Abstract
Lipid phosphate phosphatases (LPPs) regulate cell signaling by modifying the concentrations of lipid phosphates versus their dephosphorylated products. The ecto-activity regulates the availability of extracellular lysophosphatidate (LPA) and sphingosine 1-phosphate (S1P) and thereby signaling by their respective receptors. LPP products (monoacylglycerol or sphingosine) are taken up by cells and rephosphorylated to produce LPA and S1P, respectively, which activate intracellular signaling cascades. The proposed integrin binding domain on the external surface of LPP3 modifies cell/cell interactions. Expression of LPPs on internal membranes controls signaling depending on the access of lipid phosphates to their active sites. Different LPPs perform distinct functions, probably based on integrin binding, their locations, and their abilities to metabolize different lipid phosphates in vivo.
Keywords: autotaxin, ceramide 1-phosphate, diacylglycerol, lysophosphatidate, phosphatidate, sphingosine 1-phosphate
Mammalian lipid phosphate phosphatases (LPPs) were formerly known as Mg2+-independent and N-ethylmaleimide-insensitive phosphatidate phosphatases (PAP2) (1–3). There are three LPPs (LPP1, LPP2, and LPP3 and a splice variant, LPP1a), which hydrolyze lipid phosphates, including phosphatidate (PA), lysophosphatidate (LPA), sphingosine 1-phosphate (S1P), ceramide 1-phosphate (C1P), and diacylglycerol pyrophosphate (3). They belong to a phosphatase/phosphotransferase family that includes S1P phosphatases, glucose 6-phosphatase, and the sphingomyelin synthases (1, 2). LPPs possess six transmembrane domains, three conserved active site domains, and a glycosylation site on an hydrophilic loop between the first and second active site domains (1, 2, 4).
The active sites are located on the outer surface of plasma membranes or the lumenal surface of internal membranes (1, 5). Mammalian LPPs form homo- and heterooligomers, which are catalytically active compared with the monomeric forms (6). These complexes could control their activities and subcellular distributions. However, work with Wunen, a Drosophila homolog of mammalian LPPs, showed that dimerization is not required for biological activity (7).
This review will first assess how mammalian LPPs control signaling by the extracellular lipid phosphates.
FORMATION AND SIGNIFICANCE OF EXTRACELLULAR LYSOPHOSPHATIDYLCHOLINE, LPA, AND S1P
LPA is present at up to 10 μM in the blood (8, 9). Extracellular LPA is implicated in cancer, and its concentration is high in ascites fluid and plasma of patients with ovarian tumors (10). LPA is also involved in wound repair and tissue development (1, 11) by promoting cell growth, proliferation, differentiation, motility, and survival (1, 10) through at least six G-protein-coupled receptors (12, 13). These activate the phosphatidylinositol 3-kinase and extracellular signal-regulated kinase (ERK) pathways and small G-proteins that affect cytoskeletal arrangements, and they decrease the abundance of the p53 tumor suppressor (14).
A major route for synthesizing extracellular LPA (Fig. 1A) is through the action of secreted autotaxin (ATX) on lysophosphatidylcholine (LPC), which is present in blood at up to 200 μM (8). ATX expression promotes tumor progression, metastasis, and angiogenesis, and it protects tumor cells from apoptosis (1). This occurs predominantly from the generation of LPA (15). Saturated LPC is produced mainly by lecithin:cholesterol acyltransferase in circulating high-density lipoproteins (1). However, a large proportion of circulating LPC is polyunsaturated, and this is partly derived from hepatocytes (16).
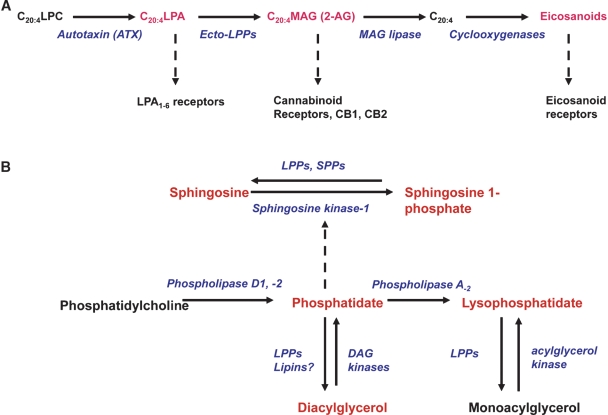
Fig. 1.
Metabolism of extracellular and intracellular bioactive lipids. A: LPC is metabolized by ATX to LPA, which is converted to MAG by the ecto-activities of the LPPs. Production of 2-arachidonoylglycerol (2-AG) could activate cannabinoid receptors (CB1 and CB2). Hydrolysis of 2-AG can produce arachidonate, which can be converted to eicosanoids. Lipids that stimulate receptors are shown in red and by dashed arrows. A similar pathway occurs with other molecular species of LPC, except that the MAG that is formed is not bioactive and will not produce eicosanoids. B: The possible formation of bioactive lipids (in red) following activation of PLD1 and PLD2. PA also activates sphingosine kinase-1 to produce S1P, as indicated by the dashed line. LPP1 may also decrease PLD activity (46).
A minor pathway for the production of saturated LPA is through secretory phospholipase A (PLA2), which hydrolyzes PA in microvesicles that are shed from cells during inflammation (17) and platelet aggregation (18).
S1P is a sphingolipid analog of LPA. S1P activates a family of five G-protein-coupled receptors, and it is important in regulating angiogenesis and immune responses (19). S1P is present in blood at 0.2–0.9 μM where it is bound to albumin and lipoproteins. S1P is released from platelets to facilitate tissue repair and angiogenesis (20). Red blood cells provide a major reservoir of S1P (19). Circulating S1P can be derived from the extracellular action of sphingosine kinase, and cells can secrete S1P as part of an autocrine/paracrine signaling loop (19).
DEPHOSPHORYLATION OF EXTRACELLULAR LIPID PHOSPHATES
Extracellular LPA and S1P are metabolized mainly by the ecto-activities of the LPPs (1, 2, 21). Increasing LPP1 expression increases the dephosphorylation of exogenous PA, LPA, and C1P (22). Ecto-LPP activities appeared to be involved in signaling, as LPP1 overexpression in fibroblasts attenuated LPA-induced activation of ERK, phospholipase D (PLD), Ca2+ transients, and cell division (22). Gonadotropin-releasing hormone increased ecto-LPP expression in ovarian cancer cells, and this explained its antiproliferative effects (23). LPP3 overexpression decreases growth, survival, and tumorigenesis of ovarian cancer cells, and it also decreases the ability of the parental cells to form colonies (24). The effects of LPP-3 on colony-forming activity were substantially reversed by an LPP-resistant LPA analog. In other studies, exogenous LPA increased ecto-LPP1 activity in platelets, and this decreased further LPA accumulation and LPA-induced shape changes and aggregation (25). Ecto-LPP activities also regulate extracellular LPA accumulation and proliferation of preadipocytes (26). This combined work establishes a role for the ecto-LPP activities in regulating cell signaling by extracellular lipid phosphates.
Significantly, LPP1 expression is decreased in a majority of ovarian cancers (27). Increased expression of LPP1 in ovarian cancer cell lines increased LPA hydrolysis, decreased cell proliferation and colony-forming activity, and increased apoptosis. It was concluded that the LPA-rich environment of ovarian cancer cells in vivo results from a combined increase in LPA production by ATX and decreased LPA removal by the LPPs (27).
A further dimension of the ecto-LPP activity is that dephosphorylated products readily enter cells (1, 2). The uptake of monoacylglycerol (MAG) following LPA dephosphorylation can increase intracellular LPA production by acylglycerol kinase (28). Intracellular LPA can then activate internal signaling cascades (Table 1).
TABLE 1.
LPPs modify cell signaling by dephosphorylating bioactive lipid phosphates and by producing DAG and ceramide
| Agonist and effect | References |
|---|---|
| PA | |
| Stimulates NADPH oxidase, H2O2 production, protein kinase C-ζ, phosphatidylinositol-4-phosphate kinase, and phospholipase C-γ | (1, 4, 19, 30) |
| Activates Ras-GTP, Raf, ERK, mTOR, and sphingosine kinase-1 | |
| Increases stress fiber formation | |
| Inhibits protein phosphatase-1 | |
| Relative LPA and PA in membranes control their curvature, vesicle budding, and transport | |
| DAG | |
| Stimulates classical and novel protein kinase Cs | (29) |
| Activation of RasGRP (guanine nucleotide releasing proteins) | |
| LPA | |
| Activates PPAR-γ and nuclear LPA1 receptors | (1, 4, 29, 43) |
| S1P | |
| Mobilizes intracellular Ca2+ | (1, 4, 19, 21, 31) |
| Increases ERK activity, cell division, and actin stress fiber formation | |
| Protects against apoptosis | |
| Increases COX-2 activity and eicosanoid synthesis | |
| C1P | |
| Involved in synaptic vesicle movement and neutrophil phagocytosis | (1, 4, 29, 31, 50) |
| Stimulates cell division and survival | |
| Activates cytosolic phospholipase A2, production of arachidonate, and prostaglandin E2 | |
| Blocks apoptosis by inhibiting acidic sphingomyelinase activity | |
| Ceramide | |
| Induces cell differentiation, apoptosis, or senescence in most cells | (1, 4, 29, 31, 50) |
| Increases survival of fibroblasts, some neurons, and other cells | |
| Inhibits PLD activity and vesicle movement | |
| Stimulates serine/threonine kinase, protein kinase C-ζ, and phosphoprotein phosphatase activities | |
Most MAGs are devoid of biological activity except for 2-AG, which is an activator of cannabinoid (CB1 and CB2) receptors (Fig. 1) (29). A significant proportion of the circulating LPC contains arachidonate (1, 16), and its metabolism by ATX should yield archidonoyl-LPA (Fig. 1A). Ecto-LPP activities could theoretically contribute to endocannabinoid production in addition to other pathways that have been described (29). Also, the 2-AG produced by the LPPs could then be metabolized to arachidonate, which regulates cell activation after the production of eicosanoids (Fig. 1A). Although the individual reactions shown in Fig. 1A can occur, there is no direct evidence at present to link the LPPs to cannabinoid signaling.
Ecto-LPP1 activity also promotes uptake of sphingosine derived from S1P by human lung endothelial cells (30). This increases intracellular S1P formation through sphingosine kinase-1 (30). Internal S1P is then able to activate internal signaling cascades (Table 1). The balance between the formation of ceramide versus S1P is a critical regulator of cell death versus survival (19, 31). Therefore, dephosphorylation of internal S1P by an LPP or SPP could help to determine the fate of the cell.
Work with FTY720 also demonstrates a role for the LPPs. FTY720 is a sphingosine analog that is used as an immunomodulatory drug for treating multiple sclerosis. FTY720 is converted to FTY720-P by sphingosine kinase-2 (32). Lysates from cells that overexpressed LPP1, LPP2, and LPP3 showed that only LPP3 dephosphorylated FTY720-P, and between the SPPs, only SPP1 showed activity. In intact cells, LPP3 acted as an ectophosphatase to control the equilibrium between FTY720 and FTY720-P that was observed in vivo (32). This result is surprising compared with the broad substrate preference of the LPPs for lipid phosphates (1, 2). In other studies, LPP1a had the highest activity and affinity for FTY720-P (33), suggesting that the first extracellular loop, which is different in LPP1a compared with LPP1, plays a role in substrate recognition.
LPP1 dephosphorylates exogenous C1P (22) to produce ceramides that can be converted to C1P once they enter the cell. This mechanism could explain how exogenous C1P increases intracellular C1P, thus activating cytosolic PLA2 and leading to arachidonate and prostaglandin E2 production (34).
Despite this weight of evidence for the importance of ecto-LPP activities, it has been questioned whether the LPPs regulate cell signaling based on conceptual problems. Namely, LPPs are not specific for their substrates, and their Km values are far beyond the Kd values for the LPA receptors (8). The fact that LPPs can dephosphorylate several lipid substrates when these are solubilized in vitro does not exclude them from having greater specificity in vivo. Even if LPPs were not specific in vivo, this would not preclude them from having a regulatory function. Ecto-LPP activities degrade LPA in proportion to its physiological concentration (22), which would be expected of an enzyme that modulates circulating LPA concentrations. Comparison of Km and Vmax values of LPPs with the Kd values estimated for the LPA receptors in vitro versus their kinetics in the outer surface of the plasma membrane in vivo is extremely difficult because the aggregation state of LPA will affect these parameters. For example, extracellular Ca2+ concentrations are ∼2 mM, and Ca2+ severely decreases LPA dephophorylation by LPP1 (22). This is probably because Ca2+ causes cross-bridging of LPA and its aggregation. Ca2+ is normally omitted from the assays of the Kd values of LPA receptors, but this does not reflect the situation in vivo.
Studies with transgenic mice that overexpress LPP1 unexpectedly demonstrated no significant differences in circulating LPA concentrations compared with control mice (9). However, this work did not evaluate LPA turnover, which could be a self-regulating process because both LPA and S1P are product inhibitors of ATX (35). This observation shows that the regulation of LPA and S1P metabolism can interact at the levels of both ATX and the LPPs.
Recent work determined the turnover of circulating LPA in vivo using LPP1 hypomorph mice (Ppap2atr/tr) that have depleted LPP1 expression in most tissues (36). LPA concentrations in the plasma were higher in Ppap2atr/tr mice compared with the controls. The half-life of LPA in the blood was ∼12 min in the Ppap2atr/tr mice compared with 3 min in controls. The results demonstrate the rapid turnover of circulating LPA and the physiological role of LPP1 in regulating this process.
Recent work also shows that exogenously added S1P is cleared from blood within 15–30 min, and this process depends on a cell-associated phosphatase activity (37).
NONCATALYTIC ACTIONS OF THE LPPS ON THE CELL SURFACE
Human LPP3 contains an exposed arginine-glycine-aspartate (RGD) cell adhesion sequence. hLPP3 expression increases cell/cell interactions through αvβ3 and anti-α5β1 integrins, but mutation to arginine-glycine-glutamate (RGE) did not produce this effect (38). Mouse and rat LPP3 contain RGE instead of RGD, but subsequent work showed that murine LPP3 also interacted with α5β1 and αvα3 integrins (39). Also, anti-LPP3 antibody blocked basic fibroblast growth factor (bFGF)- and vascular endothelial growth factor (VEGF)-induced capillary morphogenesis of endothelial cells. This suggests a role for LPP3 in controlling angiogenesis through integrin interactions (40) as well as integrin-independent mechanisms.
INTRACELLULAR FUNCTIONS OF THE LPPs
Lipid phosphates and their dephosphorylated products regulate cell signaling (Table 1); therefore, the LPPs could regulate this balance (1). For example, phospholipase D1 and D2 activities are increased by G-protein-coupled receptors and receptor tyrosine kinases. This produces PA, which the LPPs convert to diacylglycerol (DAG) (Fig. 1B). Increasing LPP activity decreases PA concentrations (1) and/or increases those of DAG (1, 2, 41). PA activates a variety of intracellular signaling targets (Table 1). It is proposed that LPP2, but not LPP3, could be functionally linked to phospholipase D1, which produces a PA-dependent recruitment of sphingosine kinase-1 to produce S1P in the perinuclear compartment (21) (Fig. 1B). S1P activates ERK, stimulates cell division, and protects against apoptosis. Although most actions of S1P are mediated through cell surface receptors, S1P can stimulate intracellular signaling cascades, including the mobilization of intracellular Ca2+ (19).
DAG produced from PA can theoretically activate classical and novel protein kinase Cs and RasGRP (Table 1). However, it was proposed that the fatty acid compositions of DAGs derived from PC do not activate the protein kinase Cs compared with polyunsaturated DAGs produced from phosphatidylinositol 4,5-bisphosphate (42).
PA can also be metabolized to LPA (Fig. 1B). Intracellular LPA can activate nuclear LPA1 receptors that regulate pro-inflammatory gene expression. Polyunsaturated LPA stimulates PPAR-γ receptors (Table 1) (43), although this latter conclusion is disputed (44). Additionally, LPA can be released by cells (4), and the level of LPP expression could regulate this process.
LPPs can also degrade C1P, which is involved in inflammation. C1P production activates PLA2 to release arachidonate (34). Consequently, there are several intracellular targets through which the LPPs could modify signal transduction.
Definitive evidence for intracellular actions of LPP on cell signaling came from work showing that LPPs control ERK activation by extracellular thrombin (4). This effect correlated with decreased intracellular PA, and it could not be explained by ecto-LPP activity. The effects of LPP2 and LPP3 on intracellular PA and S1P concentrations, respectively, control cell survival (45). Increased LPP1 activity also attenuates Ca2+-transients and interleukin-8 production downstream of LPA receptor activation (46). HEK 293 cells that overexpress LPP3 exhibited greater DAG formation following the stimulation of PLD (2). PLD2 and LPP3 are both present in caveolin-1-enriched microdomains (2). It was postulated that chronic increases in DAG concentrations following LPP1 overexpression decreased the expression of PKCs and thereby ERK activation. This could decrease cell division (45) and platelet-derived growth factor (PDGF)-induced cell migration (41).
The active sites of LPPs are outside the cell or on the lumenal surface of endoplasmic reticulum or Golgi membranes (1). The PLDs should produce PA on the cytosolic surface of membranes. Thus, there could be a barrier to PA hydrolysis unless the PA is efficiently transported to the active sites of the LPPs. Although the LPPs metabolize PA formed after PLD action (1), recent work showed that a major effect of LPP1 in decreasing PA accumulation is upstream of PLD activation (47). LPA-induced cell migration requires PLD2, and LPP1 inhibits this process by blocking PA formation (47). Also, LPP1 overexpression decreased the stimulation of migration by a nonhydrolysable LPA analog, showing that ecto-LPP1 action was not involved.
Increasing LPP2 activity in fibroblasts increases entry into S-phase of the cell cycle, whereas decreasing the activity has the opposite effect (48). LPP1 and LPP3 did not modify S-phase entry, demonstrating the specificity of the LPP2 effect. The premature entry into S-phase caused by increased LPP2 activity resulted in G2/M arrest after 15 to 35 passages. These cells eventually exited the cell cycle, and they exhibited a senescent phenotype (48). Some oncogenes induce premature senescence after initially stimulating cell proliferation, and this may prevent malignancy (48).
LESSONS FROM ANIMAL MODELS
Drosophila expresses two Wunen proteins, wun and wun2, that are homologous to LPP3 (2). Wun and wun2 have cell-specific and cell-autonomous actions. They act redundantly in germ cells as repellant factors that guide migrating germ cells in Drosophila embryos. Wunens are normally expressed in somatic tissues that germ cells avoid. However, in mutants where both genes are disrupted, germ cells scatter throughout the embryo and eventually die. Overexpression of wun or wun2 in somatic tissues that normally attract germ cells causes germ cell repulsion and death. The repulsive effect was suggested to result from the degradation of an unknown lipid factor that guides the germ cells and acts as a survival factor. It is significant that Drosophila does not appear to express receptors for lipid phosphates (2).
Work from mammalian models strongly implicates the LPPs as regulators of cell migration. Knockout of LPP3 is embryonically lethal (2). Embryos from LPP3 knockout mice failed to form a chorio-allantoic placenta and yolk sac vasculature. Some embryos showed shortening of the anterior-posterior axis similar to axin deficiency, a critical regulator of Wnt signaling. It was proposed that LPP3 functions as a Wnt signaling antagonist.
Mice that overexpress LPP1 have decreased birth weight, sparse curly hair, and defective spermatogenesis causing infertility (9). Fibroblasts from these mice showed decreased LPA-induced migration (41, 47) and increased DAG concentrations after stimulation with phorbol ester (9). There were no significant differences in ERK activation in response to stimulating the cells with LPA, S1P, EGF, or PDGF (9). However, in subsequent work, ERK activation in response to LPA, S1P, and PDGF was decreased (41). The combined results support the hypothesis that LPP1 regulates intracellular signaling. However, circulating LPA concentrations were not lower in the LPP1 overexpressing mice, and the proposed role of the ecto-LPP1 activity was not demonstrated (9). By contrast, studies described above with transgenic mice that have decreased LPP1 expression (Ppap2atr/tr) showed that LPP1 controls LPA removal from the blood, which increases circulating LPA levels (36). Otherwise, these mice display no obvious phenotype.
LPP2 knockout mice are fertile and viable with no obvious phenotype (49). This may appear to be incompatible with the proposal that LPP2 regulates cell cycle progression (48). LPP2 regulates the timing of S-phase entry, but it is not essential for cell cycle progression. Several genes that regulate progression into late G1 or entry into S-phase have been knocked out in mice without lethality or major phenotype, including genes that encode for CDK4, CDK6, CDK2, and cyclins D1, D2, D3, E1, or E2 (48). Likewise, deletion of LPP2 should not result in lethality or a major phenotype.
CONCLUSIONS
The LPPs participate in cell signaling by modifying the balance between the effects of lipid phosphates versus their dephosphorylated products. They act as ecto-enzymes in isolated cells. Evidence is now appearing that the LPPs degrade extracellular LPA and S1P in vivo, but more work is required using suitable animal models. This ecto-activity regulates the turnover and availability of circulating LPA and S1P, thus controlling the activation of families of G-protein-coupled receptors for these lipids. Second, the MAG and sphingosine that is produced is taken up by cells and rephosphorylated. The resulting intracellular LPA and S1P can then activate their own signaling cascades. In the case of LPP3, the presence of external integrin binding domains modifies cell/cell interactions.
LPPs are also expressed on internal membranes, and they potentially act on a wide variety of lipid phosphates to control signaling. The extent to which they can do this should be regulated by the access of the lipid phosphate to the active sites of the LPPs, which is thought to be on the lumenal surface of internal membranes. This access is likely to limit the use of various lipid substrates for the different LPPs and enforce greater substrate specificity for lipid phosphates than is seen in assays in vitro. Relatively little is known about the physiological substrate specificity for the different LPPs. Although there is possible redundancy in the actions of the LPPs, the different phenotypes of knockout and transgenic mice indicate that the different LPP perform distinct functions. This could depend on where the LPPs are expressed in different cells and which lipid phosphates they degrade. For LPP3, its role in cell adhesion could provide a further dimension to its action. Further work is needed to establish how the individual LPPs differentially control the metabolism of exogenous and endogenous lipid phosphates and thereby elicit their different functions in regulating cell signaling.
Abbreviations
2-AG, 2-arachidonoylglycerol
ATX, autotaxin
C1P, ceramide 1-phosphate
DAG, diacylglycerol
LPA, lysophosphatidate
LPC, lysophosphatidylcholine
LPP, lipid phosphate phosphatase
MAG, monoacylglycerol
PA, phosphatidate
PLA, phospholipase A
PLD, phospholipase D
S1P, sphingosine 1-phosphate
The work was supported by the Canadian Institutes of Health Research.
Published, JLR Papers in Press, December 9, 2008.
References
- 1.Brindley D. N. 2004. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J. Cell. Biochem. 92 900–912. [DOI] [PubMed] [Google Scholar]
- 2.Sigal Y. J., M. I. McDermott, and A. J. Morris. 2005. Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse functions. Biochem. J. 387 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brindley D. N., and D. W. Waggoner. 1998. Mammalian lipid phosphate phosphohydrolases. J. Biol. Chem. 273 24281–24284. [DOI] [PubMed] [Google Scholar]
- 4.Pyne S., K. C. Kong, and P. I. Darroch. 2004. Lysophosphatidic acid and sphingosine 1-phosphate biology: the role of lipid phosphate phosphatases. Semin. Cell Dev. Biol. 15 491–501. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q. X., C. S. Pilquil, J. Dewald, L. G. Berthiaume, and D. N. Brindley. 2000. Identification of structurally important domains of lipid phosphate phosphatase-1: implications for its sites of action. Biochem. J. 345 181–184. [PMC free article] [PubMed] [Google Scholar]
- 6.Long J. S., N. J. Pyne, and S. Pyne. 2008. Lipid phosphate phosphatases form homo- and hetero-oligomers: catalytic competency, subcellular distribution and function. Biochem. J. 411 371–377. [DOI] [PubMed] [Google Scholar]
- 7.Burnett C., P. Makridou, L. Hewlett, and K. Howard. 2004. Lipid phosphate phosphatases dimerise, but this interaction is not required for in vivo activity. BMC Biochem. 5 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moolenaar W. H., L. A. van Meeteren, and B. N. Giepmans. 2004. The ins and outs of lysophosphatidic acid signaling. Bioessays. 26 870–881. [DOI] [PubMed] [Google Scholar]
- 9.Yue J., K. Yokoyama, L. Balazs, D. L. Baker, D. Smalley, C. Pilquil, D. N. Brindley, and G. Tigyi. 2004. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell. Signal. 16 385–399. [DOI] [PubMed] [Google Scholar]
- 10.Mills G. B., and W. H. Moolenaar. 2003. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer. 3 582–591. [DOI] [PubMed] [Google Scholar]
- 11.Moolenaar W. H. 2002. Lysophospholipids in the limelight: autotaxin takes center stage. J. Cell Biol. 158 197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tigyi G., and A. L. Parrill. 2003. Molecular mechanisms of lysophosphatidic acid action. Prog. Lipid Res. 42 498–526. [DOI] [PubMed] [Google Scholar]
- 13.Rivera R., and J. Chun. 2008. Biological effects of lysophospholipids. Rev. Physiol. Biochem. Pharmacol. 160 25–46. [DOI] [PubMed] [Google Scholar]
- 14.Murph M. M., J. Hurst-Kennedy, V. Newton, D. N. Brindley, and H. Radhakrishna. 2007. Lysophosphatidic acid decreases the nuclear localization and cellular abundance of the p53 tumor suppressor in A549 lung carcinoma cells. Mol. Cancer Res. 5 1201–1211. [DOI] [PubMed] [Google Scholar]
- 15.Samadi, N., C. Gaetano, I. S. Goping, and D. N. Brindley. Autotaxin protects MCF-7 breast cancer and MDA-MB-435 melanoma cells against Taxol-induced apoptosis. Oncogene. Epub ahead of print. December 15, 2008. [DOI] [PubMed]
- 16.Brindley D. N. 1993. Hepatic secretion of lysphosphatidylcholine: a novel transport system for polyunsaturated fatty acids and choline. J. Nutr. Biochem. 4 442–449. [Google Scholar]
- 17.Fourcade O., M. F. Simon, C. Viode, N. Rugani, F. Leballe, A. Ragab, B. Fournie, L. Sarda, and H. Chap. 1995. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 80 919–927. [DOI] [PubMed] [Google Scholar]
- 18.Sano T., D. Baker, T. Virag, A. Wada, Y. Yatomi, T. Kobayashi, Y. Igarashi, and G. Tigyi. 2002. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J. Biol. Chem. 277 21197–21206. [DOI] [PubMed] [Google Scholar]
- 19.Takabe K., S. W. Paugh, S. Milstien, and S. Spiegel. 2008. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 60 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.English D., Z. Welch, A. T. Kovala, K. Harvey, O. V. Volpert, D. N. Brindley, and J. G. Garcia. 2000. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 14 2255–2265. [DOI] [PubMed] [Google Scholar]
- 21.Pyne S., J. S. Long, N. T. Ktistakis, and N. J. Pyne. 2005. Lipid phosphate phosphatases and lipid phosphate signalling. Biochem. Soc. Trans. 33 1370–1374. [DOI] [PubMed] [Google Scholar]
- 22.Jasinska R., Q. X. Zhang, C. Pilquil, I. Singh, J. Xu, J. Dewald, D. A. Dillon, L. G. Berthiaume, G. M. Carman, D. W. Waggoner, et al. 1999. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 340 677–686. [PMC free article] [PubMed] [Google Scholar]
- 23.Imai A., T. Furui, T. Tamaya, and G. B. Mills. 2000. A gonadotropin-releasing hormone-responsive phosphatase hydrolyses lysophosphatidic acid within the plasma membrane of ovarian cancer cells. J. Clin. Endocrinol. Metab. 85 3370–3375. [DOI] [PubMed] [Google Scholar]
- 24.Tanyi J. L., A. J. Morris, J. K. Wolf, X. Fang, Y. Hasegawa, R. Lapushin, N. Auersperg, Y. J. Sigal, R. A. Newman, E. A. Felix, et al. 2003. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 63 1073–1082. [PubMed] [Google Scholar]
- 25.Smyth S. S., V. A. Sciorra, Y. J. Sigal, Z. Pamuklar, Z. Wang, Y. Xu, G. D. Prestwich, and A. J. Morris. 2003. Lipid phosphate phosphatases regulate lysophosphatidic acid production and signaling in platelets: studies using chemical inhibitors of lipid phosphate phosphatase activity. J. Biol. Chem. 278 43214–43223. [DOI] [PubMed] [Google Scholar]
- 26.Simon M. F., A. Rey, I. Castan-Laurel, S. Gres, D. Sibrac, P. Valet, and J. S. Saulnier-Blache. 2002. Expression of ectolipid phosphate phosphohydrolases in 3T3F442A preadipocytes and adipocytes. Involvement in the control of lysophosphatidic acid production. J. Biol. Chem. 277 23131–23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanyi J. L., Y. Hasegawa, R. Lapushin, A. J. Morris, J. K. Wolf, A. Berchuck, K. Lu, D. I. Smith, K. Kalli, L. C. Hartmann, et al. 2003. Role of decreased levels of lipid phosphate phosphatase-1 in accumulation of lysophosphatidic acid in ovarian cancer. Clin. Cancer Res. 9 3534–3545. [PubMed] [Google Scholar]
- 28.Bektas M., S. G. Payne, H. Liu, S. Goparaju, S. Milstien, and S. Spiegel. 2005. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J. Cell Biol. 169 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brindley, D. N., and A. U. Bräuer. 2009. Lipid mediators and modulators of neural function. In Handbook of Neurochemistry and Molecular Biology: Lysophosphatidate and Lysolipids. A. Lajtha, G. Gorraci, and G. Tettamanti, editor. Springer, New York. In press.
- 30.Zhao Y., S. K. Kalari, P. V. Usatyuk, I. Gorshkova, D. He, T. Watkins, D. N. Brindley, C. Sun, R. Bittman, J. G. Garcia, et al. 2007. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J. Biol. Chem. 282 14165–14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannun Y. A., and L. M. Obeid. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9 139–150. [DOI] [PubMed] [Google Scholar]
- 32.Mechtcheriakova D., A. Wlachos, J. Sobanov, F. Bornancin, G. Zlabinger, T. Baumruker, and A. Billich. 2007. FTY720-phosphate is dephosphorylated by lipid phosphate phosphatase 3. FEBS Lett. 581 3063–3068. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka M., Y. Anada, Y. Igarashi, and A. Kihara. 2008. A splicing isoform of LPP1, LPP1a, exhibits high phosphatase activity toward FTY720 phosphate. Biochem. Biophys. Res. Commun. 375 675–679. [DOI] [PubMed] [Google Scholar]
- 34.Pettus B. J., K. Kitatani, C. E. Chalfant, T. A. Taha, T. Kawamori, J. Bielawski, L. M. Obeid, and Y. A. Hannun. 2005. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol. Pharmacol. 68 330–335. [DOI] [PubMed] [Google Scholar]
- 35.van Meeteren L. A., P. Ruurs, E. Christodoulou, J. W. Goding, H. Takakusa, K. Kikuchi, A. Perrakis, T. Nagano, and W. H. Moolenaar. 2005. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J. Biol. Chem. 280 21155–21161. [DOI] [PubMed] [Google Scholar]
- 36.Tomsig, J. L., A. H. Snyder, E. V. Berdyshev, A. Skobeleva, C. Mataya, V. Natarajan, D. N. Brindley, and K. R. Lynch. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. Epub ahead of print. February 12, 2009. [DOI] [PMC free article] [PubMed]
- 37.Peest U., S. C. Sensken, P. Andreani, P. Hanel, P. P. Van Veldhoven, and M. H. Graler. 2008. S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J. Cell. Biochem. 104 756–772. [DOI] [PubMed] [Google Scholar]
- 38.Humtsoe J. O., S. Feng, G. D. Thakker, J. Yang, J. Hong, and K. K. Wary. 2003. Regulation of cell-cell interactions by phosphatidic acid phosphatase 2b/VCIP. EMBO J. 22 1539–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humtsoe J. O., R. A. Bowling, Jr., S. Feng, and K. K. Wary. 2005. Murine lipid phosphate phosphohydrolase-3 acts as a cell-associated integrin ligand. Biochem. Biophys. Res. Commun. 335 906–919. [DOI] [PubMed] [Google Scholar]
- 40.Wary K. K., and J. O. Humtsoe. 2005. Anti-lipid phosphate phosphohydrolase-3 (LPP3) antibody inhibits bFGF- and VEGF-induced capillary morphogenesis of endothelial cells. Cell Commun. Signal. 3 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long J. S., K. Yokoyama, G. Tigyi, N. J. Pyne, and S. Pyne. 2006. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid- and platelet-derived-growth-factor-induced cell migration. Biochem. J. 394 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettitt T. R., A. Martin, T. Horton, C. Liossis, J. M. Lord, and M. J. Wakelam. 1997. Diacylglycerol and phosphatidate generated by phospholipases C and D, respectively, have distinct fatty acid compositions and functions. Phospholipase D-derived diacylglycerol does not activate protein kinase C in porcine aortic endothelial cells. J. Biol. Chem. 272 17354–17359. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C., D. L. Baker, S. Yasuda, N. Makarova, L. Balazs, L. R. Johnson, G. K. Marathe, T. M. McIntyre, Y. Xu, G. D. Prestwich, et al. 2004. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J. Exp. Med. 199 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon M. F., D. Daviaud, J. P. Pradere, S. Gres, C. Guigne, M. Wabitsch, J. Chun, P. Valet, and J. S. Saulnier-Blache. 2005. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J. Biol. Chem. 280 14656–14662. [DOI] [PubMed] [Google Scholar]
- 45.Long J., P. Darroch, K. F. Wan, K. C. Kong, N. Ktistakis, N. J. Pyne, and S. Pyne. 2005. Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools. Biochem. J. 391 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y., P. V. Usatyuk, R. Cummings, B. Saatian, D. He, T. Watkins, A. Morris, E. W. Spannhake, D. N. Brindley, and V. Natarajan. 2005. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid-induced calcium release, NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem. J. 385 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilquil C., J. Dewald, A. Cherney, I. Gorshkova, G. Tigyi, D. English, V. Natarajan, and D. N. Brindley. 2006. Lipid phosphate phosphatase-1 regulates lysophosphatidate-induced fibroblast migration by controlling phospholipase D2-dependent phosphatidate generation. J. Biol. Chem. 281 38418–38429. [DOI] [PubMed] [Google Scholar]
- 48.Morris K. E., L. M. Schang, and D. N. Brindley. 2006. Lipid phosphate phosphatase-2 activity regulates S-phase entry of the cell cycle in Rat2 fibroblasts. J. Biol. Chem. 281 9297–9306. [DOI] [PubMed] [Google Scholar]
- 49.Zhang N., J. P. Sundberg, and T. Gridley. 2000. Mice mutant for Ppap2c, a homolog of the germ cell migration regulator wunen, are viable and fertile. Genesis. 27 137–140. [DOI] [PubMed] [Google Scholar]
- 50.Gómez-Muñoz A. 2006. Ceramide 1-phosphate/ceramide, a switch between life and death. Biochim. Biophys. Acta. 1758 2049–2056. [DOI] [PubMed] [Google Scholar]