Abstract
Macrophage apoptosis is an important feature of atherosclerotic plaque development. Research directed at understanding the functional consequences of macrophage death in atherosclerosis has revealed opposing roles for apoptosis in atherosclerotic plaque progression. In early lesions, macrophage apoptosis limits lesion cellularity and suppresses plaque progression. In advanced lesions, macrophages apoptosis promotes the development of the necrotic core, a key factor in rendering plaques vulnerable to disruption and in acute lumenal thrombosis. The first section of this review will examine the role of phagocytic clearance of apoptotic macrophages, a process known as efferocytosis, in the dichotomous roles of macrophage apoptosis in early vs. advanced lesions. The second section will focus on the molecular and cellular mechanisms that are thought to govern macrophage death during atherosclerosis. Of particular interest is the complex and coordinated role that the endoplasmic reticulum (ER) stress pathway and pattern recognition receptors (PRRs) may play in triggering macrophage apoptosis.
Keywords: innate immunity, efferocytosis, plaque necrosis, ER stress
CONSEQUENCES OF MACROPHAGE DEATH
Macrophages play crucial roles as a primary line of defense against infectious pathogens and foreign material and by ridding tissues of apoptotic debris. However, under pathological conditions, macrophages can promote a number of important disease processes, including insulin resistance, cancer, and atherosclerosis (1, 2). In the case of atherosclerosis, the topic of this review, a macrophage-dominant maladaptive inflammatory response develops as a reaction to the subendothelial retention and modification of apolipoprotein B-containing lipoproteins (3). In all stages of atherosclerotic lesions, activated macrophages, probably dominated by the “classically” activated M1 subset (4), secrete inflammatory cytokines and other molecules that contribute to lesion progression (5). Therefore, processes that increase macrophage accumulation in lesions, notably influx and proliferation, can promote lesion development, while those that decrease macrophage accumulation, such as apoptosis coupled with phagocytic clearance and macrophage egress, can retard lesion progression. In advanced lesions, macrophage apoptosis is not properly coupled with phagocytic clearance, and so in this setting macrophage death is associated with a detrimental role: plaque necrosis (6, 7). This process leads to expansion of the necrotic core of advanced plaques, which contributes to plaque disruption and acute thrombosis (8). Thus, depending on the efficiency of apoptotic cell clearance, macrophage death can be a process that limits lesion cellularity or promotes plaque necrosis. In this review, we summarize the evidence supporting this dichotomous model of lesional macrophage death and discuss new concepts related to mechanisms of macrophage apoptosis and phagocytic clearance of apoptotic cells.
Macrophage death as a factor that limits lesion cellularity
Macrophage apoptosis occurs during all stages of atherosclerosis (9, 10). Apoptotic cells have been identified in vivo using a variety of techniques, including annexin V staining, which is indicative of phosphatidylserine externalization; condensed nuclei; terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL), which signifies DNase-mediated DNA fragmentation; and caspase activation. Over the last few years, mouse models have been developed to explore the functional consequences of macrophage death in atherosclerosis. In early lesions (i.e., prior to necrotic core development), there is an inverse relationship between macrophage apoptosis and lesion size. For example, reconstitution of APOE*3-Leiden mice with p53−/− bone marrow resulted in reduced macrophage apoptosis, while macrophage content and lesion area were significantly increased (11). Another study demonstrated reduced macrophage apoptosis and increased lesion size in Ldlr−/− mice reconstituted with Bax-deficient bone marrow (12). The beneficial aspect of early lesional macrophage death has also been documented using mice deficient in the prosurvival molecule AIM (apoptosis inhibitor expressed by macrophages; also called Spα or Api6). This study found that Aim−/−;Ldlr−/− macrophages were more susceptible to oxLDL-induced apoptosis, and double knockout mice exhibited accelerated macrophage death and a significant reduction in early lesion area (13). Taken together, these findings support the concept that macrophage apoptosis in early lesions is beneficial by suppressing lesion cellularity.
Macrophage death as a factor that promotes advanced plaque necrosis
As a prelude to this section, we wish to clarify the use of the term “necrosis” when referring to processes related to advanced atherosclerosis. On a cellular level, “necrosis” refers to a type of cell perturbation in which membranes become leaky and organelles swell, ultimately leading to cellular death. In vivo, cell necrosis can result when apoptotic cells, a programmed form of cell death in which membranes are initially intact and organelles are condensed, are not rapidly ingested by neighboring phagocytes. When this happens, the noningested apoptotic cells eventually become leaky and swollen. This type of cell death is often called “postapoptotic,” or “secondary,” necrosis. At a tissue level, “necrosis” refers to collections of cell debris resulting from necrotic cell death. For example, in tuberculosis this process is referred to as “caseating necrosis.” For the purpose of this review on atheromata, we refer to cell necrosis as “postapoptotic macrophage necrosis” and tissue necrosis as “plaque necrosis.” “Necrotic core” is often referred to in the literature as “lipid core,” because the dying macrophages are filled with lipid, mostly cholesterol, which becomes incorporated as extracellular lipid into the areas of plaque necrosis.
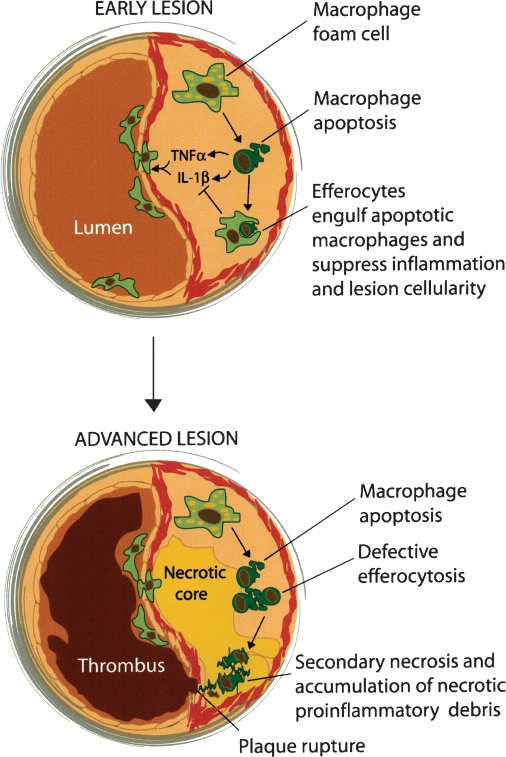
Observational studies of advanced atherosclerotic lesions have shown that apoptotic macrophages accumulate in focal areas surrounding the developing necrotic core (14). Rather than simply “guilt by association,” the fact that necrotic cores contain predominantly macrophage debris has given rise to a concept alluded to above, namely, that plaque necrosis develops as a direct consequence of postapoptotic macrophage necrosis (7, 14). The necrotic debris is a source of proinflammatory stimuli and proteases and thus can elicit an inflammatory response and cause damage to nearby cells. These events, together with stresses on the fibrous cap caused by the physical nature of the necrotic core (15), can contribute to fibrous cap rupture, exposure of tissue factor, and subsequent lumenal thrombosis (7) (Fig. 1).
Fig. 1.
Model of the functional consequences of macrophage apoptosis in early and advanced lesions. In early lesions, monocytes surveying the vascular wall are recruited to the developing plaque. The monocytes differentiate into macrophages in areas where modified and remnant lipoproteins are retained in the extracellular matrix. Macrophages become foam cells by ingesting lipoproteins and storing these lipids in droplets. The engorged foam cells secrete a variety of proinflammatory cytokines and then eventually undergo apoptosis. Rapid efferocytic clearance of the apoptotic cells leads to suppression of the proinflammatory response. The overall effect is a reduction in lesion cellularity and size. In advanced lesions, the apoptotic macrophages are not efficiently cleared by efferocytosis. The apoptotic macrophages that accumulate eventually undergo secondary necrosis. The buildup of necrotic debris promotes inflammation, plaque instability, and acute thrombosis.
To support this overall concept using a molecular and genetic approach, investigators have turned to mouse models in which proteins involved in macrophage apoptosis have been genetically altered. Because the traditional models of murine atherosclerosis, namely, Western diet-fed Apoe−/− and Ldlr−/− mice, do not develop plaque disruption or acute thrombosis, advanced lesional macrophage apoptosis and plaque necrosis are often used as endpoints to test causation.
As will be discussed in more detail later, endoplasmic reticulum (ER) stress is a likely factor that promotes advanced lesional macrophage death. Markers of ER stress have been shown to occur in advanced atherosclerotic lesions of human and mouse, and thin or ruptured advanced human plaque have the highest accumulation of ER-stressed macrophages (16–19). These ER-stressed macrophages occur in areas of high TUNEL reactivity (18). Moreover, recent studies designed to look at the contribution of the ER stress pathway to macrophage apoptosis have shown a correlative relationship between macrophage death and necrotic core development. For example, macrophages with haploinsufficiency of the cholesterol trafficking protein NPC1 are protected from apoptosis induced by unesterified cholesterol, an ER stress-inducing agent (16, 20). When compared with Apoe−/− mice, Npc1+/−;Apoe−/− have a marked reduction in apoptosis and necrotic area (20). A similar reduction in macrophage apoptosis and plaque necrosis was observed using Ldlr−/− mice reconstituted with Stat1−/− bone marrow (21). STAT1 is a transcription factor that is phosphorylated and activated in human plaques and is necessary for ER stress-mediated macrophage apoptosis in vitro (21). As another example, thiazolidinediones have recently been shown to enhance ER stress-induced macrophage apoptosis in vitro. When administered to nondiabetic LDL receptor-deficient mice with pre-established nonnecrotic lesions, there was a substantial increase in lesional macrophage apoptosis and enhanced plaque necrosis (22). In yet another example, in vitro studies have shown that macrophages with defective insulin signaling, including those from insulin-resistant mice, are more susceptible to ER stress-induced apoptosis (23). When Ldlr−/− mice were reconstituted with insulin receptor-deficient bone marrow as a proof-of-concept model of macrophage insulin resistance, an increase in advanced lesional macrophage apoptosis and plaque necrosis was observed. These combined data provide strong evidence in support of the hypothesis that macrophage apoptosis in advanced atheromata promote plaque necrosis.
The role of efferocytosis in modulating plaque necrosis
As described above, whether apoptosis leads to a decrease in cellularity or an increase in tissue necrosis depends to a large extent on the efficiency of apoptotic cell clearance by phagocytes, a process known as efferocytosis (24, 25). In early atherosclerotic lesions, low levels of apoptotic macrophages suggest a normal or unperturbed efferocytic process (9). However in advanced lesions, the large number of apoptotic macrophages that accumulate almost certainly suggests perturbed efferocytosis even if the rate if apoptosis were also increased. Indeed, recent work by Schrijvers et al. (6) has demonstrated that efferocytosis is defective in advanced lesions. In vitro work has also shown that modified lipoproteins such as oxidized-LDL, which are abundant in advanced plaque, inhibit efferocytosis (26). Apoptosis may also be influenced by the stage of lesion progression. For example, as the necrotic core develops and cellular debris accumulates, the macrophage environment becomes enriched in proinflammatory cytokines and pattern recognition receptor ligands that, as discussed in more detail in the following section, may further enhance macrophage death. Therefore, necrotic core development likely occurs through the combination of defective efferocytosis and enhanced macrophage death (7). The importance of efferocytosis has recently been documented using in vivo mouse models that are deficient in efferocytic receptors. Two independent studies have shown that deficiency of Mertk, a phagocytic receptor for apoptotic cells, leads to a marked increase in apoptotic macrophages in lesions and enhanced plaque necrosis (27–29). Similar results of enhanced apoptotic macrophage accumulation and necrosis were also shown with deficiencies of other molecules thought to play roles in efferocytosis, including apolipoprotein E, Fas, transglutaminase-2, complement protein C1q, and lactadherin (28).
MECHANISMS OF MACROPHAGE DEATH
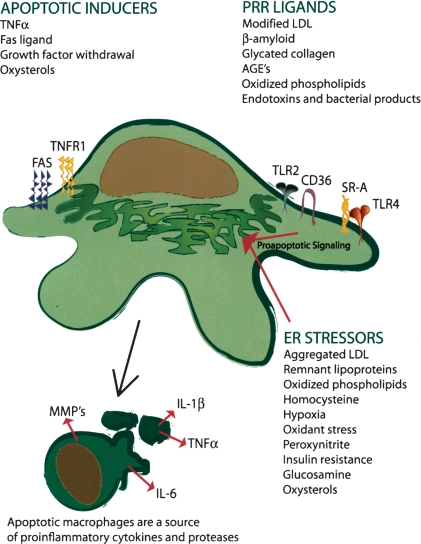
Induction of macrophage apoptosis in atherosclerotic lesions likely involves the chronic, cumulative effect of several subtle, subthreshold “hits” rather than a single acute, catastrophic event. Examples of proapoptotic processes that occur in atheromata are oxidant stress (30), high concentrations of cytokines such as TNFα (31), unesterified cholesterol or oxysterols (16, 18), oxLDL, activation of the Fas death pathway by Fas ligand (32), and ER stress (16–19) (Fig. 2). ER stress in particular, through activation of the unfolded protein response (UPR), is strongly correlated with advanced lesional macrophage apoptosis and plaque necrosis in murine lesions and in human coronary artery vulnerable plaques (16–19).
Fig. 2.
Potential inducers of macrophage apoptosis in atherosclerotic lesions. Macrophage apoptosis can be triggered by a variety of factors that work alone or, most likely, in combination to trigger macrophage death. The buildup of endogenous ligands that are recognized by SRA, CD36, and toll-like receptors (TLRs) trigger a proinflammatory and apoptotic response during ER stress. See text for details. AGE, advanced glycation end-products; MMP, matrix metalloproteinase; PRR, pattern recognition receptors; TNF-α, tumor necrosis factor-α.
The roles of ER stress and pattern recognition receptors in advanced lesional macrophage apoptosis
The UPR exists as a means to protect cells from the accumulation and detrimental effects of misfolded proteins and therefore functions in normal physiology as an adaptive survival pathway (33). In particular, when misfolded proteins accumulate, a unique set of signal transduction events take place to simultaneously halt further protein translation and up-regulate chaperones and transcription factors that serve to increase the capacity for the ER to process client proteins. When the amount of misfolded proteins exceeds the capacity for proper protein folding, or are not degraded through the ER-associated degradation pathway, apoptosis can ensue. Apoptosis in this context is highly dependent on prolonged expression of the UPR effector CHOP (Gadd153) (16, 34).
There are many potential causes of ER stress in atherosclerotic plaques that are likely to influence advanced plaque progression. Examples of athero-relevant ER stress inducers include oxidant stress and peroxynitrite (30); insulin resistance (23); glucosamine (35); saturated fatty acids (36); hypoxia (37); homocysteine (38); oxidized phospholipids (17); oxysterols such as 7-ketocholesterol (18); serum starvation; and unesterified cholesterol accumulation from the uptake of modified, aggregated, and remnant lipoproteins (16, 34). However, because ER stress is usually a protective response, high levels and prolonged activation of ER stress would be needed to induce apoptosis. A more likely scenario that may be occurring in advanced lesional macrophage apoptosis is that more physiologic levels of ER stress combine with one or more additional noxious hits to trigger apoptosis. In vitro work has supported this concept by showing that additional hits can enhance macrophage apoptosis during low levels of ER stress.
One category of atherosclerosis-relevant “second hits” that trigger apoptosis in macrophages undergoing low-level ER stress is engagement of pattern recognition receptors (PRRs). PRRs are cell-surface receptors that bind pathogens, foreign antigens, endogenous proteins, and modified lipids through their ability to recognize a variety of structural or molecular motifs called pathogen-associated molecular patterns (PAMPs). Examples of PRRs include scavenger receptors and toll-like receptors (TLRs). When cells, particularly macrophages, bind PAMPs, inflammation, and other processes are triggered as part of an innate immunity host defense mechanism.
Many endogenous PRR ligands have been found to accumulate in atherosclerotic plaques and have the potential to contribute to macrophage apoptosis by acting as second hits during ER stress. Examples of ligands that trigger macrophage apoptosis during ER stress include lesion-modified forms of LDL, advanced glycation end-products, β-amyloid, and oxidized phosphatidylcholine, which engage scavenger receptors such as SRA and CD36 (34, 39) (T. Seimon and I. Tabas, unpublished data) (Fig. 2). Mechanistic studies have shown that scavenger receptors and TLRs provide combinatorial proapoptotic signals that are necessary to trigger apoptosis in ER-stressed macrophages. Examples include the combination of SRA and TLR4 activation by SRA ligands (39) and the combination of CD36 and TLR2 activation by oxidized phospholipids (T. Seimon and I. Tabas, unpublished data). In the case of the SRA-TLR4 pathway, apoptosis is dependent on SRA-mediated suppression of the macrophage survival protein interferon-β and on TLR4-mediated activation of the proapoptotic signal transducer STAT1 (21, 39).
Two in vivo causation experiments have provided support for this model. The key role of macrophage STAT1, which is activated in advanced human coronary atheromata, was recently demonstrated to have a causal role in advanced lesional macrophage apoptosis and plaque necrosis in Western diet-fed Ldlr−/− mice (21). More recently, we found that the combined deficiency of CD36 and SRA protected Apoe−/− mice from advanced lesional macrophage apoptosis and plaque necrosis (J. Tobin-Manning, K. Moore, T. Seimon, and I. Tabas, manuscript under revision). These data suggest that SRA and/or CD36 contribute to macrophage apoptosis and plaque necrosis in advanced lesions. Studies are currently underway to determine whether TLR2 or TLR4 can directly participate in macrophage apoptosis and necrotic core formation in vivo.
A possible teleology for induction of apoptosis in ER-stressed macrophages by PRR ligands
As mentioned above, ER stress is considered to be an adaptive pathway to protect macrophages from the increased burden of client protein overload. Thus, the ability to commit suicide during ER stress by simply engaging a subset of PRRs would seem counterproductive to the goal of keeping macrophages alive to fight infection. However, there are classes of infectious organisms that depend upon living macrophages to survive. Examples include viruses and intracellular bacteria, such as Mycobacterium tuberculosis and Brucella species (40, 41). Thus, the triggering of apoptosis could actually be part of the innate host defense system to prevent chronic infection by these organisms. In this regard, there is evidence that intracellular organisms activate the UPR as a mechanism to support synthesis of pathogen proteins (41–43), and these organisms also display PAMPs that can activate scavenger receptors and TLRs. Most interestingly, in vitro studies have shown that macrophage apoptosis is associated with control of infection by M. tuberculosis (40), and genetic studies in mice have shown an association between resistance to infection by M. tuberculosis and induction of macrophage apoptosis (44). Recent work from our lab has shown that TLR-dependent macrophage apoptosis induced by Mycobacterium is enhanced by ER stress (T. Seimon, I. Tabas, and C. Nathan, unpublished data). Although ER stress PRR-induced macrophage apoptosis may be a highly detrimental process in advanced atherosclerosis, a postreproductive disease with little or no evolutionary pressure, it may represent an evolutionarily conserved process that is important for host defense.
CONCLUSIONS AND FUTURE DIRECTIONS
Macrophage apoptosis in atherosclerotic lesions likely occurs through a combination of cellular stresses and events that differ depending on the stage of lesion progression. Apoptosis may be induced through a variety of ER stressors working alone or in combination with PRR ligands, or by the accumulation of additional cellular stresses such as oxidized lipids and death receptor ligands (Fig. 2). The consequence of macrophage apoptosis, whether beneficial by suppressing cellularity in early lesions, or detrimental by contributing to necrotic core formation in advanced lesion, is likely dependent on the efficiency of lesional phagocytes to clear these dead cells. An important goal therefore is to determine the cause of defective phagocytosis in advanced lesions. Understanding the switch between the beneficial versus detrimental consequence of macrophage apoptosis, and whether the switch is driven by a defect in efferocytosis, remain crucial areas of future research into the cause of acute atherothrombotic vascular disease.
Acknowledgments
The authors wish to acknowledge the outstanding researchers involved in contributing to the efferocytosis and macrophage apoptosis studies cited herein, particularly Drs. Dongying Cui, Bo Feng, Wahseng Lim, Yankun Li, Carl Nathan, Dorien Schrijvers, and Ed Thorp.
This work was supported by the American Heart Association SDG (0735594T) to T.S.; National Institute of Health grants HL087123, HL075662, HL054591, and US Army Medical Research and Materiel Command grant W81XWH-06-1-0212 to I.T.
Published, JLR Papers in Press, October 25, 2008.
References
- 1.Mantovani A., P. Allavena, A. Sica, and F. Balkwill. 2008. Cancer-related inflammation. Nature. 454 436–444. [DOI] [PubMed] [Google Scholar]
- 2.Zhang K., and R. J. Kaufman. 2008. From endoplasmic-reticulum stress to the inflammatory response. Nature. 454 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabas I., K. J. Williams, and J. Boren. 2007. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 116 1832–1844. [DOI] [PubMed] [Google Scholar]
- 4.Yan Z-Q., and G. K. Hansson. 2008. Innate immunity, macrophage activation, and atherosclersis. Immunol. Rev. 219 187–203. [DOI] [PubMed] [Google Scholar]
- 5.Libby P., and S. K. Clinton. 1993. The role of macrophages in atherogenesis. Curr. Opin. Lipidol. 4 355–363. [Google Scholar]
- 6.Schrijvers D. M., G. R. De Meyer, A. G. Herman, and W. Martinet. 2007. Phagocytosis in atherosclerosis: molecular mechanisms and implications for plaque progression and stability. Cardiovasc. Res. 73 470–480. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I. 2005. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25 2255–2264. [DOI] [PubMed] [Google Scholar]
- 8.Naghavi M., P. Libby, E. Falk, S. W. Casscells, S. Litovsky, J. Rumberger, J. J. Badimon, C. Stefanadis, P. Moreno, G. Pasterkamp, et al. 2003. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation. 108 1664–1672. [DOI] [PubMed] [Google Scholar]
- 9.Kockx M. M., G. R. De Meyer, J. Muhring, W. Jacob, H. Bult, and A. G. Herman. 1998. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 97 2307–2315. [DOI] [PubMed] [Google Scholar]
- 10.Kockx M. M., and A. G. Herman. 2000. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc. Res. 45 736–746. [DOI] [PubMed] [Google Scholar]
- 11.van Vlijmen B. J., G. Gerritsen, A. L. Franken, L. S. Boesten, M. M. Kockx, M. J. Gijbels, M. P. Vierboom, M. Van Eck, W. B. van De, T. J. van Berkel, et al. 2001. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ. Res. 88 780–786. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., D. P. Thewke, Y. R. Su, M. F. Linton, S. Fazio, and M. S. Sinensky. 2005. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 25 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arai S., J. M. Shelton, M. Chen, M. N. Bradley, A. Castrillo, A. L. Bookout, P. A. Mak, P. A. Edwards, D. J. Mangelsdorf, P. Tontonoz, et al. 2005. A role for the apoptosis inhibitory factor AIM/Spα/Api6 in atherosclerosis development. Cell Metab. 1 201–213. [DOI] [PubMed] [Google Scholar]
- 14.Ball R. Y., E. C. Stowers, J. H. Burton, N. R. Cary, J. N. Skepper, and M. J. Mitchinson. 1995. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 114 45–54. [DOI] [PubMed] [Google Scholar]
- 15.Ohayon J., G. Finet, A. M. Gharib, D. A. Herzka, P. Tracqui, J. Heroux, G. Rioufol, M. S. Kotys, A. Elagha, and R. I. Pettigrew. 2008. Necrotic core thickness and positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am. J. Physiol. Heart Circ. Physiol. 295 H717–H727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng B., P. M. Yao, Y. Li, C. M. Devlin, D. Zhang, H. P. Harding, M. Sweeney, J. X. Rong, G. Kuriakose, E. A. Fisher, et al. 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5 781–792. [DOI] [PubMed] [Google Scholar]
- 17.Gargalovic P. S., N. M. Gharavi, M. J. Clark, J. Pagnon, W. P. Yang, A. He, A. Truong, T. Baruch-Oren, J. A. Berliner, T. G. Kirchgessner, et al. 2006. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26 2490–2496. [DOI] [PubMed] [Google Scholar]
- 18.Myoishi M., H. Hao, T. Minamino, K. Watanabe, K. Nishihira, K. Hatakeyama, Y. Asada, K. Okada, H. Ishibashi-Ueda, G. Gabbiani, et al. 2007. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 116 1226–1233. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J., S. Lhotak, B. A. Hilditch, and R. C. Austin. 2005. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 111 1814–1821. [DOI] [PubMed] [Google Scholar]
- 20.Feng B., D. Zhang, G. Kuriakose, C. M. Devlin, M. Kockx, and I. Tabas. 2003. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc. Natl. Acad. Sci. USA. 100 10423–10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim W. S., J. M. Timmins, T. A. Seimon, A. Sadler, F. D. Kolodgie, R. Virmani, and I. Tabas. 2008. STAT1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 117 940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorp E., G. Kuriakose, Y. M. Shah, F. J. Gonzalez, and I. Tabas. 2007. Pioglitazone increases macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions of nondiabetic low-density lipoprotein receptor-null mice. Circulation. 116 2182–2190. [DOI] [PubMed] [Google Scholar]
- 23.Han S., C. P. Liang, T. DeVries-Seimon, M. Ranalletta, C. L. Welch, K. Collins-Fletcher, D. Accili, I. Tabas, and A. R. Tall. 2006. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 3 257–266. [DOI] [PubMed] [Google Scholar]
- 24.deCathelineau A. M., and P. M. Henson. 2003. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 38 105–117. [DOI] [PubMed] [Google Scholar]
- 25.Vandivier R. W., P. M. Henson, and I. S. Douglas. 2006. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 129 1673–1682. [DOI] [PubMed] [Google Scholar]
- 26.Miller Y. I., S. Viriyakosol, C. J. Binder, J. R. Feramisco, T. N. Kirkland, and J. L. Witztum. 2003. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 278 1561–1568. [DOI] [PubMed] [Google Scholar]
- 27.Ait-Oufella H., V. Pouresmail, T. Simon, O. Blanc-Brude, K. Kinugawa, R. Merval, G. Offenstadt, G. Leseche, P. L. Cohen, A. Tedgui, et al. 2008. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28 1429–1431. [DOI] [PubMed] [Google Scholar]
- 28.Tabas, I. Apoptosis and efferocytosis in mouse models of atherosclerosis. Curr. Drug Targets. Epub ahead of print. December 8, 2007. [DOI] [PubMed]
- 29.Thorp E., D. Cui, D. M. Schrijvers, G. Kuriakose, and I. Tabas. 2008. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe−/− mice. Arterioscler. Thromb. Vasc. Biol. 28 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickhout J. G., G. S. Hossain, L. M. Pozza, J. Zhou, S. Lhotak, and R. C. Austin. 2005. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 25 2623–2629. [DOI] [PubMed] [Google Scholar]
- 31.Canault M., F. Peiretti, F. Kopp, B. Bonardo, M. F. Bonzi, J. C. Coudeyre, M. C. Alessi, I. Juhan-Vague, and G. Nalbone. 2006. The TNF alpha converting enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: Possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis. 187 82–91. [DOI] [PubMed] [Google Scholar]
- 32.Zadelaar A. S., J. H. Thnsen, M. Boesten, R. C. Hoeben, M. M. Kockx, M. A. Versnel, T. J. C. van Berkel, L. M. Havekes, L. Biessen, and B. J. M. van Vlijmen. 2005. Increased vulnerability of pre-existing atherosclerosis in ApoE-deficient mice following adenovirus-mediated Fas ligand gene transfer. Atherosclerosis. 183 244–250. [DOI] [PubMed] [Google Scholar]
- 33.Rutkowski D., S. M. Arnold, C. M. Miller, J. Wu, J. Li, K. M. Gussinson, K. Mori, A. A. S. Akha, D. Raden, and R. J. Kaufman. 2006. Adaptation to ER stress is mediated by differential stabilities of prosurvival and proapoptotic mRNAs and proteins. PLoS Biol. 4 2024–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeVries-Seimon T., Y. Li, P. M. Yao, E. Stone, Y. Wang, R. J. Davis, R. Flavell, and I. Tabas. 2005. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 171 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werstuck G. H., M. I. Khan, G. Femia, A. J. Kim, V. Tedesco, B. Trigatti, and Y. Shi. 2006. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 55 93–101. [PubMed] [Google Scholar]
- 36.Borradaile N. M., X. Han, J. D. Harp, S. E. Gale, D. S. Ory, and J. E. Schaffer. 2006. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 47 2726–2737. [DOI] [PubMed] [Google Scholar]
- 37.Thuerauf D. J., M. Marcinko, N. Gude, M. Rubio, M. A. Sussman, and C. C. Glembotski. 2006. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ. Res. 99 275–282. [DOI] [PubMed] [Google Scholar]
- 38.Werstuck G. H., S. R. Lentz, S. Dayal, G. S. Hossain, S. K. Sood, Y. Y. Shi, J. Zhou, N. Maeda, S. Krisans, M. R. Malinow, et al. 2001. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Invest. 107 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seimon T. A., A. Obstfeld, K. J. Moore, D. T. Golenbock, and I. Tabas. 2006. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc. Natl. Acad. Sci. USA. 103 19794–19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kornfeld H., G. Mancino, and V. Colizzi. 1999. The role of macrophage cell death in tuberculosis. Cell Death Differ. 6 71–78. [DOI] [PubMed] [Google Scholar]
- 41.Roy C. R., S. P. Salcedo, and J. P. Gorvel. 2006. Pathogen-endoplasmic-reticulum interactions: in through the out door. Nat. Rev. Immunol. 6 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He B. 2006. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 13 393–403. [DOI] [PubMed] [Google Scholar]
- 43.Lee S-Y., M-S. Lee, R. Cherla, and V. Tesh. 2008. Siga toxin1 induces apoptosis through the endoplasmic reticulum stress response in human monocyte cells. Cell. Microbiol. 10 770–780. [DOI] [PubMed] [Google Scholar]
- 44.Pan H., B. S. Yan, M. Rojas, Y. V. Shebzukhov, H. Zhou, L. Kobzik, D. E. Higgins, M. J. Daly, B. R. Bloom, and I. Kramnik. 2005. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 434 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]