Abstract
The outer monolayer of the outer membrane of Gram-negative bacteria consists of the lipid A component of lipopolysaccharide (LPS), a glucosamine-based saccharolipid that is assembled on the inner surface of the inner membrane. The first six enzymes of the lipid A pathway are required for bacterial growth and are excellent targets for the development of new antibiotics. Following assembly, the ABC transporter MsbA flips nascent LPS to the periplasmic side of the inner membrane, whereupon additional transport proteins direct it to the outer surface of the outer membrane. Depending on the bacterium, various covalent modifications of the lipid A moiety may occur during the transit of LPS to the outer membrane. These extra-cytoplasmic modification enzymes are therefore useful as reporters for monitoring LPS trafficking. Because of its conserved structure in diverse Gram-negative pathogens, lipid A is recognized as foreign by the TLR4/MD2 receptor of the mammalian innate immune system, resulting in rapid macrophage activation and robust cytokine production.
Keywords: Francisella tularensis, endotoxin, vaccine adjuvant
DISCOVERY OF LIPID A BIOSYNTHESIS
Although lipid A was recognized over 50 years ago as the hydrophobic moiety of lipopolysaccharide (LPS) (Fig. 1), the elucidation of lipid A biosynthesis was delayed until 1983. A key breakthrough was the discovery and structural characterization of a novel Escherichia coli lipid, termed 2,3-diacylglucosamine 1-phosphate or “lipid X” (Fig. 2) (1). This substance was overlooked in earlier work with E. coli because of its low levels in wild-type cells, but it accumulates in certain phosphatidylglycerol-deficient mutants (2). The recognition of lipid X coincided with the determination of the correct structure of lipid A (1, 3, 4). The identification of 2,3-diacylglucosamine 1-phosphate as a precursor of the proximal (right) subunit of lipid A (Fig. 2) was critical for developing testable hypotheses regarding the enzymology and genetics of lipid A assembly (5).
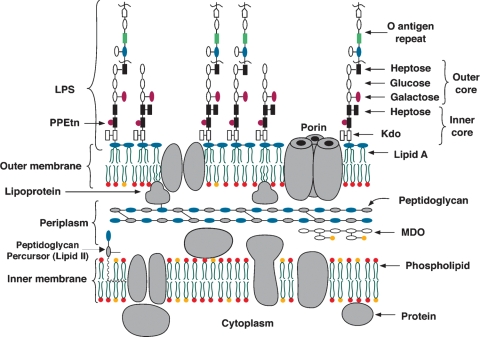
Fig. 1.
Schematic representation of the E. coli cell envelope.
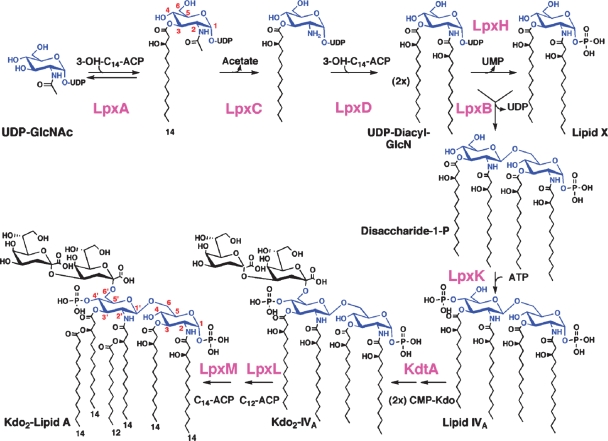
Fig. 2.
The constitutive pathway of lipid A biosynthesis in E. coli.
Given the importance of structure determination for the discovery of new pathways, as illustrated by the lipid A story (5), the LIPID MAPS Consortium is attempting to identify additional novel lipids, using mass spectrometry as the starting point (6). Many as yet unknown lipid pathways probably exist in all organisms with possible roles in signal transduction, regulation, and membrane assembly.
THE CONSTITUTIVE ENZYMATIC PATHWAY FOR LIPID A ASSEMBLY
Lipid A, the hydrophobic anchor of LPS, is a glucosamine-based lipid that makes up the outer monolayer of the outer membrane (OM) (Fig. 1) of Gram-negative bacteria (5, 7). An E. coli cell contains ∼106 lipid A residues and ∼107 glycerophospholipids (7). The lipid A and Kdo domains of LPS (Figs. 1 and 2) are usually required for growth (7), but Kdo can be eliminated in the presence of suppressors (8). Wild-type LPS contains additional core and O-antigen sugars, which are not needed for growth, but protect against antibiotics and complement (7).
The constitutive enzymes of the lipid A pathway are conserved (7). They are located in the cytoplasm or on the inner surface of the inner membrane (IM) (7). The first step is the acylation of UDP-GlcNAc (Fig. 2), catalyzed by LpxA. In E. coli LpxA is selective for β-hydroxymyristoyl acyl carrier protein (9). The active site of E. coli LpxA functions as an accurate hydrocarbon ruler that incorporates a β-hydroxymyristoyl chain two orders of magnitude faster than a β-hydroxylauroyl or a β-hydroxypalmitoyl chain (10). Other LpxA orthologs are designed to incorporate longer or shorter β-hydroxyacyl chains (7).
The equilibrium constant (∼0.01) for UDP-GlcNAc acylation is unfavorable (11). The deacetylation of UDP-3-O-(acyl)-GlcNAc by LpxC is therefore the committed step. LpxC is a Zn2+-dependent enzyme (12), conserved in diverse Gram-negative bacteria, and is an excellent target for new antibiotics (13, 14). Slow, tight-binding inhibitors of LpxC, such as CHIR-090, have antibiotic activity comparable to ciprofloxacin and are effective against almost all Gram-negatives (14), including E. coli, Pseudomonas aeruginosa, and Francisella novicida. Following deacetylation, a second β-hydroxymyristoyl chain is added by E. coli LpxD to make UDP-2,3-diacyl-GlcN (Fig. 2) (15), which is cleaved by LpxH to form 2,3-diacyl-GlcN-1-phosphate (lipid X) (16). A β,1′-6 linked disaccharide is then generated by LpxB, which condenses another molecule of UDP-2,3-diacyl-GlcN with lipid X (17). LpxA, LpxC, and LpxD are soluble proteins with known structures (18–21). LpxH and LpxB are peripheral membrane proteins; their structures have not been reported (16, 17).
The IM proteins LpxK, KdtA, LpxL, and LpxM catalyze the last five steps (Fig. 2) (7). Each contains one predicted membrane-spanning segment. LpxK phosphorylates the 4′-position (Fig. 2) to generate the intermediate lipid IVA (22), which is an endotoxin antagonist in human cells, but an agonist in mouse (23). This differential pharmacology is determined by the lipid A receptor of the mammalian innate immune system, the TLR4/MD2 complex (24, 25). Next, two Kdo residues are incorporated by the bifunctional enzyme KdtA (Fig. 2) (26). The labile nucleotide CMP-Kdo, derived from arabinose 5-phosphate (not shown), is the Kdo donor (26). The last steps of E. coli lipid A biosynthesis involve the addition of the secondary laurate and myristate chains (Fig. 2) by LpxL and LpxM (27), which display sequence similarity to each other and are related to the lysophosphatidic acid acyltransferases (28). The product, hexa-acylated lipid A (Fig. 2), is a TLR4/MD2 agonist in diverse animal species. The lpxM gene is usually nonessential (27). Salmonella typhimurium and Shigella lpxM mutants make penta-acylated lipid A and are attenuated (29).
Why lipid A structure is relatively conserved and required for growth in most Gram-negatives remains uncertain. Neisseria meningitidis is unusual in that its lpxA gene can be deleted; these mutants grow slowly without lipid A or LPS, but still assemble an OM (30).
UNUSUAL STRUCTURE OF FRANCISELLA LIPID A
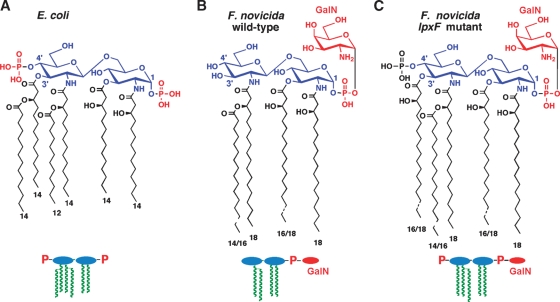
Despite the conservation of the constitutive pathway in diverse bacterial genomes, lipid A structure can vary considerably. In F. novicida, a mouse-specific strain that is a model for the type A human pathogen Francisella tularensis, the 4′-phosphate moiety and the 3′-hydroxyacyl chain are missing (Fig. 3A versus 3B) (31–33). Furthermore, the phosphate group at the 1-position is modified with a galactosamine (GalN) unit (Fig. 3B) (32, 33). We have discovered that over 90% of the lipid A in F. novicida is in a “free” form, i.e., not linked to Kdo, core sugars, and O-antigen (33). The small amount of LPS that is present in F. novicida contains only a single Kdo residue (31). A portion of the LPS (31), but not the free lipid A (33), also lacks the usual lipid A 1-phosphate group (Fig. 4).
Fig. 3.
The structure of lipid A in E. coli versus F. novicida.
Fig. 4.
Lipid A modification enzymes in F. novicida.
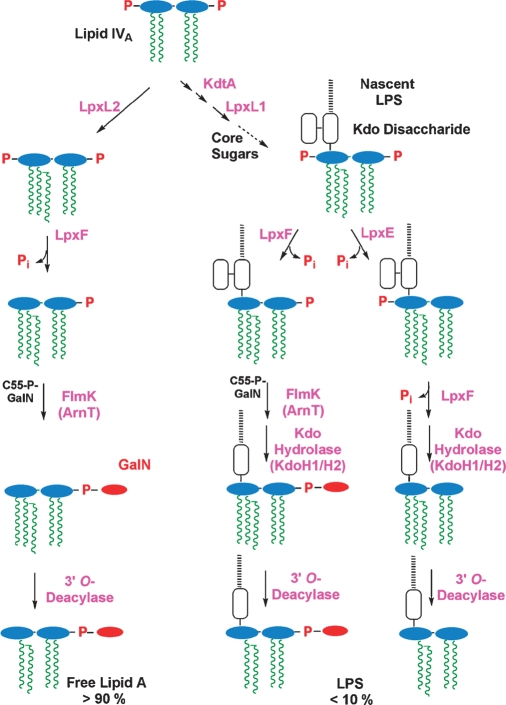
We have recently elucidated the enzymatic basis for many of the structural differences between F. novicida and E. coli lipid A. Like E. coli, F. novicida first makes lipid IVA (Fig. 2), albeit with longer hydroxyacyl chains (5). If the lipid IVA is glycosylated by KdtA, it is then acylated by the Kdo-dependent acyltransferase LpxL1 and converted to LPS (Fig. 4, right side). Alternatively, F. novicida lipid IVA can acquire its 2′ secondary acyl chain directly without Kdo addition (Fig. 4, left side), because of a second LpxL ortholog (LpxL2), which is Kdo-independent (D. Six and C. Raetz, unpublished observations). LpxL2 may function more rapidly than KdtA in F. novicida. The LpxL2 penta-acylated product is exported by MsbA (Fig. 5), possibly accounting for the large pool of free lipid A. No hexa-acylated lipid A is made because F. novicida lacks LpxM (Fig. 2).
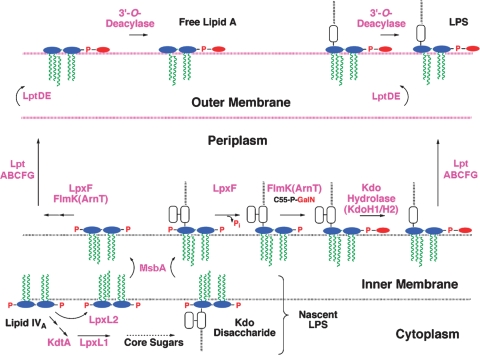
Fig. 5.
Topography of lipid A export and modification in F. novicida.
Removal of the phosphate groups by LpxF (34) and LpxE (35) (Figs. 4 and 5) occurs on the outer surface of the IM. The order of processing is not fully established. The phosphatase genes have been cloned and expressed in E. coli, where their function in cells requires MsbA (the IM flippase for LPS) (34, 35). In F. novicida, the 4′-phosphate group is removed quantitatively in both free lipid A (33) and LPS-bound lipid A (Fig. 4) (31, 32). However, LpxE, the 1-phosphatase, is Kdo-dependent (Fig. 4) (35); thus, the 1-phosphate group is present in free lipid A, but is missing in some of the LPS (Fig. 4, right bottom) (31). Deletion of lpxF results in retention of the 4′-phosphate group and the 3′-hydroxyacyl chain (36) (Fig. 3C), implying sequential processing (Fig. 4, bottom). LpxF mutants are highly attenuated (36) and can be used to immunize mice against wild-type F. novicida (R. Ernst and C. Raetz, unpublished observations).
The GalN moiety (Fig. 3), which is present on both free lipid A and on LPS-bound lipid A (Fig. 4), is attached by FlmK (an ortholog of E. coli ArnT) on the outer surface of the IM (33, 37). The GalN donor is undecaprenyl-phosphate (C55-P)-GalN (Fig. 4), which is generated from C55P and UDP-GalNAc (X. Wang, F. Song, and C. Raetz, unpublished observations). The removal of the 3′-hydroxyacyl chain (Fig. 4) probably occurs in the OM (Fig. 5), as in the case with Salmonella LpxR (38), but the relevant F. novicida 3′-O-deacylase gene has not yet been identified. Interestingly, F. novicida KdtA is bifunctional (J. Zhao and C. Raetz, unpublished observations), but its LPS contains only one Kdo residue (31). This anomaly is explained in part by the presence of a Kdo hydrolase in F. novicida (35), which consists both of a catalytic and a membrane-anchoring subunit, termed KdoH1 and KdoH2, respectively (J. Zhao and C. Raetz, unpublished observations). This type of hydrolase is also found in Helicobacter (39). Deletion of KdtA in F. novicida is not lethal, but it eliminates LPS and causes modest accumulation of free lipid A (J. Zhao and C. Raetz, unpublished observations).
DIVERSITY AND INTERCHANGEABILITY OF LIPID A MODIFICATION SYSTEMS
Whereas the constitutive lipid A pathway is conserved, the systems for lipid A modification are diverse and are usually not required for growth (5). What the modification enzymes have in common is their localization (Fig. 5) (5). With few exceptions (5), removal or modification of the lipid A phosphate groups occurs on the outer surface of the IM, whereas removal of acyl chains generally occurs in the OM (5) (Fig. 5). The distinct localization of the modification enzymes, in contrast to the intracellular constitutive enzymes (Fig. 2), makes the former excellent reporters for LPS trafficking (40, 41).
Lipid A modification enzymes can be transferred from one bacterial species to another. Expression of LpxE in E. coli or Salmonella results in nearly quantitative 1-dephosphorylation of lipid A without obvious growth impairment (35). Expression of F. novicida LpxF in E. coli or Salmonella LpxM mutants (which synthesize penta-acylated lipid A) results in the complete removal of the 4′-phosphate group (34). However, wild-type F. novicida LpxF cannot dephosphorylate hexa-acylated lipid A (34).
The attenuation of F. novicida LpxF mutants results from their hypersensitivity to cationic antimicrobial peptides, because of the negative charge presented by the LpxF mutant lipid A (Fig. 3) (36). Consequently, it is now possible to construct novel attenuated bacterial strains by interchanging or mutating lipid A modification enzymes (34, 36).
LIPID A AS AN ACTIVATOR OF INNATE IMMUNITY
Many Gram-negatives synthesize lipid A species resembling those of E. coli (Fig. 2) (5). As noted above, lipid A is detected by the TLR4/MD2 receptor, the crystal structure of which was recently determined (24, 25). E. coli lipid A induces macrophages to make potent mediators of inflammation, such as tumor necrosis factor-α and interleukin-1β (4). These events are desirable for clearing local infections, but when overproduced systemically during sepsis, these proteins can damage small blood vessels and contribute to septic shock (42). The characteristic structural features of E. coli lipid A (Fig. 2), especially its two phosphate groups and acyloxyacyl chains, are needed to trigger full TLR4/MD2 activation (4). With the availability of modification enzymes like LpxE, the lipid A moiety of live bacteria can now be altered by heterologous expression (35) to be a TLR4/MD2 partial agonist, thereby retaining its desirable adjuvant properties while greatly reducing its toxicity (43).
DIRECTIONS FOR THE FUTURE
The following questions regarding lipid A biology and chemistry remain to be answered: 1) Why is the lipid A moiety of LPS required for growth in most Gram-negative bacteria? Studies of unusual systems, like N. meningitidis, in which lipid A is not required for growth, might provide the answer (30); 2) Can in vitro assays for LPS flip-flop and intermembrane transport be developed? The genetic evidence for the role of the MsbA transporter and the Lpt protein complex is compelling, but it needs to be strengthened with in vitro biochemistry employing purified components; 3) Can new antibiotics be developed by targeting LpxC and other enzymes of the lipid A pathway? The best available LpxC inhibitors (14) are potent enough for clinical trials, but studies of bioavailability, pharmacokinetics and toxicology have not been published; and 4) Can lipid A modification enzymes be exploited for vaccine development? The opportunities afforded by attenuated lpxF mutants of F. tularensis (36) or strains lacking lpxM (msbB) are under investigation (29). The properties of Salmonella strains expressing LpxE, which synthesize the mono-phosphorylated, nontoxic adjuvant form of lipid A, will be of great interest.
Abbreviations
GalN, galactosamine
IM, inner membrane
LPS, lipopolysaccharide
OM, outer membrane
This research is supported by the National Institutes of Health (NIH) grants GM-51310 and GM-51796 to C.R.H.R. The mass spectrometry facility in the Department of Biochemistry at the Duke University Medical Center is supported by the LIPID MAPS Large Scale Collaborative Grant GM-069338 from the NIH.
Published, JLR Papers in Press, October 29, 2008.
References
- 1.Takayama K., N. Qureshi, P. Mascagni, M. A. Nashed, L. Anderson, and C. R. H. Raetz. 1983. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A: complete structure of a diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J. Biol. Chem. 258 7379–7385. [PubMed] [Google Scholar]
- 2.Nishijima M., and C. R. H. Raetz. 1979. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J. Biol. Chem. 254 7837–7844. [PubMed] [Google Scholar]
- 3.Raetz C. R. H. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59 129–170. [DOI] [PubMed] [Google Scholar]
- 4.Rietschel E. T., T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zähringer, U. Seydel, F. Di Padova, et al. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8 217–225. [DOI] [PubMed] [Google Scholar]
- 5.Raetz C. R. H., C. M. Reynolds, M. S. Trent, and R. E. Bishop. 2007. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 76 295–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahy E., S. Subramaniam, H. A. Brown, C. K. Glass, A. H. Merrill, Jr., R. C. Murphy, C. R. H. Raetz, D. W. Russell, Y. Seyama, W. Shaw, et al. 2005. A comprehensive classification system for lipids. J. Lipid Res. 46 839–862. [DOI] [PubMed] [Google Scholar]
- 7.Raetz C. R. H., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71 635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meredith T. C., P. Aggarwal, U. Mamat, B. Lindner, and R. W. Woodard. 2006. Redefining the requisite lipopolysaccharide structure in Escherichia coli. ACS Chem. Biol. 1 33–42. [DOI] [PubMed] [Google Scholar]
- 9.Anderson M. S., and C. R. H. Raetz. 1987. Biosynthesis of lipid A precursors in Escherichia coli: a cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J. Biol. Chem. 262 5159–5169. [PubMed] [Google Scholar]
- 10.Wyckoff T. J. O., S. Lin, R. J. Cotter, G. D. Dotson, and C. R. H. Raetz. 1998. Hydrocarbon rulers in UDP-N-acetylglucosamine acyltransferases. J. Biol. Chem. 273 32369–32372. [DOI] [PubMed] [Google Scholar]
- 11.Anderson M. S., H. S. Bull, S. M. Galloway, T. M. Kelly, S. Mohan, K. Radika, and C. R. H. Raetz. 1993. UDP-N-acetylglucosamine acyltransferase of Escherichia coli: the first step of endotoxin biosynthesis is thermodynamically unfavorable. J. Biol. Chem. 268 19858–19865. [PubMed] [Google Scholar]
- 12.Jackman J. E., C. R. H. Raetz, and C. A. Fierke. 1999. UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase of Escherichia coli is a zinc metalloenzyme. Biochemistry. 38 1902–1911. [DOI] [PubMed] [Google Scholar]
- 13.Onishi H. R., B. A. Pelak, L. S. Gerckens, L. L. Silver, F. M. Kahan, M. H. Chen, A. A. Patchett, S. M. Galloway, S. A. Hyland, M. S. Anderson, et al. 1996. Antibacterial agents that inhibit lipid A biosynthesis. Science. 274 980–982. [DOI] [PubMed] [Google Scholar]
- 14.McClerren A. L., S. Endsley, J. L. Bowman, N. H. Andersen, Z. Guan, J. Rudolph, and C. R. H. Raetz. 2005. A slow, tight-binding inhibitor of the zinc-dependent deacetylase LpxC of lipid A biosynthesis with antibiotic activity comparable to ciprofloxacin. Biochemistry. 44 16574–16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartling C. M., and C. R. H. Raetz. 2008. Steady-state kinetics and mechanism of LpxD, the N-acyltransferase of lipid A biosynthesis. Biochemistry. 47 5290–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babinski K. J., A. A. Ribeiro, and C. R. H. Raetz. 2002. The Escherichia coli gene encoding the UDP-2,3-diacylglucosamine pyrophosphatase of lipid A biosynthesis. J. Biol. Chem. 277 25937–25946. [DOI] [PubMed] [Google Scholar]
- 17.Radika K., and C. R. H. Raetz. 1988. Purification and properties of lipid A disaccharide synthase of Escherichia coli. J. Biol. Chem. 263 14859–14867. [PubMed] [Google Scholar]
- 18.Raetz C. R. H., and S. L. Roderick. 1995. A left-handed parallel β helix in the structure of UDP-N-acetylglucosamine acyltransferase. Science. 270 997–1000. [DOI] [PubMed] [Google Scholar]
- 19.Coggins B. E., A. L. McClerren, L. Jiang, X. Li, J. Rudolph, O. Hindsgaul, C. R. H. Raetz, and P. Zhou. 2005. Refined solution structure of the LpxC-TU-514 complex and pKa analysis of an active site histidine: insights into the mechanism and inhibitor design. Biochemistry. 44 1114–1126. [DOI] [PubMed] [Google Scholar]
- 20.Whittington D. A., K. M. Rusche, H. Shin, C. A. Fierke, and D. W. Christianson. 2003. Crystal structure of LpxC, a zinc-dependent deacetylase essential for endotoxin biosynthesis. Proc. Natl. Acad. Sci. USA. 100 8146–8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buetow L., T. K. Smith, A. Dawson, S. Fyffe, and W. N. Hunter. 2007. Structure and reactivity of LpxD, the N-acyltransferase of lipid A biosynthesis. Proc. Natl. Acad. Sci. USA. 104 4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrett T. A., J. L. Kadrmas, and C. R. H. Raetz. 1997. Identification of the gene encoding the Escherichia coli lipid A 4′-kinase. Facile synthesis of endotoxin analogs with recombinant LpxK. J. Biol. Chem. 272 21855–21864. [DOI] [PubMed] [Google Scholar]
- 23.Golenbock D. T., R. Y. Hampton, N. Qureshi, K. Takayama, and C. R. H. Raetz. 1991. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J. Biol. Chem. 266 19490–19498. [PubMed] [Google Scholar]
- 24.Ohto U., K. Fukase, K. Miyake, and Y. Satow. 2007. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 316 1632–1634. [DOI] [PubMed] [Google Scholar]
- 25.Kim H. M., B. S. Park, J. I. Kim, S. E. Kim, J. Lee, S. C. Oh, P. Enkhbayar, N. Matsushima, H. Lee, O. J. Yoo, et al. 2007. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 130 906–917. [DOI] [PubMed] [Google Scholar]
- 26.Belunis C. J., and C. R. H. Raetz. 1992. Biosynthesis of endotoxins: purification and catalytic properties of 3-deoxy-D-manno-octulosonic acid transferase from Escherichia coli. J. Biol. Chem. 267 9988–9997. [PubMed] [Google Scholar]
- 27.Vorachek-Warren M. K., S. Ramirez, R. J. Cotter, and C. R. H. Raetz. 2002. A triple mutant of Escherichia coli lacking secondary acyl chains on lipid A. J. Biol. Chem. 277 14194–14205. [DOI] [PubMed] [Google Scholar]
- 28.Six D. A., S. M. Carty, Z. Guan, and C. R. H. Raetz. 2008. Purification and mutagenesis of LpxL, the lauroyltransferase of Escherichia coli lipid A biosynthesis. Biochemistry. 47 8623–8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Hauteville H., S. Khan, D. J. Maskell, A. Kussak, A. Weintraub, J. Mathison, R. J. Ulevitch, N. Wuscher, C. Parsot, and P. J. Sansonetti. 2002. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J. Immunol. 168 5240–5251. [DOI] [PubMed] [Google Scholar]
- 30.Bos M. P., V. Robert, and J. Tommassen. 2007. Biogenesis of the gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61 191–214. [DOI] [PubMed] [Google Scholar]
- 31.Vinogradov E., M. B. Perry, and J. W. Conlan. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269 6112–6118. [DOI] [PubMed] [Google Scholar]
- 32.Phillips N. J., B. Schilling, M. K. McLendon, M. A. Apicella, and B. W. Gibson. 2004. Novel modification of lipid A of Francisella tularensis. Infect. Immun. 72 5340–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X., A. A. Ribeiro, Z. Guan, S. McGrath, R. Cotter, and C. R. H. Raetz. 2006. Structure and biosynthesis of free lipid A molecules that replace lipopolysaccharide in Francisella tularensis subsp. novicida. Biochemistry. 45 14427–14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., S. C. McGrath, R. J. Cotter, and C. R. H. Raetz. 2006. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4′-phosphatase LpxF. J. Biol. Chem. 281 9321–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., M. J. Karbarz, S. C. McGrath, R. J. Cotter, and C. R. H. Raetz. 2004. MsbA transporter-dependent lipid A 1-dephosphorylation on the periplasmic surface of the inner membrane: topography of Francisella novicida LpxE expressed in Escherichia coli. J. Biol. Chem. 279 49470–49478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., A. A. Ribeiro, Z. Guan, S. N. Abraham, and C. R. H. Raetz. 2007. Attenuated virulence of a Francisella mutant lacking the lipid A 4′-phosphatase. Proc. Natl. Acad. Sci. USA. 104 4136–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanistanon D., A. M. Hajjar, M. R. Pelletier, L. A. Gallagher, T. Kalhorn, S. A. Shaffer, D. R. Goodlett, L. Rohmer, M. J. Brittnacher, S. J. Skerrett, et al. 2008. A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLoS Pathog. 4 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds C. M., A. A. Ribeiro, S. C. McGrath, R. J. Cotter, C. R. H. Raetz, and M. S. Trent. 2006. An outer membrane enzyme encoded by Salmonella typhimurium lpxR that removes the 3′-acyloxyacyl moiety of lipid A. J. Biol. Chem. 281 21974–21987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stead C., A. Tran, D. Ferguson, Jr., S. McGrath, R. Cotter, and S. Trent. 2005. A novel 3-deoxy-D-manno-octulosonic acid (Kdo) hydrolase that removes the outer Kdo sugar of Helicobacter pylori lipopolysaccharide. J. Bacteriol. 187 3374–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doerrler W. T., H. S. Gibbons, and C. R. H. Raetz. 2004. MsbA-dependent translocation of lipids across the inner membrane of Escherichia coli. J. Biol. Chem. 279 45102–45109. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz N., L. S. Gronenberg, D. Kahne, and T. J. Silhavy. 2008. Identification of two inner-membrane proteins required for the transport of lipopolysaccharide to the outer membrane of Escherichia coli. Proc. Natl. Acad. Sci. USA. 105 5537–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell J. A. 2006. Management of sepsis. N. Engl. J. Med. 355 1699–1713. [DOI] [PubMed] [Google Scholar]
- 43.Baldridge J. R., P. McGowan, J. T. Evans, C. Cluff, S. Mossman, D. Johnson, and D. Persing. 2004. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin. Biol. Ther. 4 1129–1138. [DOI] [PubMed] [Google Scholar]