietary fat group, as indicated by accumulation of cholesteryl ester in the aorta. Genetic deletion of ACAT2 in this mouse model greatly reduced atherosclerosis extent and abolished any dietary fat-associated differences in atherosclerosis, such that levels of atherosclerosis were low and comparable among the six different dietary fat groups. The findings validate the essential role of ACAT2 in facilitating atherosclerosis, an effect apparently mediated by modifications of plasma lipoprotein composition more than cholesterol concentration, for which the differences were smaller.
Taken together, these studies indicate that dietary monounsaturated fat promotes the accumulation of hepatic oleoyl-CoA, which, serving as the preferential substrate for ACAT2, gets esterified to cholesterol, incorporated into apoB-containing lipoproteins, and secreted into the plasma compartment (Fig. 2). Plasma cholesteryl oleate enrichment of LDL particles results from the intravascular conversion of cholesteryl oleate enriched VLDL into LDL, and the resulting modification of LDL (increase in particle size and cholesteryl oleate content) has been shown in nonhuman primates and genetically engineered mouse models of atherosclerosis to be positively correlated with increased coronary artery or aortic atherosclerosis, respectively. Plasma cholesteryl oleate also has been positively associated with CHD in human beings.
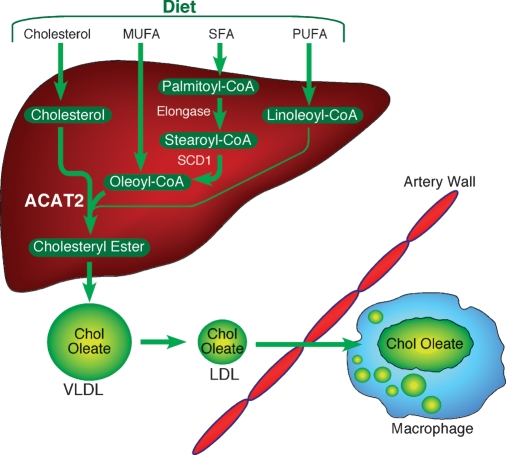
Fig. 2.
Proposed mechanism by which dietary MUFA promote plasma cholesteryl oleate enrichment and enhanced atherosclerosis. Oleoyl-CoA, from dietary MUFA and that newly synthesized by SCD1 from stearoyl-CoA, serves as the preferred and most abundant substrate for acyl-CoA:cholesterol acyltransferase (ACAT)2-driven esterification of dietary cholesterol (thick arrows). Dietary polyunsaturated fatty acids (PUFA) can lead to linoleyl-CoA formation in liver, but this acyl-CoA is less-efficiently incorporated into cholesteryl esters by ACAT2 (thin arrow) and, hence, typically does not result in cholesteryl-ester-enriched VLDL and LDL particles. ACAT2-derived cholesteryl oleate is abundant and is incorporated efficiently into nascent VLDL particles. The cholesteryl oleate enrichment is retained in LDL. The resulting LDL particles enriched with cholesteryl oleate are typically larger than normal and appear to be more active in binding to arterial proteoglycans, thereby favoring arterial retention and subsequent foam-cell formation typical of early atherosclerosis.
EVIDENCE FOR ATHEROGENICITY OF CHOLESTERYL OLEATE FROM HUMAN STUDIES
Some still debate whether dietary n-6 PUFA is a better substitute for dietary SFA than MUFA. The early studies by Mattson and Grundy (7) showed that replacing SFA with PUFA resulted in LDL-C and HDL-C lowering. These authors recommended oleic acid-rich fat as better replacement for saturated fat than linoleic acid-rich fat because HDL-C decreased with the latter. The recommendation for dietary MUFA over PUFA based on the effects on HDL-C may be premature because it has not yet been shown that the PUFA-mediated lowering of HDL, if and when it occurs, increases the risk of CHD (24). Another factor often cited is that dietary fat may influence the risk of CHD by altering the susceptibility of lipoproteins to oxidation. Substitution of MUFA in the diet instead of PUFA to replace dietary SFA results in LDL particles that are less susceptible to oxidation in vitro (25). On the other hand, the enhanced in vitro oxidation of LDL promoted by PUFA, as well as the HDL decrease, occur in spite of the overall ability of PUFA to provide relative protection from atherosclerosis in animals and people.
Evidence that alterations in dietary fatty acid composition can effectively alter the cholesteryl ester fatty acid distribution of LDL and HDL was found by Reaven et al. (26). When mildly hypercholesterolemic subjects were fed oleate- or linoleate-enriched diets for 8 weeks, diets enriched with the former fatty acid led to LDL particles relatively enriched in cholesteryl oleate. The percent of LDL cholesteryl ester as cholesteryl oleate was higher in oleate- compared with linoleate-enriched diets (32.0% vs. 13.8%). This shift is likely due to the proportion of cholesteryl esters derived from lecithin cholesterol-acyl-transferase, the source of the majority of cholesteryl linoleate in plasma, versus ACAT2, the primary product of which is cholesteryl oleate. While these investigations were aimed at addressing whether the fatty acid composition of LDL could be adjusted to be less oxidizable (oleate is not a fatty acid readily susceptible to oxidation), they showed the remarkable elasticity of LDL cholesteryl ester fatty acid composition in human subjects. One should also consider, as noted above, that enrichment of cholesteryl oleate in the core of LDL particles has been shown in nonhuman primates fed SFA or MUFA to be highly predictive of the extent of CAA (15). By contrast, dietary PUFA minimized the amount of cholesteryl oleate in LDL, and was atheroprotective.
A positive correlation between cholesteryl oleate and atherosclerosis has also been clearly shown in the prospective cohort study, Atherosclerosis Risk in Communities Study (27). This was a population-based study that investigated the association of the fatty acid composition of plasma phospholipids and cholesteryl esters with preclinical carotid atherosclerosis, assessed as carotid artery wall intima-medial thickness in middle-aged, CHD-free subjects. In men and women, the adjusted average carotid intima-medial thickness was independently and positively associated (P < 0.01) with saturated and monounsaturated cholesterol esters, and negatively associated with polyunsaturated cholesteryl esters. Additional evidence for serum cholesteryl ester fatty acids as predictors for total and cardiovascular mortality has recently been provided by a population-based cohort study, the Uppsala Longitudinal Study of Adult Men (28). Of the individual serum cholesteryl ester fatty acids, the greatest risk of cardiovascular disease mortality was associated with increased proportions of oleic acid (18:1) and palmitoleic acid (16:1) as well as with palmitic acid (16:0). In contrast a high proportion of cholesteryl linoleate (18:2) was associated with a protective effect against cardiovascular disease-associated mortality whereby some 461 CVD deaths were recorded in over 30 years of observations in approximately 2,000 men.
Collectively, the data from human studies do not make certain whether or not dietary monounsaturated fat would provide an overall benefit for protection against CHD. However, an increasing body of evidence suggests that oleate enrichment of plasma cholesteryl esters, which can be driven by dietary monounsaturated fat, is positively and significantly associated with increased CHD and its correlates.
REDUCTION OF OLEATE ENRICHMENT IN PLASMA: A MECHANISM OF ATHEROPROTECTION BY N-3 AND N-6 PUFA
When comparing the effects of dietary MUFA and PUFA on atherosclerosis and CHD risk, it is important to distinguish between n-6 and n-3 PUFA. A large body of epidemiological studies suggests that a higher intake of n-6 PUFA reduces risk of CHD. In the Kuopio Heart Study, it was reported that middle-aged men with proportions of linoleic acid in serum in the upper third were up to three times less likely to die of CHD than men with proportions in the lower third (29). These findings agree with those from the Western Electric Study and the Nurses Health Study whereby PUFA was associated with reduced coronary death and myocardial infarction, respectively (30). Benefit of n-6 intake on CAA has been shown in experimental animal studies as well (15, 19, 20, 23). Despite the HDL-C lowering in monkeys fed a diet enriched in n-6 PUFA compared with MUFA, PUFA fed animals were consistently protected from CAA (12, 14, 15). Further, the monkeys fed n-6 PUFA exhibited the lowest cholesteryl oleate content in the core of apoB-containing lipoproteins (15), a finding reminiscent of observations in human studies, as reported by Reaven et al. (26).
Beginning with the study by Dyerberg et al. (31) involving Greenland Eskimos in the late 1970s, the body of evidence supporting intake of n-3 PUFAs from fish in the prevention of CHD has continued to grow. In the Zutphen Study, an increase in fish consumption from 0 to 45 g/day was associated with a progressive decrease in the risk of CHD death after 20 years (P < 0.05). When mortality rates for the duration of the Multiple Risk Factor Intervention Trial were divided into quintiles, there was a significant inverse relationship between intake of n-3 fatty acids and death from CHD, all coronary artery disease, and all-cause mortality (32). Similar outcomes were found in a prospective cohort study, the Physicians' Health Study, whereby dietary fish intake was associated with a reduced risk of sudden death (−52%, P = 0.03). Known biological activities of n-3 PUFAs important in atherosclerosis include cholesterol lowering (usually minor in humans), triglyceride lowering (good at higher doses), decreased platelet aggregation, reduced blood viscosity, favorable decreases in eicosanoid production responsible for vasodilator and antithrombotic properties, and anti-inflammatory effects (33).
Several studies in experimental animals have also shown that n-3 PUFA-enriched diets decrease the extent of diet-induced atherosclerosis (17, 34–36). Diets enriched in fish oil containing the n-3 PUFA, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) had a protective effect similar to or better than n-6 PUFA, as reported in nonhuman primates (17). Monkeys fed a fish-oil-enriched diet showed similar trends to those seen with diets enriched with n-6 PUFA. The fish-oil group had the lowest total plasma cholesterol level, hepatic cholesteryl ester concentration, and cholesteryl ester secretion in apoB-containing lipoproteins. The extent of CAA was significantly lower in the monkeys fed fish oil relative to the group fed a diet enriched with lard. Compared with the lard-based diet, fish oil feeding was associated with smaller LDL particles containing fewer cholesteryl ester molecules per particle and having lower cholesteryl ester transition temperatures due to a relative enrichment of n-3 fatty acids in the neutral lipid fraction (37).
When liver perfusion studies were carried out to determine metabolic alterations that contribute to the observed differences in plasma lipoprotein concentration and composition, significantly less cholesterol (P < 0.055) and triglyceride (P < 0.05) were secreted by the livers of monkeys fed fish oil compared with the lard fed control group, while hepatic apoB secretion was similar for both dietary groups (38). Enrichment of LDL particles with n-3 PUFA together with a reduced cholesteryl ester and triglyceride content in the nascent apoB-containing lipoproteins secreted by the liver was associated with less coronary artery and aortic atherosclerosis (36). It is important to reiterate that the decrease in atherosclerosis appeared related to the significant reduction of cholesteryl oleate in the core of LDL particles, which occurred in spite of a significant effect of dietary fish oil to decrease HDL-C in these animals. Further, no change in the number of VLDL particles secreted in plasma was found even though VLDL triglyceride secretion was decreased (38).
Similar beneficial effects of dietary n-3 PUFA on lipoprotein metabolism and atherosclerosis have been found in genetically engineered mouse models. Compared with monounsaturated and n-6 polyunsaturated fat-fed animals, mice fed fish oil had the lowest total plasma cholesterol and triglyceride levels; the lowest VLDL-C, LDL-C, and cholesteryl oleate in LDL particles; the lowest percentage of oleoyl-CoA in the liver; and the greatest protection from deposition of cholesteryl ester in the aorta (19, 22, 23). The two major n-3 PUFAs, EPA and DHA, are found in fish oil but also can be formed from the 18 carbon n-3 fatty acid, α-linolenic acid (ALA), through elongation and desaturation reactions occurring at the level of the endoplasmic reticulum and peroxisomes. The major sources of ALA are plant oils (e.g., flaxseed oil, perilla oil, and canola oil). When the relative effects of equivalent amounts of n-3 fatty acids from fish oil vs. flaxseed oil on lipids and atherosclerosis were compared in a mouse model of atherosclerosis, fish oil was more effective in reducing cholesterol and LDL cholesteryl oleate content and offered better protection from aortic atherosclerosis (23). The relatively inefficient conversion of ALA into EPA and DHA was apparent although data showing that ALA does get converted into EPA in phospholipids of red blood cell and liver membranes was obtained in mice (Rudel et al., unpublished data). The findings suggested that newly synthesized vs. exogenous EPA might have different metabolic fates leading to an intracellular localization that, in turn, might account for a different extent of atheroprotection (23). More studies comparing marine- and plant-derived n-3 PUFA for their effects on plasma and liver lipid metabolism are needed to delineate the potential mechanisms, if any, of cardioprotection by ALA.
CONCLUSIONS
The idea that a diet rich in MUFA and poor in SFA is atheroprotective is supported by studies examining the effects of mononunsaturated fat primarily on surrogate markers of CHD risk, such as total cholesterol, LDL-C, and HDL-C (7). However, studies in experimental animals and humans have shown that dietary MUFA enrich plasma lipids in cholesterol oleate; in experimental animals an increase in hepatic cholesteryl oleate was also observed. Increased atherosclerosis has been consistently aligned with LDL cholesteryl oleate enrichment in animal models. Cholesteryl oleate enrichment occurs via hepatic secretion of cholesteryl ester enriched LDL precursor particles promoted by dietary MUFA, while dietary PUFA does not promote cholesteryl oleate secretion. However, the mechanism that underlies the association of the cholesteryl oleate enriched LDL with atherosclerosis is presently only a matter for speculation. We hypothesize that LDL enriched in MUFA may be more active in binding to arterial proteoglycans, a property that could contribute to increased arterial retention as the mechanism for promoting atherosclerosis progression (39–41). In fact, we have provided evidence for altered proteoglycan binding of LDL from monkeys fed different dietary fats, with LDL from monkeys fed MUFA and SFA forming increased numbers of insoluble complexes with arterial chondroitin sulfate proteoglycans than LDL from monkeys fed n-3 and n-6 PUFA (41). These associations are in line with differences in atherosclerosis but do not exactly follow the pattern for LDL-C concentrations (i.e., LDL composition may determine at least a part of the relative degree of LDL atherogenicity). Future studies are needed to provide more definitive mechanistic information about how an increase in LDL cholesteryl oleate promotes atherogenesis.
The work presented here was made possible with the support of the National Institutes of Health grants HL-24736, HL-49373, and AT-002782.
Published, JLR Papers in Press, November 22, 2008.
References
- 1.Kinsell L. W., J. Partridge, L. Boling, S. Margen, and G. Michaels. 1952. Dietary modification of serum cholesterol and phospholipids levels. J. Clin. Endocrinol. Metab. 12 909–913. [DOI] [PubMed] [Google Scholar]
- 2.Groen J., B. K. Tjiong, C. E. Kammingra, and A. F. Willebrands. 1952. The influence of nutrition, individuality and some other factors including various forms of stress, on serum cholesterol; an experiment of nine months duration in 60 normal human volunteers. Voeding. 13 556. [Google Scholar]
- 3.Keys A. 1997. Coronary heart disease in seven countries 1970. Nutrition. 13 250–252. [DOI] [PubMed] [Google Scholar]
- 4.Mensink R. P., and M. B. Katan. 1992. Effects of dietary fatty acids on serum lipids anad lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. 12 911–919. [DOI] [PubMed] [Google Scholar]
- 5.Gardner C. D., and H. C. Kraemer. 1995. Monounsaturated versus polyunsaturated dietary fat and serum lipids. A meta-analysis. Arterioscler. Thromb. Vasc. Biol. 15 1917–1927. [DOI] [PubMed] [Google Scholar]
- 6.Hu F. B., M. J. Stampfer, J. E. Manson, E. Rimm, G. A. Colditz, B. A. Rosner, C. H. Hennekens, and W. C. Willett. 1997. Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med. 337 1491–1499. [DOI] [PubMed] [Google Scholar]
- 7.Mattson F. H., and S. M. Grundy. 1985. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J. Lipid Res. 26 194–202. [PubMed] [Google Scholar]
- 8.Katan M. B., S. M. Grundy, and W. C. Willett. 1997. Should a low-fat, high carbohydrate diet be recommended for everyone? Beyond low-fat diets. N. Engl. J. Med. 337 563–569. [PubMed] [Google Scholar]
- 9.Brown J. M., G. S. Shelness, and L. L. Rudel. 2007. Monounsaturated fatty acids and atherosclerosis: opposing views from epidemiology and experimental animal models. Curr. Atheroscler. Rep. 9 494–500. [DOI] [PubMed] [Google Scholar]
- 10.Rudel L. L., L. L. Pitts, and C. A. Nelson. 1977. Characterization of plasma low density lipoproteins of nonhuman primates fed dietary cholesterol. J. Lipid Res. 18 211–222. [PubMed] [Google Scholar]
- 11.Rudel L. L., and L. L. Pitts. 1978. Male-female variability in the dietary cholesterol-induced hyperlipoproteinemia of cynomolgus monkeys (Macaca fascicularis). J. Lipid Res. 19 992–1003. [PubMed] [Google Scholar]
- 12.Rudel L. L., J. S. Parks, C. C. Hedrick, M. Thomas, and K. Williford. 1998. Lipoprotein and cholesterol metabolism in diet-induced coronary artery atherosclerosis in primates. Role of cholesterol and fatty acids. Prog. Lipid Res. 37 353–370. [DOI] [PubMed] [Google Scholar]
- 13.Rudel L. L., F. L. Johnson, J. K. Sawyer, M. D. Wilson, and J. S. Parks. 1995. Dietary polyunsaturated fat modifies low-density lipoproteins and reduces atherosclerosis of nonhuman primates with high and low diet responsiveness. Am. J. Clin. Nutr. 62 463S–470S. [DOI] [PubMed] [Google Scholar]
- 14.Rudel L. L., M. G. Bond, and B. C. Bullock. 1985. LDL heterogeneity and atherosclerosis in non-human primates. Ann. N. Y. Acad. Sci. 454 248–253. [DOI] [PubMed] [Google Scholar]
- 15.Rudel L. L., J. S. Parks, and J. K. Sawyer. 1995. Compared with dietary monounsaturated and saturated fat, polyunsaturated fat protects African Green monkeys from coronary artery atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 15 2101–2110. [DOI] [PubMed] [Google Scholar]
- 16.Lee R. G., M. C. Willingham, M. A. Davis, K. A. Skinner, and L. L. Rudel. 2000. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J. Lipid Res. 41 1991–2001. [PubMed] [Google Scholar]
- 17.Carr T. P., J. S. Parks, and L. L. Rudel. 1992. Hepatic ACAT activity in African Green monkeys is highly correlated to plasma plasma LDL cholesteryl ester enrichment and coronary artery atherosclerosis. Arterioscler. Thromb. 12 1274–1283. [DOI] [PubMed] [Google Scholar]
- 18.Rudel L. L., J. Haines, J. K. Sawyer, R. Shah, M. D. Wilson, and T. P. Carr. 1997. Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J. Clin. Invest. 100 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudel L. L., K. Kelley, J. K. Sawyer, R. Shah, and M. D. Wilson. 1998. Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human apoB100-overexpressing transgenic mice. Arterioscler. Thromb. Vasc. Biol. 18 1818–1827. [DOI] [PubMed] [Google Scholar]
- 20.Merkel M., W. Velez-Carrasco, L. C. Hudgins, and J. A. Breslow. 2001. Compared with saturated fatty acids, dietary monounsaturated fatty acids and carbohydrates increase atherosclerosis and VLDL cholesterol levels in LDL receptor-deficient, but not apolipoprotein E-deficient, mice. Proc. Natl. Acad. Sci. USA. 98 13294–13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parini P., M. A. Davis, A. T. Lada, S. K. Erickson, T. L. Wright, U. Gustafsson, S. Sahlin, C. Einarsson, M. Eriksson, B. Angelin, et al. 2004. ACAT2 is localized to hepatocytes and is the major cholesterol esterifying enzyme in human liver. Circulation. 110 2017–2023. [DOI] [PubMed] [Google Scholar]
- 22.Bell III T. A., M. D. Wilson, K. Kelley, J. K. Sawyer, and L. L. Rudel. 2007. Monounsaturated fatty acyl-coenzyme A is predictive of atherosclerosis in human apoB-100 transgenic, LDLr−/− mice. J. Lipid Res. 48 1122–1131. [DOI] [PubMed] [Google Scholar]
- 23.Bell III T. A., K. Kelley, M. D. Wilson, J. K. Sawyer, and L. L. Rudel. 2007. Dietary fat-induced alterations in atherosclerosis are abolished by ACAT2-deficiency in apoB100-only, LDLr−/− mice. Arterioscler. Thromb. Vasc. Biol. 27 1396–1402. [DOI] [PubMed] [Google Scholar]
- 24.Lada A. T., and L. L. Rudel. 2003. Dietary monounsaturated versus polyunsaturated fatty acids: which is really better for protection from coronary heart disease? Curr. Opin. Lipidol. 14 41–46. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Miranda J., and P. Mata. 2002. Protective effect of dietary monounsaturated fat on atherosclerosis: beyond cholesterol. Atherosclerosis. 163 385–398. [DOI] [PubMed] [Google Scholar]
- 26.Reaven P., S. Parthasarathy, B. J. Grasse, E. Miller, D. Steinberg, and J. L. Witztum. 1993. Effects of oleate-rich and linoleate-rich diets on the susceptibility of low density lipoprotein to oxidative modification in midly hypercholesterolemic subjects. J. Clin. Invest. 91 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J., A. R. Folsom, L. Lewis, and J. H. Eckfeldt. 1997. Relation of plasma phospholipids and cholesterol ester fatty acid composition to carotid artery intima-media thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 65 551–559. [DOI] [PubMed] [Google Scholar]
- 28.Warensjo E., J. Sundstrom, B. Vessby, T. Cederholm, and U. Riserus. 2008. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am. J. Clin. Nutr. 88 203–209. [DOI] [PubMed] [Google Scholar]
- 29.Laaksonen D. E., K. Nyyssonen, L. Niskanen, T. H. Rissanen, and J. T. Salonen. 2005. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch. Intern. Med. 165 193–199. [DOI] [PubMed] [Google Scholar]
- 30.Kris-Etherton P. M., K. D. Hecker, and A. E. Binkoski. 2004. Polyunsaturated fatty acids and cardiovascular health. Nutr. Rev. 62 414–426. [DOI] [PubMed] [Google Scholar]
- 31.Dyerberg J., H. O. Bang, E. Stoffersen, S. Moncada, and J. R. Vane. 1978. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 2 117–119. [DOI] [PubMed] [Google Scholar]
- 32.Caggiula A. W., and V. A. Mustad. 1997. Effects of dietary fat and fatty acids on coronary artery disease risk and total and lipoprotein cholesterol concentrations: epidemiologic studies. Am. J. Clin. Nutr. 65 (Suppl): 1597S–1610S. [DOI] [PubMed] [Google Scholar]
- 33.Demaison L., and D. Moreau. 2002. Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: a possible mechanism of action. Cell. Mol. Life Sci. 59 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis H. R., R. T. Bridenstine, D. Vesselinovitch, and R. W. Wissler. 1987. Fish oil inhibits development of atherosclerosis in rhesus monkeys. Atherosclerosis. 7 441–449. [DOI] [PubMed] [Google Scholar]
- 35.Zhu B., D. L. Smith, R. E. Sievers, W. M. Isenberg, and W. W. Parmley. 1988. Inhibition of atherosclerosis by fish oil in cholesterol-fed rabbits. J. Am. Coll. Cardiol. 12 1073–1078. [DOI] [PubMed] [Google Scholar]
- 36.Parks J. S., J. Kaduck-Sawyer, B. C. Bullock, and L. L. Rudel. 1990. Effect of dietary fish oil on coronary artery and aortic atherosclerosis in African green monkeys. Arteriosclerosis. 7 1102–1112. [DOI] [PubMed] [Google Scholar]
- 37.Parks J. S., and B. C. Bullock. 1987. Effect of fish oil vs lard diets on the chemical and physical properties of low density lipoproteins of non-human primates. J. Lipid Res. 28 173–182. [PubMed] [Google Scholar]
- 38.Parks J. S., M. D. Wilson, F. L. Johnson, and L. L. Rudel. 1989. Fish oil decreases hepatic cholesteryl ester secretion but not apoB secretion in African green monkeys. J. Lipid Res. 30 1535–1544. [PubMed] [Google Scholar]
- 39.Camejo G., S. Waich, L. Mateu, H. Acquatella, F. Lalaguna, G. Quintero, and M. L. Berrizbeitia. 1976. Differences in the structure of plasma low-density lipoproteins and their relationship to the extent of interaction with arterial wall components. Ann. N. Y. Acad. Sci. 275 153–168. [DOI] [PubMed] [Google Scholar]
- 40.Williams K. J., and I. Tabas. 1995. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manning J. M., A. K. Gebre, I. J. Edwards, W. D. Wagner, L. L. Rudel, and J. S. Parks. 1994. Dietary polyunsaturated fat decreases interaction between low density lipoproteins and arterial proteoglycans. Lipids. 29 635–641. [DOI] [PubMed] [Google Scholar]