Abstract
Cytochrome P450 (CYP) epoxygenases convert arachidonic acid to four epoxyeicosatrienoic acid (EET) regioisomers, 5,6-, 8,9-, 11,12-, and 14,15-EET, that function as autacrine and paracrine mediators. EETs produce vascular relaxation by activating smooth muscle large-conductance Ca2+-activated K+ channels (BKCa). In addition, they have anti-inflammatory effects on blood vessels and in the kidney, promote angiogenesis, and protect ischemic myocardium and brain. CYP epoxygenases also convert eicosapentaenoic acid to vasoactive epoxy-derivatives, and endocannabinoids containing 11,12- and 14,15-EET are formed. Many EET actions appear to be initiated by EET binding to a membrane receptor that activates ion channels and intracellular signal transduction pathways. However, EETs also are taken up by cells, are incorporated into phospholipids, and bind to cytosolic proteins and nuclear receptors, suggesting that some functions may occur through direct interaction of the EET with intracellular effector systems. Soluble epoxide hydrolase (sEH) converts EETs to dihydroxyeicosatrienoic acids (DHETs). Because this attenuates many of the functional effects of EETs, sEH inhibition is being evaluated as a mechanism for increasing and prolonging the beneficial actions of EETs.
Keywords: dihydroxyeicosatrienoic acid, eicosapentaenoic acid, endocannabinoids, epoxyeicosatrienoic acid, 2-epoxyeicosatrienoylglycerol, fatty acid binding protein, peroxisome proliferator-activated receptor, phospholipids, soluble epoxide hydrolase
Epoxyeicosatrienoic acids (EET) are epoxide derivatives of arachidonic acid. They are formed by cytochrome P450 (CYP) epoxygenases and function as lipid mediators. Epoxidation can occur at any of the four double bonds of arachidonic acid, giving rise to four regioisomers, 5,6-, 8,9-, 11,12-, and 14,15-EET. EETs are synthesized in the endothelium and activate large-conductance Ca2+-activated K+ channels (BKCa), causing hyperpolarization of the vascular smooth muscle and vasorelaxation. Thus, EETs function as an endothelium-derived hyperpolarizing factor (EDHF) in a number of vascular beds, including the coronary and renal circulations, producing a decrease in blood pressure. Soluble epoxide hydrolase (sEH), which converts EETs to dihydroxyeicosatrienoic acids (DHETs), attenuates many of the functional effects of EETs. These seminal findings have been described in a number of detailed reviews (1–4). Recent results with cultured cells and animal models indicate that EETs have additional potentially beneficial effects on the vascular system, heart, kidneys, and nervous system, and many current studies are directed at these actions (5–9). The other current emphasis is on sEH inhibition as a therapeutic strategy for increasing the beneficial effects of EETs (10, 11).
EET SYNTHESIS, METABOLISM, AND FUNCTION
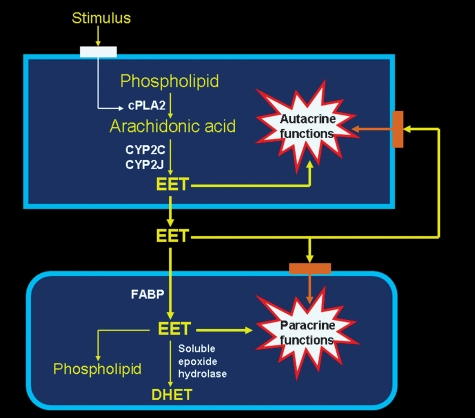
EETs are synthesized by cells that express CYP epoxygenase activity. As illustrated in Fig. 1, these enzymes act on arachidonic acid released from phospholipids when cytosolic phospholipase A2 (cPLA2) is activated (12). The epoxygenase inserts an oxygen atom on a carbon attached to one of the double bonds of arachidonic acid, and the double bond is reduced as the epoxide forms. Each CYP epoxygenase produces several regioisomers, with one form usually predominating. Thus, epoxygenases that mainly produce 14,15-EET also produce a moderate amount of 11,12-EET and a small amount of 8,9-EET. Each regioisomer contains two R/S enantiomeric forms in different proportions (2, 4). Because the regioisomers have a number of similar metabolic and functional properties, EETs are generally considered as a single class of compounds. This is an oversimplification, and there are quantitative and even qualitative differences in the actions of the various regioisomers (4). For example, 14,15-EET is the best substrate for sEH (4), and 11,12-EET is the only regioisomer that inhibits basolateral K+ channels in the renal cortical collecting duct (13).
Fig. 1.
Epoxyeicosatrienoic acid (EET) synthesis, metabolism, and function. EETs are synthesized by cells that express cytochrome P450 (CYP) epoxygenase. When cytosolic phospholipase A2 (cPLA2) is activated, the arachidonic acid hydrolyzed from intracellular phospholipids is converted to EETs. The EETs are released into the extracellular fluid and produce paracrine effects on other cells in the local environment. This may occur through EET binding to a receptor that is coupled to a signal transduction system, or through uptake and direct interaction of the EET with intracellular effector systems. Cytosolic fatty acid binding protein (FABP) may facilitate the uptake of EETs and modulate their incorporation into cell phospholipids and conversion to dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH). EETs also can produce autacrine effects through similar intracellular or receptor-mediated mechanisms.
EETs are taken up by many different kinds of cells (4, 12), and purified heart and liver cytoplasmic fatty acid binding proteins (FABP) bind EETs (14). This suggests that FABP may increase EET desorption from the cell membrane and thereby facilitate its uptake into the cell, as well as modulate EET incorporation into phospholipids and its availability to sEH for DHET formation (4, 12). Because conversion to DHET attenuates many of the physiological actions of EETs, binding to FABP may increase the intracellular retention of EETs and thereby prolong their functional effectiveness. Although not shown in Fig. 1, the cells that synthesize EETs also have the capacity to incorporate EETs into phospholipids and convert them to DHETs.
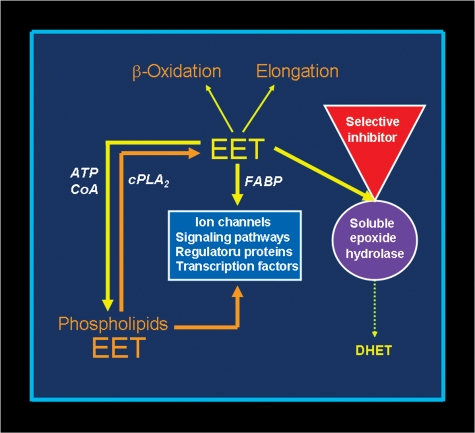
EETs undergo β-oxidation, forming 16-carbon epoxy-derivatives that accumulate in the extracellular fluid, and they can be chain-elongated to form 22-carbon derivatives that are incorporated into phospholipids (15). As illustrated in Fig. 2, the availability of EETs to these metabolic pathways increases when sEH is inhibited (16). The 22-carbon elongation product of 14,15-EET, which is an epoxide derivative of adrenic acid, relaxes bovine coronary artery preparations by hyperpolarizing smooth muscle in a manner similar to EETs (17).
Fig. 2.
sEH inhibition enhances EET function. Conversion of EET to DHET by sEH is the main pathway of EET metabolism. This attenuates most functional effects of EETs, making sEH a logical target for increasing and prolonging the actions of EETs. sEH inhibition decreases DHET formation and leads to intracellular EET accumulation. This results in more EET incorporation into phospholipids and utilization by other metabolic pathways, including β-oxidation and chain-elongation. Functional responses are increased because of the larger amounts of intracellular unesterified EET and EET-containing phospholipids. Furthermore, more EET is released when intracellular phospholipids are hydrolyzed, maintaining the increased intracellular concentration of unesterified EET.
EETs produce autacrine and paracrine effects, but these actions often are overlooked in experimental studies because CYP epoxygenases are labile in cultured cells and are inhibited under conditions where H2O2 is formed (18). The paracrine effects are produced by the EET released into the extracellular fluid (Fig. 1). While the autocrine effects probably also are produced by the EET initially released into the extracellular fluid, the possibility that they result from intracellular actions of EET that is retained in the cell following synthesis cannot be excluded.
CELLULAR MECHANISM OF ACTION
Three mechanisms have been proposed to explain the cellular actions of EETs (4). Two involve EET binding to cell-surface receptors, and the other is an intracellular mechanism. A substantial amount of chemical and functional data supports the likelihood that EETs bind to a selective EET receptor that is coupled by a G-protein to intracellular signal transduction pathways (4, 19). However, this possibility remains open to question because the putative EET receptor has not yet been identified or cloned. A second mechanism involves EET binding to receptors for other lipid-soluble agonists that also function by coupling to intracellular signaling pathways. The third possibility, an intracellular mechanism, is based on the fact that EETs have many characteristics of long-chain fatty acids. This mechanism involves uptake of the EET by the cell, with the cell-associated EET directly interacting with ion channels, signaling proteins, or transcription factors. Support for an intracellular mechanism of action stems from the fact that EETs are incorporated into cell phospholipids and bind to cytoplasmic FABPs and peroxisome proliferator-activated receptor (PPAR)γ (4, 12, 14, 20).
INCORPORATION INTO PHOSPHOLIPIDS
The functional significance of EET incorporation into cell phospholipids is uncertain. EETs are incorporated primarily into the sn-2 position of the phospholipids, and cell culture studies indicate that a substantial amount of the incorporation is subsequently released even when no stimulus is applied to the cultures (4, 12). This suggests that EET incorporation may temporarily alter the properties of membrane microdomains and thereby transiently affect the function of proteins located in these domains. Alternatively, the incorporation of EETs may modulate phospholipid-dependent signal transduction systems. EETs also are hydrolyzed rapidly from the phospholipids by a Ca2+-stimulated mechanism (12, 16). Therefore, EETs contained in phospholipids may constitute an intracellular storage pool that is available for immediate release when the cell is activated (12).
Another possibility is that phospholipids containing EET are substrates for the production of other lipid mediators. Kidney and spleen produce 2-epoxyeicosatrienoylglycerols that contain 11,12-EET or 14,15-EET (21). 2-Epoxyeicosatrienoylglycerol are endocannabinoids that activate CB1 and CB2 receptors, and 2-(14,15)-EG produces proliferation of renal proximal tubule cells by causing the release of ligands that activate the epidermal growth factor receptor (22). In addition, phospholipids containing EET are the likely substrates for the EET-ethanolamide synthesized in the liver and kidney (23).
Finally, EET incorporation into phospholipids may serve to lower the intracellular unesterified EET concentration and thereby be part of the mechanism that terminates its functional effects. The subsequent gradual hydrolysis of the EET from the phospholipids might then make the EET available so it can be efficiently inactivated by sEH or β-oxidation.
EET-ACTIVATED SIGNALING PATHWAYS
The functional effects of EETs have been observed to occur through a number of different signal transduction pathways (4). The most effective regioisomer that produces the EDHF effect in the coronary circulation, 11,12-EET, functions through a cAMP-dependent process that activates vascular smooth muscle BKCa channels. This paracrine mechanism involves the Gαs protein, adenylyl cyclase activation, and an increase in cAMP (5). A similar pathway involving ADP-ribosylation of Gαs, an increase in cAMP, and protein kinase A activation produces EET-stimulated vasodilation of preglomerular renal microvessels (24). Likewise, a cAMP-protein kinase A mechanism mediates the EET-stimulated increase in StAR protein and steroid hormone production (25). While the EET-stimulated relaxation of renal afferent arterioles also is mediated by cAMP-dependent activation of BKCa channels, the response in this preparation involves an increase in phosphoprotein phosphatase 2A (26).
EET activation of endothelial Trp channels is an alternative mechanism proposed for the coronary EDHF response (27). Trp channel activation produces Ca2+ influx and endothelial K+ channel activation, and the hyperpolarized endothelium triggers relaxation of the vascular smooth muscle.
EETs have anti-inflammatory and antiapoptotic actions in the endothelium (7). The anti-inflammatory effect produced by 11,12-EET occurs through a signaling pathway that inhibits NF-κB activation (28). 8,9-EET, 11,12-EET, and 14,15-EET inhibit endothelial apoptosis, but this occurs through activation of a PI3K/Akt pathway that inhibits Erk1/2 dephosphorylation (29). Anti-inflammatory effects of EETs also have been observed in the kidney (10).
Several different signaling pathways have been implicated in the angiogenic effect of EETs (4, 6). 11,12-EET has been observed to stimulate angiogenesis by activating an EphB4-coupled PI3K/Akt pathway (30), whereas others find that it functions by activating sphingosine kinase-1 (31). Likewise, different pathways are reported to produce 14,15-EET-mediated angiogenesis. A Src activated PI3K/Akt pathway coupled to FGF-2 expression and mTOR-S6K1 activation has been observed in one study (32), whereas Src-stimulated tyrosine phosphorylation of STAT-3, which binds to the VEGF promoter, has been observed in another study with 14,15-EET (33).
11,12- and 14,15-EET have cardioprotective effects during reoxygenation of ischemic myocardium, and they decrease infarct size (8, 34). Several mechanisms have been reported to mediate the cardioprotective effect, including activation of myocardial KATP channels by decreasing their sensitivity to ATP (35), activation of KATP channels by triggering a burst of reactive oxygen species (36), and activation of a PI3K/Akt pro-survival pathway (37).
EETs are synthesized in the brain by astrocytes through a mechanism linked to mGluR and adenosine A(2B) receptors and are involved in neurovascular coupling (38). They also produce antinociception by activating β-endorphin and met-enkephalin that interact with μ- and δ-opioid receptors (39). In addition, EETs reduce brain ischemia and infarct size in stroke (40).
sEH INHIBITION
Inhibition of sEH is potentially beneficial because it increases and prolongs the functional effects of EETs (16). As illustrated in Fig. 2, EET accumulates in the cell when the conversion to DHET is inhibited. This increases EET availability for incorporation into phospholipids and for interaction with ion channels, signaling pathways, and transcription factors. No essential function of DHETs has so far been detected (4), and the widely held view is that sEH inhibition is not harmful. However, some caution is suggested by the findings that 11,12-DHET activates BKCa channels in coronary artery myocytes and produces coronary vasodilation (41). Furthermore, 14,15-DHET stimulates PPARα-mediated transcription in a COS-7 cell gene expression system (42). Whether any of these DHET effects are physiologically relevant remains open to question.
sEH inhibitors initially were developed as antihypertensive agents (11), but recent data indicate that they also prevent cardiac hypertrophy (43), decrease vascular smooth muscle proliferation (44), improve renal hemodynamics (3), and decrease hypertensive renal damage (10, 45). This is consistent with the finding that angiotensin II up-regulates sEH through a transcriptional mechanism, thereby reducing EET availability (46). sEH inhibitors with improved physical properties and metabolic stability have been developed (47), and compounds suitable for clinical trials are now available.
OMEGA-3 EET ANALOGS
17,18-Epoxyeicosatetraenoic acid (17,18-EETr), the main epoxide regioisomer synthesized from eicosapentaenoic acid, produces vasodilation (48). The mechanism involves activation of BKCa channels, with the 17,18-EETr targeting the pore-forming BKα subunit (49). This suggests that some beneficial effects of omega-3 fatty acid supplementation may be due to conversion of eicosapentaenoic acid to 17,18-EETr. Likewise, chemically synthesized epoxide derivatives of docosahexaenoic acid are potent dilators of coronary arterioles (50), but the formation of these derivatives has not been observed so far in biological systems.
FUTURE DIRECTIONS
EETs are now well-established as autacrine and paracrine lipid mediators. While the initial emphasis focused on the EDHF response and activation of BKCa channels, recent evidence indicates that EETs have effects on other ion channels and signal transduction pathways. EETs also are incorporated into phospholipids and affect angiogenesis, mitogenesis, apoptosis, and PPAR-transactivated gene expression. EET mimetics and antagonists, potent selective sEH inhibitors, and transgenic CYP and sEH mice are now available to further explore these and other functional effects of EETs. The discoveries of omega-3 EET analogs and EET-containing endocannabinoids open up new and potentially fruitful research directions. In addition, many new opportunities for translational studies are suggested by results in animal models indicating that EETs have anti-inflammatory effects in the vasculature and kidney, produce antinociception, and protect the myocardium and brain against ischemic damage.
Abbreviations
BKCa, large-conductance Ca2+-activated K+ channels
CYP, cytochrome P450
cPLA2, cytosolic phospholipase A2
DHET, dihydroxyeicosatrienoic acid
EDHF, endothelium-derived hyperpolarizing factor
EET, epoxyeicosatrienoic acid
17,18-EETr, 17,18-epoxyeicosatetraenoic acid
FABP, fatty acid binding protein
PPAR, peroxisome proliferator-activated receptor
sEH, soluble epoxide hydrolase
Work from my laboratory cited in this review was supported by National Institutes of Health grants HL049264 and HL072845.
Published, JLR Papers in Press, October 23, 2008.
References
- 1.Campbell W. B., and D. R. Harder. 1999. Endothelium derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ. Res. 84 484–488. [DOI] [PubMed] [Google Scholar]
- 2.Capdevila J. H., J. R. Falck, and R. C. Harris. 2000. Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of arachidonic acid monooxygenases. J. Lipid Res. 41 163–181. [PubMed] [Google Scholar]
- 3.Roman R. 2002. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82 131–185. [DOI] [PubMed] [Google Scholar]
- 4.Spector A. A., and A. W. Norris. 2007. Action of epoxyeicosatrienoic acids on cellular function. Am. J. Physiol. Cell Physiol. 292 C996–C1012. [DOI] [PubMed] [Google Scholar]
- 5.Campbell W. B., and J. R. Falck. 2007. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 49 590–596. [DOI] [PubMed] [Google Scholar]
- 6.Michaelis U. R., and I. Fleming. 2006. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol. Ther. 111 584–595. [DOI] [PubMed] [Google Scholar]
- 7.Larsen B. T., W. B. Campbell, and D. D. Gutterman. 2007. Beyond vasodilation: non-vascular roles for epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol. Sci. 28 32–38. [DOI] [PubMed] [Google Scholar]
- 8.Seubert J. M., D. C. Zeldin, K. Nithipatikom, and G. J. Gross. 2007. Role of epoxyeicosatrienoic acids in protecting myocardium following ischemic/reperfusion injury. Prostagl. other Lipid Mediat. 82 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming I. 2008. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc. Med. 18 20–25. [DOI] [PubMed] [Google Scholar]
- 10.Imig J. D. 2005. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am. J. Physiol. Renal Physiol. 289 F496–F503. [DOI] [PubMed] [Google Scholar]
- 11.Chiamvimonvat N., C. M. Ho, H. J. Tsai, and B. D. Hammock. 2007. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J. Cardiovasc. Pharmacol. 50 225–237. [DOI] [PubMed] [Google Scholar]
- 12.Spector A. A., X. Fang, G. D. Snyder, and N. L. Weintraub. 2004. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog. Lipid Res. 43 55–90. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z., Y. Wei, J. R. Falck, K. R. Atcha, and W. H. Wang. 2008. Arachidonic acid inhibits K channels in the cortical collecting duct via cytochrome P-450 epoxygenase-dependent metabolic pathways. Am. J. Physiol. Renal Physiol. 294 F1441–F1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widstrom R. L., A. W. Norris, J. Van Der Veer, and A. A. Spector. 2003. Fatty acid binding proteins inhibit hydration of epoxyeicosatrienoic acids by soluble epoxide hydrolase. Biochemistry. 42 11762–11767. [DOI] [PubMed] [Google Scholar]
- 15.Fang X., N. L. Weintraub, C. L. Oltman, L. L. Stoll, T. L. Kaduce, S. Harmon, K. C. Dellsperger, C. Morisseau, B. D. Hammock, and A. A. Spector. 2002. Human coronary endothelial cells convert 14,5-EET to a biologically active chain-shortened epoxide. Am. J. Physiol. Heart Circ. Physiol. 283 H2306–H2314. [DOI] [PubMed] [Google Scholar]
- 16.Fang X., T. L. Kaduce, N. L. Weintraub, S. Harmon, L. M. Teesch, K. C. Dellsperger, C. Morisseau, D. A. Thompson, B. D. Hammock, and A. A. Spector. 2001. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J. Biol. Chem. 276 14867–14874. [DOI] [PubMed] [Google Scholar]
- 17.Yi X. Y., K. M. Gauthier, L. Cui, K. Nithipatikom, J. R. Falck, and W. B. Campbell. 2007. Metabolism of adrenic acid to vasodilatory 1α,1β-dihomo-epoxyeicosatrienoic acids by bovine coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 292 H2265–H2274. [DOI] [PubMed] [Google Scholar]
- 18.Larsen B. T., D. D. Gutterman, A. Sato, A. Toyama, W. B. Campbell, D. C. Zeldin, V. L. Manthati, J. R. Falck, and H. Miura. 2008. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ. Res. 102 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang W., V. R. Tuniki, S. Anjaiah, J. R. Falck, C. J. Hillard, and W. B. Campbell. 2008. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125I-14,15-epoxyeicosa-8(Z)-enoic acid. J. Pharmacol. Exp. Ther. 324 1019–1027. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Y. Zhang, K. Schmelzer, T. S. Lee, X. Fang, Y. Zhu, A. A. Spector, S. Gill, C. Morisseau, B. D. Hammock, et al. 2005. The anti-inflammatory effect of laminar flow: the role of PPARγ, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc. Natl. Acad. Sci USA. 102 16747–16752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J. K., J. Chen, J. D. Imig, S. Wei, D. L. Hackey, J. S. Guthi, J. R. Falck, J. H. Capdevila, and R. C. Harris. 2008. Identification of novel endogenous cytochrome p450 arachidonate metabolites with high affinity for cannabinoid receptors. J. Biol. Chem. 283 24511–24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J., J. K. Chen, J. R. Falck, J. S. Guthi, S. Anjaiah, J. H. Capdevila, and R. C. Harris. 2007. Mitogenic activity and signaling mechanism of 2-(14,15-epoxyeicosatrienoyl)glycerol, a novel cytochrome p450 arachidonate metabolite. Mol. Cell. Biol. 27 3023–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snider N. T., A. M. Kornilov, U. M. Kent, and P. F. Hollenberg. 2007. Anandamide metabolism by human liver and kidney microsomal cytochrome p450 enzymes to form hydroxyeicosatetraenoic and epoxyeicosatrienoic acid ethanolamides. J. Pharmacol. Exp. Ther. 321 590–597. [DOI] [PubMed] [Google Scholar]
- 24.Carroll M. A., A. B. Doumod, J. Li, M. K. Cheng, J. R. Falck, and J. C. McGiff. 2006. Adenosine 2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am. J. Physiol. Renal Physiol. 291 F155–F161. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., C. L. Shen, M. T. Dyson, X. Yin, A. B. Schiffer, P. Grammas, and J. M. Stocco. 2006. The involvement of epoxygenase metabolites of arachidonic acid in cAMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J. Endocrinol. 190 871–878. [DOI] [PubMed] [Google Scholar]
- 26.Imig J. D., C. Dimitropoulou, D. S. Reddy, R. E. White, and J. R. Falck. 2008. Afferent arteriole dilation to 11,12-EET analogs involves PP2A activity and Ca2+-activated K+ channels. Microcirculation. 15 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming I., A. Reuben, R. Poop, B. Fisslthaler, S. Schrodt, A. Sander, J. Haendeler, J. R. Falck, C. Morisseau, B. D. Hammock, et al. 2007. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 27 2612–2618. [DOI] [PubMed] [Google Scholar]
- 28.Spiecker M., and J. K. Liao. 2005. Vascular protective effects of cytochrome P450 epoxygenase-derived eicosanoids. Arch. Biochem. Biophys. 433 413–420. [DOI] [PubMed] [Google Scholar]
- 29.Yang S., L. Lin, J. X. Chen, C. R. Lee, J. M. Seubert, Y. Wang, H. Wang, Z. R. Chao, D. D. Tao, J. P. Gong, et al. 2007. Cytochrome P450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor alpha via MAPK and PI3K/Akt signaling pathway. Am. J. Physiol. Heart Circ. Physiol. 293 H142–H151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webber A. C., R. Popp, U. Thomas Korff, R. Michaelis, C. Urbich, R. Busse, and I. Fleming. 2008. Cytochrome P450 2C9-induced angiogenesis is dependent on EphB4. Arterioscler. Thronb. Vasc. Biol. 28 1123–1129. [DOI] [PubMed] [Google Scholar]
- 31.Yan G., S. Chen, B. You, and J. Sun. 2008. Activation of sphingosine kinase-1 mediates induction of endothelial cell proliferation and angiogenesis by epoxyeicosatrienoic acids. Cardiovasc. Res. 78 308–314. [DOI] [PubMed] [Google Scholar]
- 32.Zhang B., H. Cao, and G. N. Rao. 2006. Fibroblast growth factor-2 is a downstream mediator of phosphatidylinositol 3-kinase-Akt signaling in 14,15-epoxyeicosatrienoic acid-induced angiogenesis. J. Biol. Chem. 281 905–914. [DOI] [PubMed] [Google Scholar]
- 33.Cheranov S. Y., M. Karpurapu, D. Wang, B. Zhang, R. C. Venema, and G. N. Rao. 2008. An essential role for SRC-activated STAT-3 in 14,15-EET-induced VGEF expression and angiogenesis. Blood. 111 5581–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross G. J., K. M. Gauthier, J. Moore, J. R. Falck, B. D. Hammock, W. B. Campbell, and K. Nithipatikom. 2008. Effect of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in canine hearts. Am. J. Physiol. Heart Circ. Physiol. 294 H2838–H2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X. L., T. Lu, S. Cao, V. H. Shah, and H. C. Lee. 2006. Inhibition of ATP binding to the carboxyl terminus of Kir 6.2 by epoxyeicosatrienoic acids. Biochim. Biophys. Acta. 1761 1041–1049. [DOI] [PubMed] [Google Scholar]
- 36.Gross G. J., A. Hsu, J. R. Falck, and K. Nithipatikom. 2007. Mechanism by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J. Mol. Cell. Cardiol. 42 687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhanasekaran A., S. K. Gruenloh, J. N. Buonaccorsi, R. Zhang, G. R. Gross, J. R. Falck, P. K. Patel, E. R. Jacobs, and M. Medhora. 2008. Multiple antiapoptotic targets of PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am. J. Physiol. Heart Circ. Physiol. 294 H724–H735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Y., X. Liu, D. Gebremedhin, J. R. Falck, D. R. Harder, and R. C. Koehler. 2008. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J. Cereb. Blood Flow Metab. 28 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terashvili M., L. F. Tseng, H. E. Wu, J. Narayanan, L. M. Hart, J. R. Falck, P. F. Pratt, and D. R. Harder. 2008. Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray matter. J. Pharmacol. Exp. Ther. 326 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W., I. P. Koerner, R. Noppens, M. Grafe, H. J. Tsai, C. Morisseau, A. Luria, B. D. Hammock, J. R. Falck, and N. J. Alkayed. 2007. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J. Cereb. Blood Flow Metab. 27 1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu T., P. V. G. Katakam, M. VanRollins, N. L. Weintraub, A. A. Spector, and H. C. Lee. 2001. Dihydroxyeicosatrienoic acids are potent activators of Ca2+-activated K+ channels in isolated rat coronary arterial myocytes. J. Physiol. 534 651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang X., S. Hu, B. Xu, G. D. Snyder, S. Harmon, J. Tao, Y. Liu, B. Sangras, J. R. Falck, N. L. Weintraub, et al. 2006. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor α. Am. J. Physiol. Heart Circ. Physiol. 290 H55–H63. [DOI] [PubMed] [Google Scholar]
- 43.Xu D., N. Li, V. Timofeyev, L. Lu, H. J. Tsai, I. H. Kim, D. Tuteja, R. K. Mateo, A. Singapuri, B. B. Davis, et al. 2006. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc. Natl. Acad. Sci USA. 103 18733–18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng V. Y., C. Morisseau, J. R. Falck, B. D. Hammock, and D. L. Kroetz. 2006. Inhibition of smooth muscle proliferation by urea-based alkanoic acids via peroxisome proliferator-activated receptor α-dependent repression of cyclin D1. Arterioscler. Thromb. Vasc. Biol. 26 2462–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H., C. Morisseau, J. Wang, T. Yang, J. R. Falck, B. D. Hammock, and M. H. Wang. 2007. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am. J. Physiol. Renal Physiol. 293 F342–F349. [DOI] [PubMed] [Google Scholar]
- 46.Ai D., Y. Fu, D. Guo, H. Tanaka, N. Wang, C. Tang, B. D. Hammock, J. Y. Shyy, and Y. Zhu. 2007. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelial cells in vitro and in vitro. Proc. Natl. Acad. Sci. USA. 104 9018–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim I. H., H. J. Tsai, K. Nishi, T. Kasagami, C. Morisseau, and B. D. Hammock. 2007. 1,3-Disubstituted ureas functionalized with ether groups are potent inhibitors of soluble epoxide hydrolase with improved pharmacokinetic properties. J. Med. Chem. 50 5217–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauterbach B., E. Barbosa-Siscard, M. H. Wang, H. Honeck, E. Kargel, J. Theuer, M. L. Schwartzman, H. Haller, F. C. Luft, M. Gollasch, et al. 2002. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension. 39 609–613. [DOI] [PubMed] [Google Scholar]
- 49.Hercule H. C., B. Salanova, K. Essin, H. Honeck, J. R. Falck, M. Sausbier, P. Ruth, W. H. Schunk, F. C. Luft, and M. Gollasch. 2007. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BKα channel subunit in rodents. Exp. Physiol. 92 1067–1076. [DOI] [PubMed] [Google Scholar]
- 50.Ye D., D. Zhang, C. Oltman, L. Dellsperger, H. C. Lee, and M. VanRollins. 2002. Cytochrome P-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 303 768–776. [DOI] [PubMed] [Google Scholar]