Abstract
Numerous lines of evidence implicate a role for myeloperoxidase (MPO) in the pathogenesis of atherosclerosis. Enriched within vulnerable plaque, MPO serves as an enzymatic source of eicosanoids and bioactive lipids and generates atherogenic forms of both low- and high-density lipoproteins. These factors likely contribute to clinical studies demonstrating that increased systemic levels of MPO and its oxidation products predict increased cardiovascular risk. As a result, interest has focused on the potential to target MPO for the development of new risk markers, imaging, and therapies to prevent cardiovascular events.
Keywords: oxidant stress, free radicals, scavenger receptor, high density lipoprotein
It has become increasingly established that inflammatory events contribute to all stages of atherosclerosis. However, the role of specific inflammatory mediators in the orchestration of these events remains to be defined. Accumulating evidence that myeloperoxidase (MPO) has effects on a range of factors that influence the arterial wall suggests that it plays a pivotal role in the natural history of atherosclerotic cardiovascular disease (CVD).
PHYSIOLOGIC ACTIVITY OF MPO AND ITS ROLE IN THE INNATE IMMUNE RESPONSE
MPO is a member of the mammalian heme peroxidase superfamily and is stored within the azurophilic granules of leukocytes (1). MPO is found within circulating neutrophils, monocytes, and some tissue macrophage populations (2). The catalytic activity of MPO results in the generation of various reactive oxidants and diffusible radical species (1). These products play an important role in killing invading parasites and pathogens. MPO-deficient humans and animals demonstrate heightened susceptibility to fungal and yeast infections (3). However, the ability of MPO-derived reactive oxidants to promote host tissue injury through lipid peroxidation (4) and posttranslational protein modifications (5) has resulted in MPO being thought as participating in a wide range of chronic inflammatory diseases (4–7).
During leukocyte activation, MPO amplifies the oxidative potential of the respiratory burst by using hydrogen peroxide as a cosubstrate to form more reactive oxidant species. This can result in the generation of a number of potent oxidant compounds capable of promoting oxidative modification of host tissues (8–12). Production of reactive chlorinating species, such as hypochlorous acid, is an activity specific to the MPO pathway (8). The antimicrobial activities of these products provide the rationale for the role of MPO in the innate immune response to foreign invasion (13). Generation of oxidized bioactive lipids provides additional mechanisms linking MPO and inflammatory pathways (4). Indeed, studies employing mice with functional deficiency in MPO reveal that the enzyme plays an important role in the formation of arachidonic acid oxidation products involved in the promotion of inflammatory cascades (4). While this provides evidence that MPO and its products are important homeostatic factors, evidence suggests that excessive activity of MPO can play a role in inflammatory tissue injury.
ROLE OF MPO IN THE GENERATION OF ATHEROGENIC LDL SPECIES
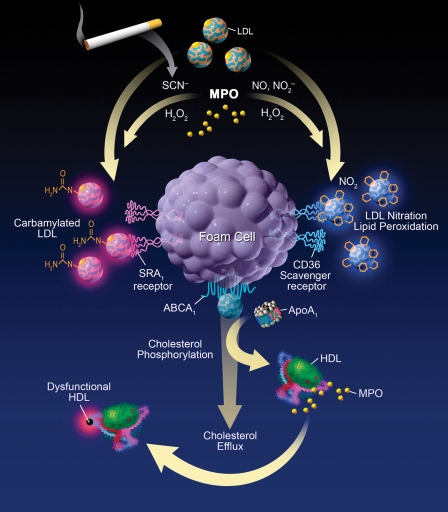
MPO has emerged as one enzymatic catalyst for LDL oxidation in vivo via several chemical processes (Fig. 1) and conversion into more atherogenic forms within the artery wall. Enrichment of LDL with markers of chlorination, such as 3-chlorotyrosine, served to identify MPO as the first enzymatic catalyst of a specific oxidative pathway operative within human atherosclerotic plaque and modifying LDL in vivo (9). Subsequent studies have expanded the repertoire of oxidant generating pathways catalyzed by MPO in the artery wall, including formation of nitric-oxide-derived oxidants and consequent nitrated LDL (14). Exposure of LDL to activated monocytes via MPO-generated reactive nitrogen species facilitates lipid peroxidation and protein nitration and converts LDL into a high uptake form (14) that is avidly taken up by macrophages via the macrophage scavenger receptor CD36 (15). The physiologic nature of this pathway for initiating lipid peroxidation is supported by studies employing MPO-knockout mice, demonstrating reduction in lipid peroxidation products following leukocyte activation at sites of inflammation (4, 11) and the observation that neutrophils isolated from individuals with MPO deficiency do not initiate lipid peroxidation when activated ex vivo in plasma but regain this ability with exogenous addition of only catalytic levels of MPO (6).
Fig. 1.
Role of MPO-catalyzed pathways in the generation of atherogenic LDL and dysfunctional HDL particles. MPO-generated products promote lipid peroxidation, conversion of LDL to a high-uptake form, and impairment of the ability of apoA-I to promote cholesterol efflux. MPO-catalyzed carbamylation has recently been reported also to be involved in generation of high-uptake forms of LDL and impaired functional activities of HDL.
More recently, MPO has been identified as an enzymatic catalyst for promoting protein and lipoprotein carbamylation, a form of posttranslational modification well characterized previously in end-stage renal disease (16). Thiocyanate, a preferred substrate for MPO that is markedly elevated in plasma of smokers, forms a reactive intermediate capable of carbamylating nucleophilic groups on proteins such as lysine residues at sites of inflammation (16). MPO-catalyzed carbamylation of LDL has been demonstrated to convert LDL into a ligand for the scavenger receptor SRA-1 (16). Thus, MPO can generate multiple high-uptake forms of LDL in vivo (Fig. 1). A role of carbamylation in atherosclerosis in vivo was supported by the findings that i) antibodies specific for carbamylated LDL colocalize with MPO in human atheroma; ii) MPO renders LDL a high-uptake form for SRA-1 at low levels of carbamylation, well within the levels observed in human plasma; iii) clinical studies showing that systemic levels of the carbamylated product, protein-bound homocitrulline (carbamyllysine), independently predict both prevalent and prospective cardiovascular risks; and iv) human MPO-transgenic mice show marked increases in atherosclerosis and increased aortic tissue content of protein-bound homocitrulline proportionate with cholesterol enrichment (16).
ROLE OF MPO IN THE GENERATION OF DYSFUNCTIONAL HDL PARTICLES
Increasing interest has focused on the relative functionality of HDL in different subjects. This is highlighted by observations that cardiovascular events can occur even in the presence of high levels of HDL cholesterol, and upon isolation, HDL from subjects with CVD can show proinflammatory activities. The recent failure of HDL-raising agents has fueled speculation that novel therapies need to be evaluated to ensure they do not have an adverse effect on HDL functionality. While early studies suggested that in vitro oxidative modification of HDL particles can serve as one global mechanism for impairment in HDL functional properties, the potential pathways resulting in the generation of dysfunctional HDL particles in vivo remain to be elucidated. Recent observations suggest a potential role for an altered “HDL-associated proteome” and MPO-catalyzed site-specific modification of apolipoprotein A-I (apoA-I) as mechanisms resulting in functional impairment.
HDL isolated from plaque contains MPO and its oxidant products (17, 18), consistent with the observation that MPO binds to a specific region of helix 8 of apoA-I (17). ApoA-I isolated from plasma of subjects with coronary heart disease contains greater amounts of nitrotyrosine and chlorotyrosine than healthy controls (17, 19, 20). In a study of outpatient cardiology subjects, individuals with highest tertile levels of apoA-I nitrotyrosine and chlorotyrosine demonstrated a 6- and 16-fold greater likelihood of having CVD compared with subjects possessing lowest tertile levels of oxidized apoA-I (17). The finding that apoA-I enrichment in chlorotyrosine was 500-fold greater than other proteins in lesions and similarly enriched within apoA-I in plasma supports the concept that oxidative modification occurs preferentially on apoA-I in the artery wall (17). This is consistent with colocalization of apoA-I with MPO, chlorinated proteins, and carbamyllysine in plaque (21, 22). The finding that the degree of apoA-I modification correlates with impairment of HDL to promote ABCA-1-dependent cholesterol efflux from macrophages is consistent with an increased risk of atherosclerotic plaque development and CVD (Fig. 1) (17, 19, 20).
Mass spectrometry has identified specific sites on apoA-I as preferred targets for MPO-derived oxidative modification (17). In vitro studies demonstrate that oxidation occurs preferentially at residues on helix 8 (Tyr-192) in close spatial proximity with where MPO is mapped to bind apoA-I of HDL (17). Subsequent studies have revealed that MPO-induced modification of apoA-I has a detrimental impact on additional aspects of the reverse cholesterol transport pathway. For example, apoA-I Tyr-166, an abundant site-specific modification on apoA-I recovered from human atherosclerotic lesions via both nitrating and chlorinating pathways, has been shown to be an essential component of an apoA-I LCAT binding loop located on nascent HDL (23). MPO-catalyzed oxidation of a single methionine of apoA-I near the LCAT activation region (Met-148) is also reported to result in functional impairment of LCAT activity. MPO-catalyzed modifications to apoA-I that inhibit LCAT binding and activity are likely to interfere with reverse cholesterol transport. Recent studies by Podrez report that HDL exposed to the MPO-H2O2-Cl− system competes with native HDL as a ligand for the scavenger receptor BI, potentially interfering further with mobilization of cholesterol from peripheral tissues to the liver. Further studies are warranted on MPO-catalyzed oxidation of HDL and the effects on plaque development.
While there is consensus in the field that MPO catalyzes oxidative modification of apoA-I and impairs its function, the precise modifications that result in impairment in different HDL functions requires further study. Structural studies have provided further insights to understand structure-function relationships of HDL and site-specific changes involved during oxidative modification of apoA-I. A refined structural model of HDL was recently generated by application of hydrogen-deuterium exchange mass spectrometry, a method for quantifying solvent accessibility through amide proton exchange throughout the polypeptide chain of apoA-I of nascent HDL (23). The so-called “solar flare model” found two apoA-I molecules arranged in an antiparallel double belt structure with protruding solvent-exposed loops corresponding to amino acid residues 159 to 170, a region shown to be involved in LCAT docking and activation (23). This docking loop also contains one of the preferred targets for MPO oxidation observed in apoA-I recovered from human plaque, Tyr-166 (23). Recent studies suggest that tyrosine modification is not required for MPO-induced loss of ABCA1-dependent cholesterol efflux activity since apoA-I mutants lacking all tyrosine are susceptible to oxidative inactivation by MPO (24). However, mutation of Tyr-166 to Phe was shown to result in loss of the majority of LCAT activity (23). Investigation of the residues involved in oxidative inactivation of apoA-I-mediated ABCA1-dependent efflux activity has similarly recently been investigated. Proteomic analyses have identified all four tryptophan residues within apoA-I as targets for oxidation. Site-directed mutagenesis to substitute all four tryptophan residues to leucine resulted in a HDL particle lacking efflux activity; however, substitution of each of the four tryptophan residues to phenylalanine generated an apoA-I and HDL particle that showed normal cholesterol efflux and LCAT activities under native conditions and that was markedly resistant to oxidative inactivation, suggesting that tryptophan modification is an essential factor involved in loss of ABCA1-mediated efflux activity of HDL (25). Paradoxically, other investigators report no effect on efflux activity with tryptophan mutation and instead invoke a key role for tryptophan and methionine in oxidative inactivation, despite reports that apoA-I lacking all tryptophan or methionine retain complete efflux and LCAT activities and sensitivities to oxidative inactivation (25).
Recent studies show that MPO-catalyzed carbamylation may also play an important role in atherosclerosis via modification of HDL. Low levels of MPO-catalyzed carbamylation of HDL ablates its nonlipid transporting influence on endothelial cell apoptosis and smooth muscle cell proliferation (16). Given that MPO adversely influences LDL atherogenicity and HDL functionality, it is possible that inhibiting MPO activity may provide a therapeutic approach to management of both LDL and HDL. The finding that statins partially reduce MPO expression (26) and reduce systemic levels of protein modification by MPO-catalyzed pathways (27) suggests that perhaps some of the so-called plieotropic benefit of statins may be due in part to influence on MPO levels and activity.
ANIMAL OBSERVATIONS OF MPO AND ATHEROSCLEROSIS
Investigation of the impact of MPO on lesion formation in animal models of atherosclerosis has produced variable results. Murine models have demonstrated the in vivo role of MPO and its products in acute inflammation, lipid peroxidation, endothelial dysfunction, and adverse ventricular remodeling following myocardial infarction (4, 11, 28, 29). However, early studies found that genetic deletion of MPO had no impact on lesion formation in apolipoprotein E knockout mice (30), and infusion of bone marrow from MPO-knockout mice into irradiated LDL receptor knockout mice demonstrated an unexpected modest increase in lesion size (30). The subsequent observations that mouse aortic lesions contained virtually no traces of MPO and its products, in contrast with observations within human atheroma, coupled with the observations that murine leukocytes contain >10- to 20-fold less MPO per cell than found in humans, indicates that many substantial species differences exist (30, 31). Several groups have consequently sought to generate “humanized” MPO/atherosclerosis models. Recent reports from multiple groups with distinct mouse strains have shown that human MPO transgenic mice have accelerated atherosclerotic plaque development (16, 32, 33). As a result, there remains hope that humanized animal models of atherosclerosis, such as those overexpressing the human MPO transgene, may be of some use in evaluating the impact of experimental MPO inhibitors on atherosclerosis models where MPO is present and catalytically active.
HUMAN STUDIES OF MPO AND CVD
The past 5 years have witnessed dramatic growth in the number of human clinical investigations exploring the role of MPO in atherosclerosis. Early studies localized MPO and its products as being enriched within human atherosclerotic plaques (2, 9, 34–36). Furthermore, individuals with total or subtotal MPO deficiency (3) or loss-of-function polymorphisms have more recently been associated with protection from coronary heart disease. As illustrated below, many studies now demonstrate associations between increasing systemic MPO levels and risks of CVD throughout the full spectrum of cardiovascular risk.
In a recent nested case-control analysis of >25,000 apparently healthy middle-aged individuals (EPIC/Norfolk study), the prospective risk of developing symptomatic coronary heart disease over the ensuing 6-year period was shown to increase in parallel with baseline MPO levels (37). In patients with stable coronary artery disease symptoms, MPO levels have been shown to predict the prevalence and extent of coronary artery disease and future risk of cardiovascular events. Case-control studies report the relationship between increasing MPO levels and the prevalence and extent of obstructive disease on coronary angiography (38, 39), consistent with the initial observations by Zhang et al. (40), who observed correlations between the content of MPO per leukocyte and angiographic evidence of coronary stenosis >50%.
Multiple studies now link systemic MPO levels and adverse cardiovascular outcomes in patients investigated in the setting of acute ischemic syndromes. For example, in a study of >600 patients presenting to the emergency room for evaluation of acute chest pain, MPO plasma levels were found to independently predict cardiovascular risk, regardless of evidence of myocardial necrosis (41). Additional reports of associations between MPO levels and incident adverse cardiovascular risks in patients with acute coronary syndromes were observed from clinical trials of antiplatelet therapies. These findings were even observed in subjects with low systemic levels of markers of myocardial necrosis or inflammation (42) and were found to have the highest sensitivity to predict risk of recurrent ischemic events when compared with a panel of current cardiovascular biomarkers (43). This relationship has also been demonstrated in patients presenting with myocardial infarction, regardless of levels of clinical risk (44, 45).
The past few years have also witnessed the extension of the diagnostic and prognostic role of MPO to the setting of stable, chronic heart failure (46). MPO levels predicted functional class and adverse outcomes, regardless of brain natriuretic protein levels and degree of systolic dysfunction (46). In a community-based screen of apparently healthy middle-aged subjects, MPO levels demonstrated the greatest sensitivity for identifying subjects with occult left ventricular dysfunction (47). The relationship between MPO and systolic heart failure is further supported by the observation of increasing activation of polymorphonuclear leukocytes in patients with impaired left ventricular systolic function (48) and the role of MPO in impaired ventricular remodeling in murine models of both chronic coronary artery ligation and ischemia-reperfusion (29, 49).
SUMMARY
A large body of evidence demonstrates that MPO and its reactive oxidant species play a role in the promotion of pathological events involved in all stages of atherosclerotic CVD. More recent observations of associations between systemic MPO levels and cardiovascular risks in humans suggest that MPO testing may play a role in clinical risk prediction. New developments with MPO functional imaging may permit imaging of vulnerable plaque and myocardial injury (50). MPO represents a potential target for the development of new therapeutic agents to prevent or retard development of CVD.
Abbreviations
apoA-I, apolipoprotein A-I
CVD, cardiovascular disease
MPO, myeloperoxidase
Published, JLR Papers in Press, December 16, 2008.
References
- 1.Klebanoff S. J. 1980. Oxygen metabolism and the toxic properties of phagocytes. Ann. Intern. Med. 93 480–489. [DOI] [PubMed] [Google Scholar]
- 2.Daugherty A., J. L. Dunn, D. L. Rateri, and J. W. Heinecke. 1994. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest. 94 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutter D., P. Devaquet, G. Vanderstocken, J. M. Paulus, V. Marchal, and A. Gothot. 2000. Consequences of total and subtotal myeloperoxidase deficiency: risk or benefit? Acta Haematol. 104 10–15. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R., M. L. Brennan, Z. Shen, J. C. MacPherson, D. Schmitt, C. E. Molenda, and S. L. Hazen. 2002. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 277 46116–46122. [DOI] [PubMed] [Google Scholar]
- 5.Podrez E. A., H. M. Abu-Soud, and S. L. Hazen. 2000. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic. Biol. Med. 28 1717–1725. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R., Z. Shen, W. M. Nauseef, and S. L. Hazen. 2002. Defects in leukocyte-mediated initiation of lipid peroxidation in plasma as studied in myeloperoxidase-deficient subjects: systematic identification of multiple endogenous diffusible substrates for myeloperoxidase in plasma. Blood. 99 1802–1810. [PubMed] [Google Scholar]
- 7.Malle E., T. Buch, and H. J. Grone. 2003. Myeloperoxidase in kidney disease. Kidney Int. 64 1956–1967. [DOI] [PubMed] [Google Scholar]
- 8.Hazen S. L., F. F. Hsu, D. M. Mueller, J. R. Crowley, and J. W. Heinecke. 1996. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J. Clin. Invest. 98 1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazen S. L., and J. W. Heinecke. 1997. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 99 2075–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Vliet A., J. P. Eiserich, B. Halliwell, and C. E. Cross. 1997. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J. Biol. Chem. 272 7617–7625. [DOI] [PubMed] [Google Scholar]
- 11.Brennan M. L., W. Wu, X. Fu, Z. Shen, W. Song, H. Frost, C. Vadseth, L. Narine, E. Lenkiewicz, M. T. Borchers, et al. 2002. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 277 17415–17427. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Soud H. M., and S. L. Hazen. 2000. Nitric oxide is a physiological substrate for mammalian peroxidases. J. Biol. Chem. 275 37524–37532. [DOI] [PubMed] [Google Scholar]
- 13.Klebanoff S. J. 1970. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 169 1095–1097. [DOI] [PubMed] [Google Scholar]
- 14.Podrez E. A., D. Schmitt, H. F. Hoff, and S. L. Hazen. 1999. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J. Clin. Invest. 103 1547–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podrez E. A., M. Febbraio, N. Sheibani, D. Schmitt, R. L. Silverstein, D. P. Hajjar, P. A. Cohen, W. A. Frazier, H. F. Hoff, and S. L. Hazen. 2000. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Invest. 105 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z., S. J. Nicholls, E. R. Rodriguez, O. Kummu, S. Horkko, J. Barnard, W. F. Reynolds, E. J. Topol, J. A. Didonato, and S. L. Hazen. 2007. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 13 1176–1184. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L., B. Nukuna, M. L. Brennan, M. Sun, M. Goormastic, M. Settle, D. Schmitt, X. Fu, L. Thomson, P. L. Fox, et al. 2004. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng L., M. Settle, G. Brubaker, D. Schmitt, S. L. Hazen, J. D. Smith, and M. Kinter. 2005. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J. Biol. Chem. 280 38–47. [DOI] [PubMed] [Google Scholar]
- 19.Bergt C., S. Pennathur, X. Fu, J. Byun, K. O'Brien, T. O. McDonald, P. Singh, G. M. Anantharamaiah, A. Chait, J. Brunzell, et al. 2004. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA. 101 13032–13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennathur S., C. Bergt, B. Shao, J. Byun, S. Y. Kassim, P. Singh, T. O. McDonald, J. Brunzell, A. Chait, J. F. Oram, et al. 2004. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 279 42977–42983. [DOI] [PubMed] [Google Scholar]
- 21.Hazell L. J., L. Arnold, D. Flowers, G. Waeg, E. Malle, and R. Stocker. 1996. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Invest. 97 1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malle E., G. Wag, J. Thiery, W. Sattler, and H. J. Grone. 2001. Hypochlorite-modified (lipo)proteins are present in rabbit lesions in response to dietary cholesterol. Biochem. Biophys. Res. Commun. 289 894–900. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z., M. A. Wagner, L. Zheng, J. S. Parks, J. M. Shy 3rd, J. D. Smith, V. Gogonea, and S. L. Hazen. 2007. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat. Struct. Mol. Biol. 14 861–868. [DOI] [PubMed] [Google Scholar]
- 24.Peng D. Q., Z. Wu, G. Brubaker, L. Zheng, M. Settle, E. Gross, M. Kinter, S. L. Hazen, and J. D. Smith. 2005. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J. Biol. Chem. 280 33775–33784. [DOI] [PubMed] [Google Scholar]
- 25.Peng D. Q., G. Brubaker, Z. Wu, L. Zheng, B. Willard, M. Kinter, S. L. Hazen, and J. D. Smith. 2008. Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler. Thromb. Vasc. Biol. 28 2063–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A. P., and W. F. Reynolds. 2005. Statins downregulate myeloperoxidase gene expression in macrophages. Biochem. Biophys. Res. Commun. 331 442–451. [DOI] [PubMed] [Google Scholar]
- 27.Shishehbor M. H., M. L. Brennan, R. J. Aviles, X. Fu, M. S. Penn, D. L. Sprecher, and S. L. Hazen. 2003. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 108 426–431. [DOI] [PubMed] [Google Scholar]
- 28.Eiserich J. P., S. Baldus, M. L. Brennan, W. Ma, C. Zhang, A. Tousson, L. Castro, A. J. Lusis, W. M. Nauseef, C. R. White, et al. 2002. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 296 2391–2394. [DOI] [PubMed] [Google Scholar]
- 29.Askari A. T., M. L. Brennan, X. Zhou, J. Drinko, A. Morehead, J. D. Thomas, E. J. Topol, S. L. Hazen, and M. S. Penn. 2003. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J. Exp. Med. 197 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan M. L., M. M. Anderson, D. M. Shih, X. D. Qu, X. Wang, A. C. Mehta, L. L. Lim, W. Shi, S. L. Hazen, J. S. Jacob, et al. 2001. Increased atherosclerosis in myeloperoxidase-deficient mice. J. Clin. Invest. 107 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauseef W. M. 2001. The proper study of mankind. J. Clin. Invest. 107 401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellani L. W., J. J. Chang, X. Wang, A. J. Lusis, and W. F. Reynolds. 2006. Transgenic mice express human MPO -463G/A alleles at atherosclerotic lesions, developing hyperlipidemia and obesity in -463G males. J. Lipid Res. 47 1366–1377. [DOI] [PubMed] [Google Scholar]
- 33.McMillen T. S., J. W. Heinecke, and R. C. LeBoeuf. 2005. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 111 2798–2804. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama S., Y. Okada, G. K. Sukhova, R. Virmani, J. W. Heinecke, and P. Libby. 2001. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am. J. Pathol. 158 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thukkani A. K., J. McHowat, F. F. Hsu, M. L. Brennan, S. L. Hazen, and D. A. Ford. 2003. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 108 3128–3133. [DOI] [PubMed] [Google Scholar]
- 36.Beckman J. S., Y. Z. Ye, P. G. Anderson, J. Chen, M. A. Accavitti, M. M. Tarpey, and R. White. 1993. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler. 375 81–88. [DOI] [PubMed] [Google Scholar]
- 37.Meuwese M. C., E. S. Stroes, S. L. Hazen, J. N. van Miert, J. A. Kuivenhoven, R. G. Schaub, N. J. Wareham, R. Luben, J. J. Kastelein, K. T. Khaw, et al. 2007. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 50 159–165. [DOI] [PubMed] [Google Scholar]
- 38.Ndrepepa G., S. Braun, J. Mehilli, N. von Beckerath, A. Schomig, and A. Kastrati. 2008. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndromes. Eur. J. Clin. Invest. 38 90–96. [DOI] [PubMed] [Google Scholar]
- 39.Duzguncinar O., B. Yavuz, T. Hazirolan, A. Deniz, S. L. Tokgozoglu, D. Akata, and E. Demirpence. 2008. Plasma myeloperoxidase is related to the severity of coronary artery disease. Acta Cardiol. 63 147–152. [DOI] [PubMed] [Google Scholar]
- 40.Zhang R., M. L. Brennan, X. Fu, R. J. Aviles, G. L. Pearce, M. S. Penn, E. J. Topol, D. L. Sprecher, and S. L. Hazen. 2001. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 286 2136–2142. [DOI] [PubMed] [Google Scholar]
- 41.Brennan M. L., M. S. Penn, F. Van Lente, V. Nambi, M. H. Shishehbor, R. J. Aviles, M. Goormastic, M. L. Pepoy, E. S. McErlean, E. J. Topol, et al. 2003. Prognostic value of myeloperoxidase in patients with chest pain. N. Engl. J. Med. 349 1595–1604. [DOI] [PubMed] [Google Scholar]
- 42.Baldus S., C. Heeschen, T. Meinertz, A. M. Zeiher, J. P. Eiserich, T. Munzel, M. L. Simoons, and C. W. Hamm. 2003. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 108 1440–1445. [DOI] [PubMed] [Google Scholar]
- 43.Morrow D. A., M. S. Sabatine, M. L. Brennan, J. A. de Lemos, S. A. Murphy, C. T. Ruff, N. Rifai, C. P. Cannon, and S. L. Hazen. 2008. Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS-TIMI 18. Eur. Heart J. 29 1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mocatta T. J., A. P. Pilbrow, V. A. Cameron, R. Senthilmohan, C. M. Frampton, A. M. Richards, and C. C. Winterbourn. 2007. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J. Am. Coll. Cardiol. 49 1993–2000. [DOI] [PubMed] [Google Scholar]
- 45.Khan S. Q., D. Kelly, P. Quinn, J. E. Davies, and L. L. Ng. 2007. Myeloperoxidase aids prognostication together with N-terminal pro-B-type natriuretic peptide in high-risk patients with acute ST elevation myocardial infarction. Heart. 93 826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang W. H., W. Tong, R. W. Troughton, M. G. Martin, K. Shrestha, A. Borowski, S. Jasper, S. L. Hazen, and A. L. Klein. 2007. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J. Am. Coll. Cardiol. 49 2364–2370. [DOI] [PubMed] [Google Scholar]
- 47.Ng L. L., B. Pathik, I. W. Loke, I. B. Squire, and J. E. Davies. 2006. Myeloperoxidase and C-reactive protein augment the specificity of B-type natriuretic peptide in community screening for systolic heart failure. Am. Heart J. 152 94–101. [DOI] [PubMed] [Google Scholar]
- 48.Rudolph V., T. K. Rudolph, J. C. Hennings, S. Blankenberg, R. Schnabel, D. Steven, M. Haddad, K. Knittel, S. Wende, J. Wenzel, et al. 2007. Activation of polymorphonuclear neutrophils in patients with impaired left ventricular function. Free Radic. Biol. Med. 43 1189–1196. [DOI] [PubMed] [Google Scholar]
- 49.Vasilyev N., T. Williams, M. L. Brennan, S. Unzek, X. Zhou, J. W. Heinecke, D. R. Spitz, E. J. Topol, S. L. Hazen, and M. S. Penn. 2005. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 112 2812–2820. [DOI] [PubMed] [Google Scholar]
- 50.Nahrendorf M., D. Sosnovik, J. W. Chen, P. Panizzi, J. L. Figueiredo, E. Aikawa, P. Libby, F. K. Swirski, and R. Weissleder. 2008. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation. 117 1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]