Abstract
The skin serves the vital function of providing a barrier between the hostile external environment and the host. While the skin has many important barrier functions, the two that are absolutely essential for survival are the barrier to the movement of water and electrolytes (permeability barrier) and the barrier against invasive and toxic microorganisms (antimicrobial barrier). Lipids play an essential role in the formation and maintenance of both the permeability and antimicrobial barriers. A hydrophobic extracellular lipid matrix in the stratum corneum composed primarily of ceramides, cholesterol, and free fatty acids provides the barrier to the movement of water and electrolytes. A variety of lipids, such as fatty alcohols, monoglycerides, sphingolipids, phospholipids, and in particular free fatty acids, have antimicrobial activity and contribute to the antimicrobial barrier. In addition to these essential functions, we will also review the ability of skin surface cholesterol to reflect alterations in systemic lipid metabolism and the risk of atherosclerosis.
Keywords: permeability barrier, infection, stratum corneum, lamellar body, cholesterol, fatty acid, ceramides, atherosclerosis
The skin is the largest organ of the body and provides the interface between the external environment and the host. Therefore, the major function of the skin is to provide a barrier between the host and the hostile external world (1). While the skin has many important barrier functions, the two that are absolutely required for survival are the barrier to the movement of water and electrolytes (permeability barrier) and the barrier against invasive and toxic microbes (antimicrobial barrier) (1). The importance of these barriers for survival is well illustrated in conditions where these barriers are markedly deficient, such as in premature infants and following extensive thermal burns. In both of these conditions, the host has great difficulty in maintaining normal fluid balance with marked dehydration and alterations in serum electrolytes. In addition, both local and systemic infections occur commonly and are a major cause of morbidity and mortality. Thus, survival is severely compromised when the permeability and antimicrobial barriers are markedly perturbed. Lipid metabolism in the skin is quite complex, and lipids play an essential role in the formation and maintenance of both the permeability and antimicrobial barriers. In this brief review, we will discuss the role of lipids in mediating these barrier functions [for more detailed reviews, see (2) and (3)]. In addition, we will also review the data suggesting that the quantity of cholesterol on the skin surface reflects alterations in systemic lipid metabolism and the risk of atherosclerosis.
PERMEABILITY BARRIER
The permeability barrier is localized to the outer layer of the epidermis, the stratum corneum (SC) (1, 2). The SC consists of corneocytes, keratinocytes that have undergone terminal differentiation, surrounded by a neutral lipid-enriched, extracellular matrix composed primarily of ceramides, cholesterol, and free fatty acids. The hydrophobic extracellular lipid matrix provides the principal barrier to the transcutaneous movement of water and electrolytes (1, 2).
Delivery of lipids to the SC
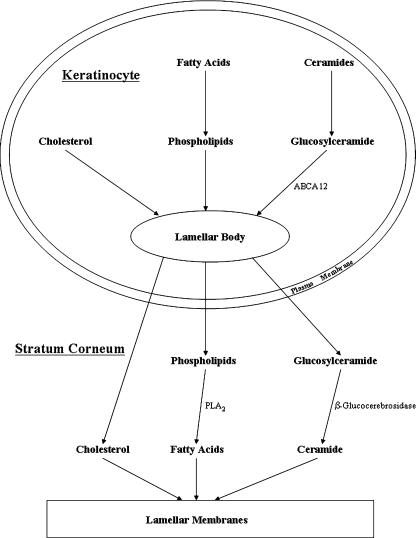
The lipids that form the extracellular lamellar membranes are secreted as their precursors from outer epidermal keratinocytes in lamellar bodies, which are ovoid, membrane bilayer-encircled secretory organelles that are unique to the epidermis (4) (Fig. 1). Lamellar bodies begin to be generated as keratinocytes differentiate and maximal numbers are present in keratinocytes in the stratum granulosum (4). In addition to phospholipids, glucosylceramides, sphingomyelin, and cholesterol, lamellar bodies contain numerous enzymes, including lipid hydrolases, such as β-glucocerebrosidase, acidic sphingomyelinase, secretory phospholipase A2 (sPLA2), and acidic/neutral lipases (4). When the permeability barrier is perturbed, both the secretion and synthesis of lamellar bodies is stimulated, which allows for the rapid repair and normalization of permeability barrier function (2). In fact, severe disruptions of the permeability barrier lead to the secretion of 70–90% of the lamellar bodies stored in stratum granulosum cells. Within the epidermis there is a calcium gradient with high levels of extracellular calcium in the upper epidermis surrounding the stratum granulosum cells. Immediately following permeability barrier disruption, the increased water movement through the compromised SC carries calcium outward toward the skin surface resulting in a reduction in the calcium concentration surrounding the stratum granulosum cells (2). This change in calcium concentration appears to be the primary signal inducing lamellar body secretion. If one prevents the reduction in calcium levels by providing exogenous calcium, lamellar body secretion does not occur and permeability barrier repair is not initiated (2). Conversely, if one lowers calcium levels surrounding stratum granulosum cells, without disrupting the permeability barrier, lamellar body secretion is stimulated (2). In addition, other nonionic signals generated in the SC and by keratinocytes may also influence the secretory response [for review, see (5)]. This robust homeostatic response assures that defects in permeability barrier function are rapidly corrected.
Fig. 1.
Formation of the permeability barrier.
Processing of secreted lipid in the SC
Following secretion, these lamellar-body-derived lipid precursors are further metabolized in the SC extracellular spaces by enzymes that are cosecreted in lamellar bodies (2). Specifically, β-glucocerebrosidase converts glucosylceramides into ceramides, acidic sphingomyelinase converts sphingomyelin into ceramides, and phospholipases convert phospholipids into free fatty acids and glycerol. Both Gaucher disease, due to a deficiency in β-glucocerebrosidase, and Niemann Pick disease, due to a deficiency in acidic sphingomyelinase, lead to defects in the extracellular lipid membranes in the SC and abnormal permeability barrier function due to the impaired conversion of lipid precursors into ceramides (2). Of note, disruption of the permeability barrier produces an increase in β-glucocerebrosidase activity and mRNA levels in the epidermis (2). Similarly, disruption of the permeability barrier also increases acidic sphingomyelinase activity in the epidermis (6). Thus, the activity of the two key enzymes that are required for the extracellular metabolism of lamellar body sphingolipids to ceramides that are required for the formation of lamellar membranes is enhanced following permeability barrier disruption. Additionally, inhibition of sPLA2 activity, which blocks the conversion of phospholipids to free fatty acids, also leads to defects in the structure of the extracellular lipid membranes and permeability barrier homeostasis (2). There are several different isoforms of sPLA2 expressed in the epidermis, and which specific isoforms are important for the extracellular catabolism of phospholipids to fatty acids in the SC remains to be determined. It should be noted that the pH optima for both β-glucocerebrosidase and acid sphingomyelinase activity is ∼5; thus, the acidification of the SC is important for normal permeability barrier homeostasis. If the pH of the SC is elevated (pH > 6), permeability barrier homeostasis is perturbed (2, 7).
Formation of lamellar bodies
The structural proteins that comprise the lamellar bodies have not yet been identified, and many of the details of lamellar body formation are not well understood. The incorporation of the lipid hydrolases and proteases into lamellar bodies requires the prior or concurrent delivery of lipids to the lamellar bodies (2, 4). If lipids are deficient, the enzymes that are characteristically found in lamellar bodies are not transported into the lamellar bodies. Recent studies have shown that mutations in ATP binding cassette transporter family A12 (ABCA12) result in the failure to form lamellar bodies and extracellular lamellar membranes due to a failure to deliver glucosylceramides to lamellar bodies (8). The expression of ABCA12 increases with keratinocyte differentiation, and recent studies have shown that peroxisome proliferator-activated receptor and LXR activators stimulate ABCA12 expression (9).
Source of lipids for lamellar body formation: locally synthesized
Cholesterol
The epidermis on a weight basis is a very active site of cholesterol synthesis, and following acute barrier disruption, there is a rapid and marked increase in epidermal cholesterol synthesis that is associated with an increase in the activity, protein, and mRNA levels of HMG-CoA reductase (2). Additionally, mRNA levels of other key enzymes in the cholesterol synthetic pathway, including HMG-CoA synthase, farnesyl diphosphate synthase, and squalene synthase, also increase following barrier disruption (2). Most importantly, if one inhibits the increase in epidermal cholesterol synthesis by topical application of statins, which inhibit HMG-CoA reductase activity and decrease cholesterol synthesis, the recovery of permeability barrier function is delayed (2). The initial wave of lamellar body secretion occurs, but the reappearance of lamellar bodies is delayed, and those organelles that do appear have an abnormal internal structure. Of note, mice with a deficiency of 3 β-hydroxysterol-delta, the enzyme that catalyzes the conversion of desmosterol to cholesterol, have abundant desmosterol but no cholesterol in the epidermis. These animals die within a few hours after birth due to impaired cutaneous permeability, providing additional evidence for the importance of cholesterol for normal permeability barrier function (10).
Free fatty acids
The epidermis is also a very active site of fatty acid synthesis, and disruption of the permeability barrier results in a rapid and marked increase in fatty acid synthesis (2). Barrier disruption increases the activity and mRNA levels of both of the key enzymes required for de novo fatty acid synthesis, acetyl CoA carboxylase, and fatty acid synthase (2). Moreover, following acute barrier disruption, inhibition of fatty acid synthesis delays the recovery of permeability barrier function (2). The initial wave of lamellar body secretion occurs normally, but the ability of the epidermis to synthesis new lamellar bodies is impaired and those lamellar bodies that are formed display abnormal lamellar membranes. These results demonstrate an important role for epidermal de novo fatty acid synthesis in permeability barrier homeostasis. Furthermore, the elongation of fatty acids is also important as animals deficient in elongation of very-long-chain fatty acid-like 4 have a severely compromised permeability barrier and do not survive after birth (11–13). Additionally, animals deficient in stearoyl-CoA desaturase 2 have abnormal lamellar bodies, a decrease in lamellar membranes, and die soon after birth due to a defective permeability barrier, indicating that the desaturation of fatty acids is also crucial (14). Interestingly, animals deficient in stearoyl-CoA desaturase 1 have normal permeability function but have abnormal sebaceous glands (15), indicating that while stearoyl-CoA desaturase 2 plays an important role in the formation of the permeability barrier by the epidermis, stearoyl-CoA desaturase 1 plays a key role in the formation of sebaceous glands.
Ceramides
Acute barrier disruption stimulates sphingolipid synthesis in the epidermis (2). However, in contrast with cholesterol and fatty acid synthesis, the increase in sphingolipid synthesis is delayed first occurring 6 h after barrier disruption. Additionally, the activity and mRNA levels of serine palmitoyl transferase, the first enzyme in the sphingolipid pathway, increase following barrier disruption (2). Most importantly, inhibition of serine palmitoyl transferase activity slowed permeability barrier recovery at the late time points and reduced the number of lamellar bodies in stratum granulosum cells and sphingolipids in the SC (2). These studies demonstrate a key role for epidermal ceramide synthesis in the latter phase of permeability barrier repair. As noted earlier, glucosylceramides are the key ceramide constituent of lamellar bodies. Glucosylceramides are synthesized from ceramides by the enzyme glucosylceramide synthase. Surprisingly, disruption of the permeability barrier does not alter glucosylceramide synthase activity (2). However, treatment with an inhibitor of glucosylceramide synthase activity delays barrier recovery following acute disruption (2). These results demonstrate that glucosylceramides are essential for permeability barrier homeostasis but that baseline epidermal glucosylceramide synthase activity appears sufficient to accommodate acute challenges to the barrier. Recent studies have confirmed the importance of glucosylceramide synthase for permeability barrier homeostasis. Mice with an epidermal-specific deficiency of glucosylceramide synthase have marked abnormalities in permeability barrier function and die soon after birth (16). Not unexpectedly, they have abnormalities in both lamellar body and SC structure (16).
Source of lipids for lamellar body formation: extracutaneous
It is clear that the lipids that form the lamellar bodies and lamellar membranes are derived not only from local synthesis but also from extracutaneous sources (2). For example, essential fatty acids are present in large quantities in the SC, and by definition these fatty acids must be derived from dietary sources. How lipids are transported into the epidermis is unknown, but studies have shown that keratinocytes have LDL and scavenger receptor class B type 1 receptors, and the expression of these receptors is increased following barrier disruption (2). Additionally, fatty acid transport proteins 1, 3, 4, and 6 and CD36 are expressed in epidermis, and fatty acid transport protein 1 and 6 and CD36 also increase following barrier disruption (2). These results suggest that lipoprotein receptors and fatty acid transporters may play a role in the transport of lipids into the epidermis required for permeability barrier formation.
SC integrity and cohesion
Normal permeability barrier function requires a cohesive, multilayered SC. Abnormal SC integrity/cohesion would increase the susceptibility to injuries that would lead to defects in permeability barrier function. However, regulated corneocyte desquamation must occur to avoid the buildup of a thick SC. Adhesion between adjacent corneocytes is facilitated by corneodesmosomes, which rivet together adjacent cells. SC integrity/cohesion normally is tightly regulated to allow the invisible, distal shedding of corneocytes (desquamation) to maintain a SC thickness sufficient to mediate barrier functions. Detachment of adjacent corneocytes begins with the progressive, proteolytic degradation of corneodesmosomes by serine proteases in the lower SC, a process that is largely completed as corneocytes reach the outer SC. A number of factors effect the degradation of corneodesmosomes, but cholesterol sulfate is a key inhibitor of serine protease activity and, hence, low levels of cholesterol sulfate will increase serine protease activity leading to increased corneodesmosome degradation (17). Cholesterol sulfate is synthesized in keratinocytes by the enzyme cholesterol sulfotransferase, and in keratinocytes, cholesterol sulfotransferase type 2B isoform 1b is the isoform that accounts for epidermal activity (18). Sulfotransferase type 2B isoform 1b expression increases with keratinocyte differentiation, and recent studies have shown that peroxisome proliferator-activated receptor and LXR activators also increase the levels of this enzyme (19). In the SC, cholesterol sulfate is broken down by steroid sulfatase. In X-linked ichthyosis, there are mutations in steroid sulfatase and a failure to catabolize cholesterol sulfate, which results in the failure of desquamation and a thickening of the SC (i.e., ichthyosis) (17).
ANTIMICROBIAL BARRIER
The barrier to pathogenic microbes is more diverse than the permeability barrier (20). The initial layer of defense comprises surface-deposited free fatty acids (3) and preformed antimicrobial peptides (21) as well as an intact (cohesive) structurally normal SC, which forms a formidable physical barrier to the entry of microbes (20). The low pH of intact SC also limits the growth of pathogens on the SC (20). For example, the normal SC pH of 5.5 encourages the growth of benign bacteria such as Staphylococcus epidermidis while inhibiting the growth of pathogens such as Staphylococcus aureus. Additionally, constituent levels of certain antimicrobial peptides and certain amphiphilic lipids, particularly sphingosine and free fatty acids, decrease the growth of microbes, thereby reducing infection (3).
Acidification of the SC
The low pH of the SC is an important contributor to antimicrobial defense. That the surface of the skin is acidic has been recognized for decades, but the mechanisms that account for its acidification are still incompletely understood (7). It is postulated that exogenous pathways (originating outside the epidermis), such as microbial metabolites, free fatty acids of pilosebaceous origin, and eccrine gland-derived products, such as lactic acid, contribute to SC acidification. Moreover, recent studies have shown that endogenous pathways are also very important for SC acidification. Free fatty acid generation from phospholipid hydrolysis catalyzed by secretory phospholipase A2 plays a role in SC acidification (7). Inhibition of sPLA2 activity increases the pH of the SC.
Epidermal antimicrobial lipids
It is well known that a variety of naturally occurring lipids, such as fatty alcohols, free fatty acids, and monoglycerides, exhibit potent antimicrobial activity against enveloped viruses, gram-positive, gram-negative bacteria, candida, and fungi (3). These lipids are presumed to play an important role in host defense in both the respiratory tract and skin. In skin, long-chain fatty acids of both epidermal and sebaceous origin appear to account for most of this endogenous antimicrobial activity (3). In the SC, fatty acids are formed during the hydrolysis of phospholipids. In addition, sebaceous glands secrete squalene, wax monoesters, triglycerides, and small amounts of cholesterol and cholesterol esters (22). These sebaceous gland-derived triglycerides undergo hydrolysis, a reaction mediated by the acid lipases in SC or by bacterial triglyceride lipases (22). Both saturated and unsaturated free fatty acids on the skin surface exhibit potent activity against S. aureus, S. pyogenes, and C. albicans, but less activity against gram-negative organisms. Sapienic and lauric acid, both of which are derived from the breakdown of sebaceous gland triglyceride, have particularly potent antibacterial properties (3). Although glucosylceramides and phospholipids also demonstrated moderate antimicrobial in vitro activity, they were less active than fatty acids in vivo.
DOES THE SKIN REFLECT ABNORMALITIES IN LIPID METABOLISM THAT LEAD TO ATHEROSCLEROSIS?
It has been recognized for many years that different types of xanthomas are markers for abnormalities in lipid metabolism (23–25). For example, eruptive xanthomas are associated with marked elevations in serum triglyceride levels, whereas planar xanthomas are associated with familial dysbetalipoproteinemia. Thus, the presence of xanthoma can be a cutaneous marker that allows for the diagnosis of disorders in lipid and lipoprotein metabolism. Several reviews are available that describe the different types of xanthomas and their relationship to various perturbations in lipid metabolism (23–25).
Recently, there have been a series of studies that suggested that measuring the cholesterol levels on the skin surface, i.e., in the SC, could provide insights into the presence and/or severity of atherosclerosis. In 2001, Zawydiwski et al. reported a positive correlation between skin surface cholesterol levels and a positive treadmill stress test, a standard procedure for diagnosing coronary artery disease (26). Soon after this study appeared, Mancini et al. reported that skin cholesterol content correlated with Framingham risk prediction (correlation coefficient 0.38 with P = 0.003) (27). Several studies then went on to demonstrate that increased skin cholesterol levels correlated with the extent of atherosclerosis measured by coronary artery angiography, coronary calcium score determined by CT scan, or carotid intima media thickness determined by ultrasound (28–31). Additionally, patients with a history of a myocardial infarction had higher skin cholesterol levels (32). In general in these studies, skin cholesterol levels did not strongly correlate with serum lipid levels, and the relationship of skin cholesterol with atherosclerosis was independent of serum lipid levels. In contrast with these positive studies Reiter et al. found no difference in skin cholesterol levels in patients with or without a history of coronary, peripheral vascular, or cerebral vascular events (33, 34), and Vaidya et al., while finding a relationship between skin cholesterol levels and coronary calcium score in Caucasians, did not find such a relationship in African Americans (31). Thus, while there is suggestive data supporting a link between skin surface cholesterol levels and atherosclerosis, additional studies are required to definitively determine whether skin surface cholesterol levels accurately reflect the presence and/or severity of atherosclerosis.
Abbreviations
ABCA12, ATP binding cassette transporter family A12
SC, stratum corneum
sPLA2, secretory phospholipase A2
These studies were supported by National Institutes of Health Grants AR-39448, AR-049932, and HD-29706 and by the Medical Research Service, Department of Veterans Affairs.
Published, JLR Papers in Press, October 31, 2008.
References
- 1.Elias, P., K. Feingold, and J. Fluhr. 2003. The skin as an organ of protection. In Dermatology in General Medicine. I. M. Friedberg, A. Z. Eisen, K. Wolff, K. F. Austen, L. A. Goldsmith, and S. I. Katz, editors. McGraw Hill, New York. 107–118.
- 2.Feingold K. R. 2007. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J. Lipid Res. 48 2531–2546. [DOI] [PubMed] [Google Scholar]
- 3.Drake D. R., K. A. Brogden, D. V. Dawson, and P. W. Wertz. 2008. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J. Lipid Res. 49 4–11. [DOI] [PubMed] [Google Scholar]
- 4.Elias, P., K. Feingold, and M. Fartasch. 2006. Epidermal lamellar body as a multifunctional secretory organelle. In Skin Barrier. P. Elias and K. Feingold, editors. Taylor & Francis, New York. 261–272.
- 5.Feingold K. R., M. Schmuth, and P. M. Elias. 2007. The regulation of permeability barrier homeostasis. J. Invest. Dermatol. 127 1574–1576. [DOI] [PubMed] [Google Scholar]
- 6.Jensen J. M., S. Schutze, M. Forl, M. Kronke, and E. Proksch. 1999. Roles for tumor necrosis factor receptor p55 and sphingomyelinase in repairing the cutaneous permeability barrier. J. Clin. Invest. 104 1761–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauro, T. 2006. SC pH: measurement, origins, and functions. In Skin Barrier. P. Elias, and K. Feingold, editors. Taylor & Francis, New York. 223–229.
- 8.Hovnanian A. 2005. Harlequin ichthyosis unmasked: a defect of lipid transport. J. Clin. Invest. 115 1708–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y. J., B. Lu, P. Kim, G. Paragh, G. Schmitz, P. M. Elias, and K. R. Feingold. 2008. PPAR and LXR activators regulate ABCA12 expression in human keratinocytes. J. Invest. Dermatol. 128 104–109. [DOI] [PubMed] [Google Scholar]
- 10.Mirza R., S. Hayasaka, Y. Takagishi, F. Kambe, S. Ohmori, K. Maki, M. Yamamoto, K. Murakami, T. Kaji, D. Zadworny, et al. 2006. DHCR24 gene knockout mice demonstrate lethal dermopathy with differentiation and maturation defects in the epidermis. J. Invest. Dermatol. 126 638–647. [DOI] [PubMed] [Google Scholar]
- 11.Cameron D. J., Z. Tong, Z. Yang, J. Kaminoh, S. Kamiyah, H. Chen, J. Zeng, Y. Chen, L. Luo, and K. Zhang. 2007. Essential role of Elovl4 in very long chain fatty acid synthesis, skin permeability barrier function, and neonatal survival. Int. J. Biol. Sci. 3 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., R. Sandhoff, M. Kono, P. Zerfas, V. Hoffmann, B. C. Ding, R. L. Proia, and C. X. Deng. 2007. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int. J. Biol. Sci. 3 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasireddy V., Y. Uchida, N. Salem, Jr., S. Y. Kim, M. N. Mandal, G. B. Reddy, R. Bodepudi, N. L. Alderson, J. C. Brown, H. Hama, et al. 2007. Loss of functional ELOVL4 depletes very long-chain fatty acids (>=C28) and the unique {omega}-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet. 16 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki M., A. Dobrzyn, P. M. Elias, and J. M. Ntambi. 2005. Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development. Proc. Natl. Acad. Sci. USA. 102 12501–12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fluhr J. W., M. Mao-Qiang, B. E. Brown, P. W. Wertz, D. Crumrine, J. P. Sundberg, K. R. Feingold, and P. M. Elias. 2003. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J. Invest. Dermatol. 120 728–737. [DOI] [PubMed] [Google Scholar]
- 16.Jennemann R., R. Sandhoff, L. Langbein, S. Kaden, U. Rothermel, H. Gallala, K. Sandhoff, H. Wiegandt, and H. J. Grone. 2007. Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J. Biol. Chem. 282 3083–3094. [DOI] [PubMed] [Google Scholar]
- 17.Elias P. M., M. L. Williams, W. M. Holleran, Y. J. Jiang, and M. Schmuth. 2008. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J. Lipid Res. 49 697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higashi Y., H. Fuda, H. Yanai, Y. Lee, T. Fukushige, T. Kanzaki, and C. A. Strott. 2004. Expression of cholesterol sulfotransferase (SULT2B1b) in human skin and primary cultures of human epidermal keratinocytes. J. Invest. Dermatol. 122 1207–1213. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y. J., P. Kim, P. M. Elias, and K. R. Feingold. 2005. LXR and PPAR activators stimulate cholesterol sulfotransferase type 2 isoform 1b in human keratinocytes. J. Lipid Res. 46 2657–2666. [DOI] [PubMed] [Google Scholar]
- 20.Elias P. M. 2007. The skin barrier as an innate immune element. Semin. Immunopathol. 29 3–14. [DOI] [PubMed] [Google Scholar]
- 21.Radek K., and R. Gallo. 2007. Antimicrobial peptides: natural effectors of the innate immune system. Semin. Immunopathol. 29 27–43. [DOI] [PubMed] [Google Scholar]
- 22.Smith K. R., and D. M. Thiboutot. 2008. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J. Lipid Res. 49 271–281. [DOI] [PubMed] [Google Scholar]
- 23.Cruz P. D., Jr., C. East, and P. R. Bergstresser. 1988. Dermal, subcutaneous, and tendon xanthomas: diagnostic markers for specific lipoprotein disorders. J. Am. Acad. Dermatol. 19 95–111. [DOI] [PubMed] [Google Scholar]
- 24.Haber C., and P. O. Kwiterovich, Jr. 1984. Dyslipoproteinemia and xanthomatosis. Pediatr. Dermatol. 1 261–280. [DOI] [PubMed] [Google Scholar]
- 25.Maher-Wiese V. L., E. L. Marmer, and J. M. Grant-Kels. 1990. Xanthomas and the inherited hyperlipoproteinemias in children and adolescents. Pediatr. Dermatol. 7 166–173. [DOI] [PubMed] [Google Scholar]
- 26.Zawydiwski R., D. L. Sprecher, M. J. Evelegh, P. Horsewood, C. Carte, and M. Patterson. 2001. A novel test for the measurement of skin cholesterol. Clin. Chem. 47 1302–1304. [PubMed] [Google Scholar]
- 27.Mancini G. B., S. Chan, J. Frohlich, L. Kuramoto, M. Schulzer, and D. Abbott. 2002. Association of skin cholesterol content, measured by a noninvasive method, with markers of inflammation and Framingham risk prediction. Am. J. Cardiol. 89 1313–1316. [DOI] [PubMed] [Google Scholar]
- 28.Sprecher D. L., S. G. Goodman, P. Kannampuzha, G. L. Pearce, and A. Langer. 2003. Skin tissue cholesterol (SkinTc) is related to angiographically-defined cardiovascular disease. Atherosclerosis. 171 255–258. [DOI] [PubMed] [Google Scholar]
- 29.Stein J. H., W. S. Tzou, J. M. DeCara, A. T. Hirsch, E. R. Mohler 3rd, P. Ouyang, G. L. Pearce, and M. H. Davidson. 2008. Usefulness of increased skin cholesterol to identify individuals at increased cardiovascular risk (from the Predictor of Advanced Subclinical Atherosclerosis study). Am. J. Cardiol. 101 986–991. [DOI] [PubMed] [Google Scholar]
- 30.Tzou W. S., M. E. Mays, C. E. Korcarz, S. E. Aeschlimann, and J. H. Stein. 2005. Skin cholesterol content identifies increased carotid intima-media thickness in asymptomatic adults. Am. Heart J. 150 1135–1139. [DOI] [PubMed] [Google Scholar]
- 31.Vaidya D., J. Ding, J. G. Hill, J. A. Lima, J. R. Crouse 3rd, R. A. Kronmal, M. Szklo, and P. Ouyang. 2005. Skin tissue cholesterol assay correlates with presence of coronary calcium. Atherosclerosis. 181 167–173. [DOI] [PubMed] [Google Scholar]
- 32.Sprecher D. L., and G. L. Pearce. 2005. Elevated skin tissue cholesterol levels and myocardial infarction. Atherosclerosis. 181 371–373. [DOI] [PubMed] [Google Scholar]
- 33.Reiter M., S. Wirth, A. Pourazim, M. Exner, M. Baghestanian, H. Rumpold, E. Minar, and R. A. Bucek. 2006. Skin cholesterol: test performance, evaluation of potential determinants and correlation analysis with cardiovascular risk factors and circulating markers of inflammation. Vasa. 35 167–173. [DOI] [PubMed] [Google Scholar]
- 34.Reiter M., S. Wirth, A. Pourazim, S. Puchner, M. Baghestanian, E. Minar, and R. A. Bucek. 2007. Skin tissue cholesterol is not related to vascular occlusive disease. Vasc. Med. 12 129–134. [DOI] [PubMed] [Google Scholar]