Abstract
Atherosclerosis can be considered as both a chronic inflammatory disease and a lipid metabolism disorder. Innate immunity pathways have long been suspected to contribute to the initiation and progression of atherosclerosis. This suggests that crosstalk between lipid metabolism and innate immunity pathways plays an important role for the development and/or the prevention of atherosclerosis. However, it is not fully defined how innate immunity affects lipid metabolism. Macrophages play a central role in atherogenesis through the accumulation of cholesterol and the production of inflammatory mediators and cytokines. Liver X receptors (LXRs) exert an important atheroprotective effect in the macrophage. In addition to regulating cholesterol metabolism, LXRs are also negative regulators of macrophage inflammatory gene responses. In this review, we will discuss the roles of LXRs in the macrophage as key factors that link innate immunity and lipid metabolism.
Keywords: liver X receptors, lipopolysaccharide, low density lipoprotein
Cardiovascular disease, including atherosclerosis, is the leading cause of morbidity and mortality in western societies. Atherosclerosis is a chronic inflammatory disease and a disorder of lipid metabolism. Although many epidemiological studies have shown that high concentrations of LDL cholesterol are a major risk factor for atherosclerosis, innate immunity pathways have long been suspected to contribute to the initiation and progression of atherosclerosis. Atherosclerotic lesion progression has been shown to depend on chronic inflammation in the artery wall (1). After induction of hyperlipidemia, a rapid influx of monocytes into the arterial intima occurs; if persistent, this influx generates the chronic inflammation characteristic of the atherosclerotic plaque (2). Many pro-inflammatory genes activated by pathogen engagement of innate immunity signaling pathways are also induced in macrophages present in atherosclerotic lesions.
Macrophages play a central role in the atherogenic process as modulators of both lipid metabolism and immune responses (1, 3). The accumulation of cholesterol-loaded macrophages in the arterial wall is the hallmark of the early atherosclerotic lesion (4, 5). In response to lipid loading, macrophages activate a compensatory pathway for cholesterol efflux mediated by the ATP binding cassette transporters A1 and G1 (ABCA1 and ABCG1) (6, 7). In the face of systemic hypercholesterolemia, however, this homeostatic mechanism is overwhelmed, leading to the development of foam cells and the fatty streak lesion. In fact, combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice (8). Cholesterol loading of macrophages stimulates the production of inflammatory mediators, such as cytokines and reactive oxygen species that recruit other cell types and contribute to the development of a complex lesion (9). Thus, processes that interfere with the intracellular cholesterol balance would be expected to exacerbate lesion formation.
Liver X receptors (LXRs) are ligand-activated transcription factors that control cellular cholesterol and fatty acid homeostasis and have been established to exert atheroprotective effects in mouse models. In fact, the pathophysiologic significance of the LXRs is illustrated by the observations that synthetic LXR ligands reduce atherosclerosis in animal models, whereas loss of macrophage LXRs expression dramatically accelerates the disease (10, 11). Furthermore, overexpression of LXRα in macrophages has significant antiatherogenic properties (12). However, it remains unclear how these functions of LXRs are working under pathological conditions. In this review, we will provide a brief overview of the role of LXRs in the control of cholesterol metabolism during inflammation.
THE ROLE OF LXRs IN MACROPHAGE
Genomic and cDNA sequencing efforts have defined at least 48 nuclear receptors that are encoded by the human and mouse genomes (13). Several nuclear receptors have been identified to be expressed in macrophages, including receptors for steroid hormones, such as estrogen and glucocorticoid receptors, receptors for diverse products of lipid metabolism, such as peroxisome proliferator-activated receptors and LXRs (LXRα and LXRβ), and orphan receptors, such as Nurr77, Nurr1, and NOR-1. In the macrophage, several of these nuclear receptors have been demonstrated to inhibit inflammatory responses that are under the control of signal-dependent transcription factors, such as activator protein-1 and nuclear factor κB (NF-κB) (14–16).
LXRs are transcriptional regulators of cholesterol absorption, transport, and elimination (17, 18). In macrophages, LXR signaling is critical for initiating the homeostatic response to cellular lipid loading (Fig. 1). Macrophage uptake of modified lipoproteins, such as oxidized LDL, leads to increased cellular concentration of oxysterols, the physiologic ligands for LXRs (19, 20). Activation of LXRs induces the expression of genes involved in cellular cholesterol trafficking, including Niemann Pick type C 1 and 2 proteins (21), and efflux, including ABCA1, ABCG1, and apolipoprotein E (17, 18). The end result of this transcriptional cascade is the transfer of excess cholesterol to extracellular acceptors, such as apolipoprotein AI and HDL. Recent studies have also revealed that the activation of LXRs by synthetic ligands in macrophages inhibits lipopolysaccharide (LPS)- or cytokine-induced expression of inflammatory genes, such as inducible nitric oxide synthase (iNOS), interleukin-1β, and monocyte chemoattractant protein-1, by interfering with NF-κB signaling (Fig. 1) (22). This LXR transrepression of inflammatory genes uses a small ubiquitin related modifier-dependent and nuclear receptor corepressor-dependent pathway (23). In response to ligand, a fraction of cellular LXR is SUMOylated and becomes associated with nuclear receptor corepressor complexes bound to promoters of inflammatory genes, such as iNOS. This interaction prevents LPS-dependent removal of these complexes as a prerequisite to transcriptional activation. As a consequence, these genes remain in a repressed state. On the other hand, LXRs have also been shown to positively regulate the expression of anti-inflammatory molecules in macrophages, such as arginase II. Arginase II catalyzes the convertion of l-arginine to l-ornithine (24). Since both arginase II and iNOS use arginine as a common substrate, induction of arginase II expression has the potential to exert anti-inflammatory effects by the inhibition of nitric oxide production. Therefore, LXR activation contributes to immunomodulatory effects by both negative and positive regulation of inflammation-related genes.
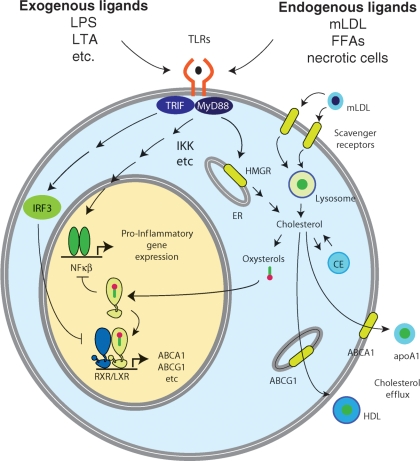
Fig. 1.
Intersections of TLR and LXR signaling pathways in the macrophage. Upon activation by exogenous or endogenous ligands, TLRs regulate gene expression through MyD88- and TRIF-dependent signal transduction pathways that control the activities of NF-κB and IRF transcription factors. These factors induce the expression of inflammatory response genes, including genes that contribute to modifications of LDL (mLDL) that enhance recognition and uptake by scavenger receptors. TLR signaling also increases cholesterol biosynthesis and promotes cholesterol accumulation. Elevated cholesterol levels give rise to elevated oxysterols, which are activating ligands of LXRs. LXRs inhibit TLR signaling at the promoter level and induce genes that promote cholesterol efflux, including ABCA1 and ABCG1. LXR activation of these genes is inhibited by IRF3, which is activated by TLRs that couple to the TRIF signaling adaptor.
INNATE IMMUNITY AND ATHEROSCLEROSIS
The search for pro-inflammatory signaling cascades activated by endogenous lipoproteins has recently focused on the Toll-like receptors (TLRs), a group of pattern recognition receptors that activate host defenses in response to microbial-derived ligands (25). These receptors are exemplified by TLR4, which is activated by Gram-negative bacterial LPS (26), and TLR2, which is activated by components of Gram-positive bacteria, such as Pam3CSK4 and lipoteichoic acid (LTA) (27). Recent studies suggest that in addition to microbially derived ligands, TLRs can also be activated by endogenously derived ligands that may be generated by pathogenic processes and include components of necrotic cells and modified lipoproteins. After binding ligands, TLRs use a downstream cascade of signaling molecules, including adaptor proteins, such as myeloid differentiation factor 88 (MyD88), and Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF), that ultimately regulate the activities of signal-dependent transcription factors, such as NF-κB and interferon regulatory factor 3 (IRF3). These in turn induce transcription of genes that encode cytokines, chemokines, and other effectors of the innate immune response (Fig. 1). Evidence that TLRs may contribute to atherosclerosis includes the demonstration that gene deletion of TLR2, TLR4, or MyD88 results in reduction in atherosclerosis in mouse models (28, 29). Intriguingly, the expression of TLR1, TLR2, and TLR4 is markedly enhanced in human atherosclerotic plaques (30). In accordance with these reports, several studies have suggested that some bacterial or viral pathogens, such as Chlamydia pneumoniae and herpesvirus, accelerate the development of atherosclerosis in mice (31, 32). In addition, peripheral administration of a ligand for TLR2 accelerated the development of atherosclerosis in mice (29). These findings link the development of atherosclerosis to a pro-inflammatory signaling cascade that is also engaged by microbial pathogens. In fact, the activation of macrophages by LPS causes an increase in intracellular concentrations of free and esterified cholesterol (33).
MACROPHAGE LIPID METABOLISM DURING INFLAMMATION
The acute-phase response at the whole-body level is characterized by increased plasma lipoprotein cholesterol levels and plasma LPS binding protein (LBP) levels. LBP is an acute-phase protein responsible for the binding and transport of LPS in circulation. Delivery of LPS by LBP to macrophage receptors initiates signal transduction pathways that lead to the increased release of pro-inflammatory cytokines (34). On the other hand, the delivery of LPS to HDL by LBP results in the attenuation of the immune response to infection (35). HDL-bound LPS is redistributed to LDL and VLDL (36) and transported to liver. Upon uptake by the liver, LPS is dephosphorylated/degraded and passed into the bile. Therefore, the uptake of LPS by HDL appears to serve as a first line of defense against the sustained activation of cellular immunity by LPS in the host. LPS stimulates hepatic lipid synthesis and increases hepatic HMG-CoA reductase activity in mice (37). This increase of hepatic cholesterol production results in an increase in LDL cholesterol (38). Taken together, it is possible that the increase in HMG-CoA reductase provides cholesterol, which allows for the production of LDL and VLDL and elevations in plasma lipid levels. These studies suggest that increases in plasma lipid and lipoprotein levels may be beneficial during infection and inflammation.
The induction of cholesterol production by LPS occurs not only in the liver, but also in the macrophage (Fig. 1), although the responsible mechanisms are not fully established. Posokhova et al. (39) reported that the intraperitoneal injection of LPS in mice led to a dramatic increase of radiolabeled oleate incorporation into cholesteryl esters and triglycerides and radiolabeled acetate incorporation into cholesterol and fatty acids in peritoneal macrophages. In accordance with their findings, the LIPID MAPS Consortium revealed that the activation of TLR4 induced HMG-CoA reductase mRNA in bone-marrow-derived macrophage as well as several intermediates in the cholesterol biosynthetic pathway (These data can be found with LIPID MAPS Consortium online at http://www.lipidmaps.org/).
Activation of macrophages by LPS also potentiates the formation of modified forms of LDL cholesterol, such as oxidized LDL cholesterol, facilitating uptake via scavenger receptors that include scavenger receptor A (SR-A), CD36, and scavenger receptor expressed by endothelial cells I (SREC-I). LPS robustly increases the mRNA expression of both SR-A and SREC-I in macrophages, but not CD36 (40). In accordance with this, the uptake of oxidized LDL induced by LPS was dramatically reduced in SR-A/SREC-I double knockout macrophages (40). Within cells, oxidized LDL-derived cholesteryl esters are hydrolyzed in lysosomes. Free cholesterol has a number of potential metabolic fates, but excess cholesterol is transported to the endoplasmic reticulum, reesterified by acyl-CoA:cholesterol acyltransferase 1, and stored as lipid droplets (41). The macrophage also has mechanisms for disposing of excess cholesterol via membrane transporters such as ABCA1 and ABCG1 (Fig. 1). ABCA1 promotes net cholesterol efflux to lipid-poor apolipoprotein AI, while ABCG1 facilitates net cholesterol efflux to HDL particles (42, 43). TLR signaling has been shown to both positively and negatively regulate ABCA1 expression (44–46). Negative regulation of ABCA1 by LPS has been proposed to result from TRIF/IRF3-depenent inhibition of the transcriptional activity of LXR (46), suggesting a mechanism by which TLR signaling promotes cholesterol accumulation in macrophages (Fig. 1).
HOW ARE LXRs ACTIVATED DURING INFLAMMATION?
Pharmacologic activation of LXRs in macrophages can reduce atherosclerosis not only by the regulation of lipid metabolism, but also by acting to limit the production of inflammatory mediators. However, it remains unclear how these functions of LXRs are working under pathological condition. In other words, what are the physiological ligands of LXRs, and how are the ligands generated during inflammation? The development of macrophage “foam cells” that contain massive amounts of cholesteryl esters is a hallmark of both early and late atherosclerotic lesions. Cholesterol accumulation in these cells is thought to be mediated by uptake of modified forms of LDL and cholesterol biosynthesis. In macrophages, endogenous oxysterols are thought to be produced in proportion to intracellular cholesterol levels. Also oxysterols are formed during oxidation of LDL (47), and LPS induced the expression of oxysterol biosynthesis-related genes, such as cholesterol 25 hydroxylase, that converts cholesterol to 25-hydroxy cholesterol (14). In fact, 25-hydroxycholesterol and 27-hydroxycholesterol reduced the DNA binding of activator protein-1 induced by LPS (48). Moreover, 22(R)-hydroxycholesterol, 24(S), 25-epoxycholesterol, and 24-hydroxycholesterol repressed iNOS activation induced by LPS (23). Therefore, candidate LXRs ligands may be generated by inflammation and may activate the LXRs, providing an additional explanation for why deletion of the LXR genes from macrophages results in increased development of atherosclerosis. Although synthetic LXR agonists exert anti-atherogenic effects, they also significantly raise circulating VLDL levels secondary to their effect on triglyceride biosynthesis in the liver (17). Clinical application of LXR agonists for prevention of cardiovascular disease will require the development of selective LXR modulators that retain the ability to promote cholesterol efflux and inhibit inflammation, but do not raise circulating triglyceride levels.
CONCLUSIONS
Atherosclerosis can be considered to be a form of chronic inflammation resulting from interactions between modified lipoproteins, monocyte-derived macrophages, T-cells, and the normal cellular elements of the arterial wall. The induction of cholesterol accumulation in macrophage by LPS has been thought to be one of the “undesired” functions of inflammation. Numerous studies in the last few years reveal that oxysterol sensors, LXRs, are one of the key regulators of macrophage biology, including the promotion of reverse cholesterol transport and the limitation of inflammation. The antagonistic functions of TLRs and LXRs with respect to macrophage cholesterol homeostasis suggest that the activation of LXRs by undesired oxysterols may be thought to be a compensatory mechanism against the excess cholesterol accumulation and the excess toxic inflammatory response. Further studies are needed to elucidate the biological roles of LXRs during inflammation and the practical possibilities of targeting LXRs for therapeutic intervention.
Abbreviations
ABCA1, ATP binding cassette transporter A1
ABCG1, ATP binding cassette transporter G1
iNOS, inducible nitric oxide synthase
IRF3, interferon regulatory factor 3
LBP, LPS binding protein
LPS, lipopolysaccharide
LXR, liver X receptor
MyD88, myeloid differentiation factor 88
NF-κB, nuclear factor κB
TLR, Toll-like receptor
TRIF, Toll/IL-1 receptor domain-containing adaptor inducing IFN-β
SR-A, scavenger receptor A
SREC-I, scavenger receptor expressed by endothelial cells I
This work was supported by the LIPID MAPS Large Scale Collaborative Grant number GM069338 from the National Institutes of Health (C.K.G.) and an American Heart Association Fellowship (N.S.).
Published, JLR Papers in Press, November 5, 2008.
References
- 1.Lusis A. J. 2000. Atherosclerosis. Nature. 407 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. 1999. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340 115–126. [DOI] [PubMed] [Google Scholar]
- 3.Glass C. K., and J. L. Witztum. 2001. Atherosclerosis. the road ahead. Cell. 104 503–516. [DOI] [PubMed] [Google Scholar]
- 4.Brown M. S., and J. L. Goldstein. 1983. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu. Rev. Biochem. 52 223–261. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. 2002. Inflammation in atherosclerosis. Nature. 420 868–874. [DOI] [PubMed] [Google Scholar]
- 6.Oram J. F. 2002. ATP-binding cassette transporter A1 and cholesterol trafficking. Curr. Opin. Lipidol. 13 373–381. [DOI] [PubMed] [Google Scholar]
- 7.Wang X., H. L. Collins, M. Ranalletta, I. V. Fuki, J. T. Billheimer, G. H. Rothblat, A. R. Tall, and D. J. Rader. 2007. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 117 2216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yvan-Charvet L., M. Ranalletta, N. Wang, S. Han, N. Terasaka, R. Li, C. Welch, and A. R. Tall. 2007. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 117 3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson G. K., P. Libby, U. Schonbeck, and Z. Q. Yan. 2002. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 91 281–291. [DOI] [PubMed] [Google Scholar]
- 10.Joseph S. B., E. McKilligin, L. Pei, M. A. Watson, A. R. Collins, B. A. Laffitte, M. Chen, G. Noh, J. Goodman, G. N. Hagger, et al. 2002. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA. 99 7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tangirala R. K., E. D. Bischoff, S. B. Joseph, B. L. Wagner, R. Walczak, B. A. Laffitte, C. L. Daige, D. Thomas, R. A. Heyman, D. J. Mangelsdorf, et al. 2002. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. USA. 99 11896–11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teupser D., D. Kretzschmar, C. Tennert, R. Burkhardt, W. Wilfert, D. Fengler, R. Naumann, A. E. Sippel, and J. Thiery. 2008. Effect of macrophage overexpression of murine liver X receptor-α (LXR-α) on atherosclerosis in LDL-receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 28 2009–2015. [DOI] [PubMed] [Google Scholar]
- 13.Duarte J., G. Perriere, V. Laudet, and M. Robinson-Rechavi. 2002. NUREBASE: database of nuclear hormone receptors. Nucleic Acids Res. 30 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa S., J. Lozach, C. Benner, G. Pascual, R. K. Tangirala, S. Westin, A. Hoffmann, S. Subramaniam, M. David, M. G. Rosenfeld, et al. 2005. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 122 707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass C. K., and S. Ogawa. 2006. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat. Rev. Immunol. 6 44–55. [DOI] [PubMed] [Google Scholar]
- 16.Bonta P. I., C. M. van Tiel, M. Vos, T. W. Pols, J. V. van Thienen, V. Ferreira, E. K. Arkenbout, J. Seppen, C. A. Spek, T. van der Poll, et al. 2006. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler. Thromb. Vasc. Biol. 26 2288–2294. [DOI] [PubMed] [Google Scholar]
- 17.Repa J. J., and D. J. Mangelsdorf. 2002. The liver X receptor gene team: potential new players in atherosclerosis. Nat. Med. 8 1243–1248. [DOI] [PubMed] [Google Scholar]
- 18.Tontonoz P., and D. J. Mangelsdorf. 2003. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 17 985–993. [DOI] [PubMed] [Google Scholar]
- 19.Janowski B. A., P. J. Willy, T. R. Devi, J. R. Falck, and D. J. Mangelsdorf. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature. 383 728–731. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann J. M., S. A. Kliewer, L. B. Moore, T. A. Smith-Oliver, B. B. Oliver, J. L. Su, S. S. Sundseth, D. A. Winegar, D. E. Blanchard, T. A. Spencer, et al. 1997. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272 3137–3140. [DOI] [PubMed] [Google Scholar]
- 21.Rigamonti E., L. Helin, S. Lestavel, A. L. Mutka, M. Lepore, C. Fontaine, M. A. Bouhlel, S. Bultel, J. C. Fruchart, E. Ikonen, et al. 2005. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ. Res. 97 682–689. [DOI] [PubMed] [Google Scholar]
- 22.Joseph S. B., A. Castrillo, B. A. Laffitte, D. J. Mangelsdorf, and P. Tontonoz. 2003. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9 213–219. [DOI] [PubMed] [Google Scholar]
- 23.Ghisletti S., W. Huang, S. Ogawa, G. Pascual, M. E. Lin, T. M. Willson, M. G. Rosenfeld, and C. K. Glass. 2007. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARγ. Mol. Cell. 25 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marathe C., M. N. Bradley, C. Hong, F. Lopez, C. M. Ruiz de Galarreta, P. Tontonoz, and A. Castrillo. 2006. The arginase II gene is an anti-inflammatory target of liver X receptor in macrophages. J. Biol. Chem. 281 32197–32206. [DOI] [PubMed] [Google Scholar]
- 25.Binder C. J., M. K. Chang, P. X. Shaw, Y. I. Miller, K. Hartvigsen, A. Dewan, and J. L. Witztum. 2002. Innate and acquired immunity in atherogenesis. Nat. Med. 8 1218–1226. [DOI] [PubMed] [Google Scholar]
- 26.Chow J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274 10689–10692. [DOI] [PubMed] [Google Scholar]
- 27.Yang R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 395 284–288. [DOI] [PubMed] [Google Scholar]
- 28.Bjorkbacka H., V. V. Kunjathoor, K. J. Moore, S. Koehn, C. M. Ordija, M. A. Lee, T. Means, K. Halmen, A. D. Luster, D. T. Golenbock, et al. 2004. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat. Med. 10 416–421. [DOI] [PubMed] [Google Scholar]
- 29.Mullick A. E., P. S. Tobias, and L. K. Curtiss. 2005. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 115 3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edfeldt K., J. Swedenborg, G. K. Hansson, and Z. Q. Yan. 2002. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 105 1158–1161. [PubMed] [Google Scholar]
- 31.Moazed T. C., L. A. Campbell, M. E. Rosenfeld, J. T. Grayston, and C. C. Kuo. 1999. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J. Infect. Dis. 180 238–241. [DOI] [PubMed] [Google Scholar]
- 32.Alber D. G., K. L. Powell, P. Vallance, D. A. Goodwin, and C. Grahame-Clarke. 2000. Herpesvirus infection accelerates atherosclerosis in the apolipoprotein E-deficient mouse. Circulation. 102 779–785. [DOI] [PubMed] [Google Scholar]
- 33.Funk J. L., K. R. Feingold, A. H. Moser, and C. Grunfeld. 1993. Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis. 98 67–82. [DOI] [PubMed] [Google Scholar]
- 34.Underhill D. M., and A. Ozinsky. 2002. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 14 103–110. [DOI] [PubMed] [Google Scholar]
- 35.Eggesbo J. B., T. Lyberg, T. Aspelin, I. Hjermann, and P. Kierulf. 1996. Different binding of 125I-LPS to plasma proteins from persons with high or low HDL. Scand. J. Clin. Lab. Invest. 56 533–543. [DOI] [PubMed] [Google Scholar]
- 36.Levels J. H., J. A. Marquart, P. R. Abraham, A. E. van den Ende, H. O. Molhuizen, S. J. van Deventer, and J. C. Meijers. 2005. Lipopolysaccharide is transferred from high-density to low-density lipoproteins by lipopolysaccharide-binding protein and phospholipid transfer protein. Infect. Immun. 73 2321–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Memon R. A., C. Grunfeld, A. H. Moser, and K. R. Feingold. 1993. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology. 132 2246–2253. [DOI] [PubMed] [Google Scholar]
- 38.Feingold K. R., I. Hardardottir, R. Memon, E. J. Krul, A. H. Moser, J. M. Taylor, and C. Grunfeld. 1993. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J. Lipid Res. 34 2147–2158. [PubMed] [Google Scholar]
- 39.Posokhova E. N., O. M. Khoshchenko, M. I. Chasovskikh, E. N. Pivovarova, and M. I. Dushkin. 2008. Lipid synthesis in macrophages during inflammation in vivo: effect of agonists of peroxisome proliferator activated receptors α and γ and of retinoid X receptors. Biochemistry. 73 296–304. [DOI] [PubMed] [Google Scholar]
- 40.Tamura Y., J. Osuga, H. Adachi, R. Tozawa, Y. Takanezawa, K. Ohashi, N. Yahagi, M. Sekiya, H. Okazaki, S. Tomita, et al. 2004. Scavenger receptor expressed by endothelial cells I (SREC-I) mediates the uptake of acetylated low density lipoproteins by macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 279 30938–30944. [DOI] [PubMed] [Google Scholar]
- 41.Buhman K. F., M. Accad, and R. V. Farese. 2000. Mammalian acyl-CoA:cholesterol acyltransferases. Biochim. Biophys. Acta. 1529 142–154. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy M. A., G. C. Barrera, K. Nakamura, A. Baldan, P. Tarr, M. C. Fishbein, J. Frank, O. L. Francone, and P. A. Edwards. 2005. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1 121–131. [DOI] [PubMed] [Google Scholar]
- 43.Wang N., D. Lan, W. Chen, F. Matsuura, and A. R. Tall. 2004. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA. 101 9774–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baranova I., T. Vishnyakova, A. Bocharov, Z. Chen, A. T. Remaley, J. Stonik, T. L. Eggerman, and A. P. Patterson. 2002. Lipopolysaccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells. Infect. Immun. 70 2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan R., X. Gan, J. G. Menke, S. D. Wright, and T. Q. Cai. 2002. Bacterial lipopolysaccharide induces expression of ABCA1 but not ABCG1 via an LXR-independent pathway. J. Lipid Res. 43 952–959. [PubMed] [Google Scholar]
- 46.Castrillo A., S. B. Joseph, S. A. Vaidya, M. Haberland, A. M. Fogelman, G. Cheng, and P. Tontonoz. 2003. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell. 12 805–816. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H. F., H. J. Basra, and U. P. Steinbrecher. 1990. Effects of oxidatively modified LDL on cholesterol esterification in cultured macrophages. J. Lipid Res. 31 1361–1369. [PubMed] [Google Scholar]
- 48.Ohlsson B. G., M. C. Englund, A. L. Karlsson, E. Knutsen, C. Erixon, H. Skribeck, Y. Liu, G. Bondjers, and O. Wiklund. 1996. Oxidized low density lipoprotein inhibits lipopolysaccharide-induced binding of nuclear factor-κB to DNA and the subsequent expression of tumor necrosis factor-α and interleukin-1β in macrophages. J. Clin. Invest. 98 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]