Abstract
There is now solid evidence that T cell adaptive immunity is involved in atherogenesis. While initial studies have focused on the pathogenic arm of the immune response, more recent work clearly suggests an important role for several subsets of regulatory T cells in the protection against lesion development. Here, we review the current knowledge on the role of both pathogenic and regulatory adaptive T cell immunity in atherosclerosis, generated mainly from the study of mouse models of the disease.
Keywords: inflammation, lymphocytes, cytokines
It is now well accepted that atherosclerosis is driven by a chronic inflammatory process within the arterial wall initiated mainly in response to endogenously modified structures, particularly oxidized lipoproteins, that stimulate both innate and adaptive immune responses, leading to further alteration of the vascular wall and promoting disease progression and complications (1, 2). Among the immune cells, monocytes/macrophages are certainly the most critical promoters and maybe the final common pathway of the immunoinflammatory response in atherosclerosis, because suppression of their vascular accumulation is associated with the most dramatic reduction in lesion development in mouse models of the disease. However, activation of the adaptive immune response that involves antigen presentation by dendritic cells (DC) and the generation/activation of antigen-specific T lymphocytes certainly deserve specific and close attention, not only because of the beauty of understanding the complex interplay of this network and its role in disease modulation, but also because of the great potential of specific and intelligent manipulation of the immune system toward antigen-specific immune downregulation or tolerance, ultimately leading to limitation of plaque progression and complications. In this review, we will briefly expose the current knowledge on the role of both pathogenic and regulatory adaptive CD4+ T cell immunity in atherosclerosis, generated mainly from the study of mouse models of the disease.
T CELLS ARE INVOLVED IN ATHEROSCLEROSIS
The adaptive immune system is involved in the development of atherosclerosis. Deficiency in both T and B cells significantly inhibits lesion development [reviewed in (1, 2)]. The protective effect is seen in the early stages of lesion development and in the absence of severe hypercholesterolemia but varies according to the site of lesion development, being more important in the aortic root and less visible in the thoracic and abdominal aorta or in the brachiocephalic trunk. Transfer of CD4+ T cells from atherosclerotic Apoe−/− mice into Apoe−/−/scid/scid mice enhances atherosclerotic lesion development to a level similar to that of immunocompetent controls, indicating a proatherogenic role for T cells.
ANTIGEN PRESENTATION IN ATHEROSCLEROSIS
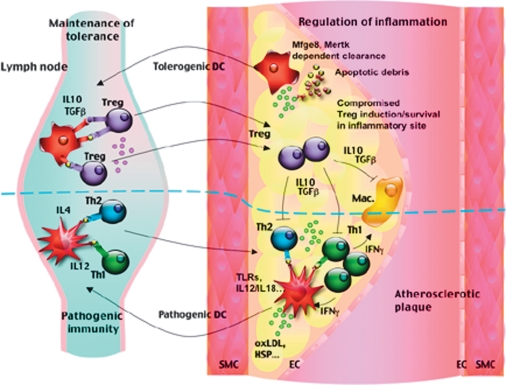
Transfer of oxLDL-reactive T cells to Apoe−/−/scid/scid mice is more efficient at lesion acceleration than the transfer of T cells with no specificity to a plaque-derived antigen (3), suggesting an important role for antigen presentation in disease development. DCs are specialized antigen-presenting cells that are required for the activation of naïve T cells and the development of antigen-specific T cell-mediated immune responses. Potential candidate T cell antigens in atherosclerosis lie within altered self-structures, such as modified lipoproteins, and among other proteins, such as heat shock proteins, β2-glycoprotein I, and probably apoptotic/necrotic debris. The exact location of initial antigen presentation in atherosclerosis has not been determined. DCs are known to acquire and process antigens for presentation to T cells in peripheral lymphoid organs, and this probably also occurs in the context of atherosclerosis. It could be argued that DCs can encounter and scavenge atherosclerosis-related antigens in the periphery, outside atherosclerotic lesions. Indeed, limited accumulation of modified lipoproteins can be encountered within the skin or in the spleen, which might be enough to induce antigen-specific pathogenic and/or regulatory T cells. Most likely, however, DCs encounter atherosclerosis-related antigens within vascular lesions. Beginning from the very early stages of lesion development, DCs accumulate within the intima of atherosclerosis-susceptible regions characterized by disturbed flow dynamics, through a mechanism involving VCAM-1 (4) and CX3CR1 (5). Their continued accumulation during lesion development is correlated with lesion progression and inflammation (5), and their clustering with T cells within the lesions suggests an important role in the modulation of T cell adaptive immunity. Scavenging of modified lipids and other potential antigenic structures within the lesions would induce DC maturation and migration to secondary lymphoid organs where they would present antigens to promote the generation of antigen-specific pathogenic and/or regulatory T cells. These antigen-specific T cells would subsequently migrate to the atherosclerotic lesions where they could be reactivated and be involved in the modulation of the immunoinflammatory response (Fig. 1). However, given the proinflammatory plaque microenvironment, the survival and maintenance of Treg cells would be compromised compared with pathogenic T cells, leading to an imbalance in T cell adaptive immunity toward increased T cell activation (Fig. 1). It could also be argued that plaque DCs would be able to initiate antigen-specific activation of T cells within the lesions (Fig. 1), although the process would be less efficient than in specialized lymphoid organs. In addition, in the case of lesion-confined activation, only antigen-specific pathogenic T cells would be generated given the proinflammatory plaque-microenvironment, which would exagerate the imbalance between pathogenic and Treg cells (Fig. 1). This model of antigen presentation allows several predictions. First, it explains the overrepresentation of pathogenic T cells compared with Treg cells. Second, in order to maintain the generation of antigen-specific Treg cells, DCs would need to present atherosclerosis-related antigens outside the lesions, in a microenvironment with reduced inflammation, and, thus, would need to continuously emigrate to regional lymphoid organs (Fig. 1). Phagocytosis of apoptotic debris by DCs through milk fat globule-EGF factor 8 (also known as lactadherin) and/or mer tyrosine kinase receptor pathways would greatly facilitate this task through modulation of their activation and their Treg-inducing potential (6, 7). In agreement with these hypotheses, impaired emigratory potential of DCs (8) and impaired clearance of apoptotic cells (6, 7) greatly enhance lesion inflammation and accelerate plaque progression. Third, with the increasing burden of dyslipidemia, oxidatively modified lipids and their related bioactive products, impaired DC emigration would ultimately lead to a reduction in the generation of antigen-specific pathogenic T cells and a reduction in the accumulation of antigen-specific T cells within the lesions, thereby reducing the impact of T cell adaptive immunity on lesion progression, which again is supported by experimental data in murine models of the disease.
Fig. 1.
Antigen presentation and induction of antigen-specific pathogenic and regulatory T cells in the context of atherosclerosis. Antigen presentation most likely occurs within the secondary lymphoid organs, and primed T cells are reactivated within the lesions. DC activation and maturation by self-altered structures, in part through toll-like receptors (TLRs), lead to induction/maintenance of pathogenic immunity. Phagocytic clearance of apoptotic debris through milk fat globule-EGF factor 8 (Mfge8) or mer tyrosine kinase receptor (mertk) is required for induction/maintenance of regulatory immunity. The proinflammatory plaque environment is permissive to the induction/maintenance of pathogenic T cells but impairs the development of regulatory immunity. Thus, in order to maintain a Treg cell response, DCs would need to continuously traffic to peripheral lymphoid organs.
TH1 RESPONSE IN ATHEROSCLEROSIS
The majority of pathogenic T cells in atherosclerosis are of the Th1 profile producing high levels of IFN-γ. Th1-driven responses are detrimental to the atherosclerotic process (1). IFN-γ is known to activate monocytes/macrophages and DCs, leading to perpetuation of the pathogenic Th1 response. In addition, IFN-γ may inhibit vascular smooth muscle cell proliferation and reduces their collagen production while upregulating the expression of matrix metalloproteinases, thereby contributing to the thinning of the fibrous cap (1). Deficiency in IFN-γR or IFN-γ significantly reduces lesion development and enhances plaque collagen content (9, 10), whereas exogenous administration of IFN-γ enhances lesion development (10). Surprisingly, one study reported reduced lesion formation in LDLR2/2 mice transplanted with IFNg-deficient bone marrow (11). The reasons for this discrepancy remain to be elucidated.
Interleukin (IL)-12 production by DCs and monocytes/macrophages plays a critical role in Th1 differentiation. IL-12 activates the transcription factor STAT4 and a unique Th1 transcription factor, T-box expressed in T cells, leading to upregulation of IFN-γ and downregulation of IL-4 and IL-5 expression in T cells. Apoe−/− mice deficient for IL-12p40 showed signifcant reduction in plaque development (12), and exogenous IL-12 administration enhances IFN-γ production and disease development (13). IL-18 synergizes with IL-12 to induce IFN-γ production (14). Treatment of Apoe−/− mice with a plasmid encoding the endogenous IL-18 inhibitor, IL-18 binding protein (15), or genetic deletion of IL-18 (16), significantly reduces lesion development, whereas exogenous administration of IL-18 clearly accelerates disease progression (17, 18). CD40-CD40L interactions also promote Th1 cell development, and inhibition of this pathway reduces lesion development and induces a ‘stable' plaque phenotype [reviewed in (19)]. However, T cell-independent effects may be involved in this pathway, because CD40L deficiency in bone marrow-derived cells did not alter atherosclerosis. Finally, T-box expressed in T cells deficiency clearly reduces lesion development (20). Collectively, these results provided convincing elements to incriminate Th1 responses in the promotion of plaque development.
TH2 RESPONSE IN ATHEROSCLEROSIS
Th2 cells secrete IL-4, IL-5, IL-10, and IL-13 and provide help for antibody production by B cells. Th2 cells are rarely detected within the atherosclerotic lesions. However, their induction is promoted in a severe hyperlipidemic context. IL-4 drives Th2 cell differentiation through STAT6, which activates the transcription factor Gata3, leading to upregulation of IL-4 and IL-5 and downregulation of IFN-γ. Thus, Th2-biased responses were proposed to antagonize proatherogenic Th1 effects and thereby confer atheroprotection. However, the role of the Th2 pathway in the development of atherosclerosis remains controversial depending on the stage and/or site of the lesion, as well as on the experimental model. In mouse models that are realtively resistant to atherosclerosis, a Th2-bias has been shown to protect against early fatty streak development (21). However, in more permissive models using LDLR−/− mice, deficiency in IL-4, the prototypic Th2-related cytokine, had no substantial effect on lesion development in one study (22) but was associated with a decrease in atherosclerotic lesion formation in a previous work by the same authors (23), suggesting a potentially proatherogenic role. Prolonged hypercholesterolemia in animal models of atherosclerosis is associated with enhanced IL-4 production, which most probably contributes to plaque progression, because deficiency in IL-4 at these advanced stages greatly hampers plaque progression (12).
However, other Th2-related cytokines, IL-5, and IL-33 appear to exhibit antiatherogenic properties. Induction of humoral immunity by immunization of hypercholesterolemic apoE−/− mice with oxLDL reduces lesion size in association with the production of high levels of IgM-type anti-oxLDL antibodies, probably from B1 cells [reviewed in (24)]. These cells appear to be stimulated by IL-5 produced by malondialdehyde-LDL-specific Th2 cells, because antibody generation and atheroprotection were significantly reduced in mice with genetic deletion of IL-5 in bone marrow cells. More recently, IL-33 has been shown to exhibit atherprotective effects, at least in part, through induction of IL-5 and the production of IgM-type anti-oxLDL antibodies (25).
TREG RESPONSE IN ATHEROSCLEROSIS
The available data suggest that Th1, and potentially Th2-mediated, responses contribute to the development and progression of atherosclerosis. Therefore, identification of the causes of Th1/Th2 dysregulation would be of great imprtance to a better understanding of the pathophysiology of atherosclerosis. Our hypothesis is that in the context of atherosclerosis, an imbalance exists between pathogenic (Th1 and/or Th2) and regulatory T cells in response to “altered” self antigens, leading to reciprocal and mutual amplification of the innate and adaptive immune responses, responsible for plaque development and progression.
Regulatory T cells and immunological homeostasis
Natural Treg cells develop in the thymus and recognize specific self-antigen. They are characterized by the expression of CD4, high levels of CD25, and the transcriptional factor Foxp3 [reviewed in (26)]. They home to peripheral tissues to maintain self-tolerance and prevent autoimmunity by inhibiting pathogenic lymphocytes. Costimulation, particularly through the CD28-CD80/CD86 pathway, is required for their maintenance. In vitro, Treg cells are anergic and are able to inhibit effector T cell proliferation. Foxp3 is not required for Treg cell development but is instrumental in mediating the suppressive propoerties of Treg cells on pathogenic T cells through its interaction with other transcription factors such as nuclear factor activated in T cells and acute myeloid leukemia 1, transcriptional repression of genes involved in T cell activation such as IL-2, and/or induction of CD25 and cytotoxic T-lymphocyte antigen 4.
Subsets of Treg cells are also generated in the periphery during active immune responses and are called induced Treg cells (iTreg). Indeed, naïve CD4+CD25− in the periphery can be converted in the presence of transforming growth factor (TGF)-β, IL10, or a low dose of antigenic peptide into CD4+CD25+FOXP3+ cells (see below). The iTreg induced by IL10 are called Tr1 cells, whereas iTreg induced by TGF-β are called Th3. These cells mediate suppressor function through the production of IL10 and TGF-β, respectively (see below).
TGF-β, Treg cell function, and atheroprotection
The importance of TGF-β in the maintenance of immunological homeostasis was highlighted by the discovery that TGF-β-deficient mice develop multiple inflammatory diseases. TGF-β inhibits the proliferation, activation, and differentiation of T cells toward Th1 and Th2. In addition, TGF-β1 has been shown to maintain Treg cells in the periphery by acting as a costimulatory factor for expression of Foxp3. DCs have the capacity to induce Treg cell formation depending on TGF-β. DCs that have phagocytosed apoptotic cells through the lactadherin-dependent pathway play an instrumental role in the maintenance of Treg cell function in the periphery (6), a process mediated through increased production of TGF-β (27). DCs also express the integrin alphavbeta8, which has the capacity to activate TGF-β and maintain a TGF-β-dependent Treg cell function (28).
Previous studies have shown that TGF-β has antiinflammatory and atheroprotective effects. Sytemic TGF-β neutralization (29, 30) or genetic deficiency in TGF-β (31), increased lesion development in Apoe−/− mice. Accelerated atherosclerosis was associated with increased infiltration of inflammatory macrophages and T cells within lesions, together with reduced collagen content, leading to the development of plaques with a ‘vulnerable' phenotype. Interestingly, specific deletion of TGF-β signaling in T cells was sufficient to induce increased lesional inflammation (32, 33) and enhanced plaque development (33), associated with increased differentiation of T cells toward both Th1 and Th2 phenotypes (32, 33), suggesting a Treg-mediated effect.
Based on these results, we directly addressed the role of Treg cells in atherogenesis. We first used mice deficient in either CD28 or CD80/CD86, known to display a marked reduction in peripheral Treg cell pool. LDLR−/− mice transplanted with CD28 or CD80/CD86-deficient marrow showed accelerated atherosclerosis and enhanced lesional inflammation. Similarly, transfer of CD28-deficient splenocytes to Apoe−/−/Rag2−/− mice led to a marked acceleration of atherosclerosis compared with the transfer of wild-type splenocytes. This was associated with decreased numbers of Foxp3+ Treg cells and impaired Treg-suppressive function. Resupplementation of CD28-deficient splenocytes with a physiological number of Treg cells improved Treg cell function and prevented lesion acceleration, identifying a major role for endogenous Treg cell response in atheroprotection. Similar studies using inducible costimulatory molecule-deficient mice clearly showed acceleration of atherosclerosis in association with a reduction in Treg cell number and function (34). Furthermore, strategies using CD25 neutralizing antibodies in young Apoe−/− mice clearly demonstrated a protective role of Treg cells against atherogenesis (35). Treg depletion did not influence lesion size or inflammatory phenotype in mice with specific deletion of TGF-β signaling in T cells, highlighting the role of TGF-β in the atheroprotective effect of Treg cells. Reduction of atherosclerosis in Apoe−/− mice has also been achieved through adoptive transfer of CD4+CD25+ regulatory T cells (36), clearly establishing an important role for Treg cell response in the taming of the inflammatory response in atherosclerosis and the control of lesion development.
IL-10, Treg cell function, and atheroprotection
IL-10 plays an important role in the control of both innate and adaptive immunity. In lymphocytes, the production of IL-10 has been associated to Th2 subset, Treg cells, and, more recently, to some Th1 cells. Among the regulatory T cells, both natural Treg and induced Tr1 cells have the capacity to produce IL-10. Tr1 cells may be independent of Foxp3 and exhibit their suppressor function through the production of IL-10 and TGF-β.
IL-10 deficiency promotes atherosclerotic lesion formation, characterized by increased infiltration of inflammatory cells, particularly activated T cells, and by increased production of proinflammatory cytokines (37, 38). Leukocyte-derived IL-10 appears to be instrumental in the prevention of atherosclerotic lesion development and in the modulation of cellular and collagen plaque composition (39). However, the effect of IL-10 disruption in specific cell subtypes (macrophages, DCs, or T cells) on lesion development and progression is still unknown. Consistent with a protective role of IL-10 in atherosclerosis, systemic or local overexpression of IL-10 by adenoviral gene transfer in collar-induced carotid atherosclerosis of LDLR−/− mice was found to be highly efficient in preventing atherosclerosis (40). Very interestingly, overexpression of IL-10 by activated T lymphocytes reduced atherosclerosis in LDLR−/− mice (41), which we believe suggests a protective effect for Tr1-like cells in atherosclerosis. This is consistent with a study from our group showing that transfer of clones of Tr1 cells reduces lesion development (42) and that promotion of endogenous adaptive Tr1 cell response plays a significant role in limiting disease development (43).
MODULATION OF ADAPTIVE T CELL IMMUNITY TO TREAT IN ATHEROSCLEROSIS
Promotion of CD4+CD25+Foxp3+ Treg cell function in vivo
Since the identification of a critical role of Treg cells in atherosclerosis, several strategies have been developed based on the enhancement of Treg cell response to limit lesion development and inflammation. Steffens et al. (44) showed that administration of a nonmitogenic anti-CD3 antibody reduced plaque development and markedly inhibited lesion progression in mice with already-established atherosclerosis. This was associated with the induction of a regulatory immune response as revealed by increased production of TGF-β and enhanced expression of Foxp3 in lymph node and spleen cells, respectively.
Other strategies may involve interference with Treg-inhibitory signals. We have recently shown that leptin receptor-deficient (db/db) mice display a marked increase in the number and suppressive function of Treg cells. Supplementation of Treg-deficient lymphocytes with Treg cells from db/db mice induces a significant reduction of lesion size and a marked inhibition of IFN-γ production compared with supplementation by Treg cells from wild-type mice (45).
Strategies involving the induction of antigen-specific Treg cells are probably the most attractive, if not the most promising ones. Induction of oral tolerance through oral administration of atherosclerosis-related antigens (oxLDL or heat shock protein-60) to LDLR−/− mice resulted in a significant attenuation of the initiation and progression of atherogenesis, associated with increased antigen-specific TGF-β and/or IL-10 production, and increased number of CD4+CD25+Foxp3+ cells in spleen and mesenteric lymph nodes (46). However, more direct evidence is still needed to relate changes in antigen-specific Treg cell response to disease limitation and to examine the requirement for Foxp3 expression in this protective effect.
Strategies to promote Foxp3-independent regulatory T cell response
Administration of antigens through the mucosal route has also been shown to induce the generation of Foxp3-independent iTreg cells. Induction of mucosal tolerance to heat shock protein has been tested as a therapeutic strategy in experimental atherosclerosis (47, 48). The studies showed a reduction in lesion size and plaque inflammation. The T cell cytokine profile was switched toward a Th2 phenotype with high production of IL-4 (47) or IL-10 (48). Thus, additional mechanistic work is required to understand the potential role of the regulatory immune response in this process.
Another way to promote endogenous Treg cell function may involve direct supplementation with clones of ex vivo-generated, antigen-specific Treg cells. We have recently shown that modulation of the peripheral immune response leading to limitation of plaque development is achievable by transfer of Tr1 cells with no specificity to a known plaque antigen and probably operates through a mechanism of bystander immune suppression (42). However, transfer of Tr1-clones specific for plaque-derived antigens would enhance the atheroprotective effects while reducing potential side effects.
CONCLUSION
T cell adaptive immunity has long been thought of as only a promoter of a proatherogenic immune response in atherosclerosis. The discovery of endogenous counter-regulators of the pathogenic immune response in atherosclerosis, particularly IL-10 and TGF-β, led to the identification of an important role for Treg cells in the control of lesion development and/or progression. However, the critical subtypes of Treg cells responsible for these protective effects and the molecular mechanisms involved in their survival, migration, homing, and suppressive function are still unknown. The precise delineation of the major determinants of the Treg cell response in atherosclerosis is currently the subject of intensive studies. A better understanding of these regulatory pathways would favor the development of novel therapeutic strategies, including vaccination-like strategies in order to promote antigen-specific Treg cell response and limit disease development and complications.
Abbreviations
DC, dendritic cell
IL, interleukin
iTreg, induced Treg cell
oxLDL, oxidized LDL
TGF, transforming growth factor
Ziad Mallat is a recipient of a Contrat d'Interface from Assistance Publique-Hôpitaux de Paris.
Published, JLR Papers in Press, December 2, 2008.
References
- 1.Tedgui A., and Z. Mallat. 2006. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 86 515–581. [DOI] [PubMed] [Google Scholar]
- 2.Weber C., A. Zernecke, and P. Libby. 2008. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 8 802–815. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X., A. K. Robertson, C. Hjerpe, and G. K. Hansson. 2006. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26 864–870. [DOI] [PubMed] [Google Scholar]
- 4.Jongstra-Bilen J., M. Haidari, S. N. Zhu, M. Chen, D. Guha, and M. I. Cybulsky. 2006. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med. 203 2073–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P., Y. R. Yu, J. A. Spencer, A. E. Johnson, C. T. Vallanat, A. M. Fong, C. Patterson, and D. D. Patel. 2008. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler. Thromb. Vasc. Biol. 28 243–250. [DOI] [PubMed] [Google Scholar]
- 6.Ait-Oufella H., K. Kinugawa, J. Zoll, T. Simon, J. Boddaert, S. Heeneman, O. Blanc-Brude, V. Barateau, S. Potteaux, R. Merval, et al. 2007. Lactadherin-defciency induces apoptotic cell accumulation, alters the regulatory immune response, and accelerates atherosclerosis in mice. Circulation. 115 2168–2177. [DOI] [PubMed] [Google Scholar]
- 7.Ait-Oufella H., V. Pouresmail, T. Simon, O. Blanc-Brude, K. Kinugawa, R. Merval, G. Offenstadt, G. Leseche, P. L. Cohen, A. Tedgui, et al. 2008. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28 1429–1431. [DOI] [PubMed] [Google Scholar]
- 8.Angeli V., J. Llodra, J. X. Rong, K. Satoh, S. Ishii, T. Shimizu, E. A. Fisher, and G. J. Randolph. 2004. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 21 561–574. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S., A. M. Pablo, X. C. Jiang, N. Wang, A. R. Tall, and C. Schindler. 1997. IFN-gamma potentiates atherosclerosis in apoE knock-out mice. J. Clin. Invest. 99 2752–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitman S. C., P. Ravisankar, H. Elam, and A. Daugherty. 2000. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am. J. Pathol. 157 1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niwa T., H. Wada, H. Ohashi, N. Iwamoto, H. Ohta, H. Kirii, H. Fujii, K. Saito, and M. Seishima. 2004. Interferon-gamma produced by bone marrow-derived cells attenuates atherosclerotic lesion formation in LDLR-deficient mice. J. Atheroscler. Thromb. 11 79–87. [DOI] [PubMed] [Google Scholar]
- 12.Davenport P., and P. G. Tipping. 2003. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 163 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee T. S., H. C. Yen, C. C. Pan, and L. Y. Chau. 1999. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 19 734–742. [DOI] [PubMed] [Google Scholar]
- 14.Gerdes N., G. K. Sukhova, P. Libby, R. S. Reynolds, J. L. Young, and U. Schonbeck. 2002. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J. Exp. Med. 195 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallat Z., A. Corbaz, A. Scoazec, P. Graber, S. Alouani, B. Esposito, Y. Humbert, Y. Chvatchko, and A. Tedgui. 2001. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ. Res. 89 E41–E45. [DOI] [PubMed] [Google Scholar]
- 16.Elhage R., J. Jawien, M. Rudling, H. G. Ljunggren, K. Takeda, S. Akira, F. Bayard, and G. K. Hansson. 2003. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 59 234–240. [DOI] [PubMed] [Google Scholar]
- 17.Whitman S. C., P. Ravisankar, and A. Daugherty. 2002. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ. Res. 90 E34–E38. [DOI] [PubMed] [Google Scholar]
- 18.Tenger C., A. Sundborger, J. Jawien, and X. Zhou. 2005. IL-18 accelerates atherosclerosis accompanied by elevation of IFN-gamma and CXCL16 expression independently of T cells. Arterioscler. Thromb. Vasc. Biol. 25 791–796. [DOI] [PubMed] [Google Scholar]
- 19.Lutgens E., D. Lievens, L. Beckers, M. Donners, and M. Daemen. 2007. CD40 and its ligand in atherosclerosis. Trends Cardiovasc. Med. 17 118–123. [DOI] [PubMed] [Google Scholar]
- 20.Buono C., C. J. Binder, G. Stavrakis, J. L. Witztum, L. H. Glimcher, and A. H. Lichtman. 2005. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl. Acad. Sci. USA. 102 1596–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber S. A., P. Sakkinen, C. David, M. K. Newell, and R. P. Tracy. 2001. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 103 2610–2616. [DOI] [PubMed] [Google Scholar]
- 22.King V. L., L. A. Cassis, and A. Daugherty. 2007. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am. J. Pathol. 171 2040–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King V. L., S. J. Szilvassy, and A. Daugherty. 2002. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 22 456–461. [DOI] [PubMed] [Google Scholar]
- 24.Binder C. J., P. X. Shaw, M. K. Chang, A. Boullier, K. Hartvigsen, S. Horkko, Y. I. Miller, D. A. Woelkers, M. Corr, and J. L. Witztum. 2005. Thematic review series: the immune system and atherogenesis. The role of natural antibodies in atherogenesis. J. Lipid Res. 46 1353–1363. [DOI] [PubMed] [Google Scholar]
- 25.Miller A. M., D. Xu, D. L. Asquith, L. Denby, Y. Li, N. Sattar, A. H. Baker, I. B. McInnes, and F. Y. Liew. 2008. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 205 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens G. L., and E. M. Shevach. 2007. Foxp3+ regulatory T cells: selfishness under scrutiny. Immunity. 27 417–419. [DOI] [PubMed] [Google Scholar]
- 27.Jinushi M., Y. Nakazaki, M. Dougan, D. R. Carrasco, M. Mihm, and G. Dranoff. 2007. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J. Clin. Invest. 117 1902–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Travis M. A., B. Reizis, A. C. Melton, E. Masteller, Q. Tang, J. M. Proctor, Y. Wang, X. Bernstein, X. Huang, L. F. Reichardt, et al. 2007. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 449 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallat Z., A. Gojova, C. Marchiol-Fournigault, B. Esposito, C. Kamate, R. Merval, D. Fradelizi, and A. Tedgui. 2001. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 89 930–934. [DOI] [PubMed] [Google Scholar]
- 30.Lutgens E., M. Gijbels, M. Smook, P. Heeringa, P. Gotwals, V. E. Koteliansky, and M. J. Daemen. 2002. Transforming growth factorbeta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler. Thromb. Vasc. Biol. 22 975–982. [DOI] [PubMed] [Google Scholar]
- 31.Grainger D. J., D. E. Mosedale, J. C. Metcalfe, and E. P. Bottinger. 2000. Dietary fat and reduced levels of TGFbeta1 act synergistically to promote activation of the vascular endothelium and formation of lipid lesions. J. Cell Sci. 113 2355–2361. [DOI] [PubMed] [Google Scholar]
- 32.Gojova A., V. Brun, B. Esposito, F. Cottrez, P. Gourdy, P. Ardouin, A. Tedgui, Z. Mallat, and H. Groux. 2003. Specific abrogation of transforming growth factor-{beta} signaling in T cells alters atherosclerotic lesion size and composition in mice. Blood. 102 4052–4058. [DOI] [PubMed] [Google Scholar]
- 33.Robertson A. K., M. Rudling, X. Zhou, L. Gorelik, R. A. Flavell, and G. K. Hansson. 2003. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J. Clin. Invest. 112 1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotsman I., N. Grabie, R. Gupta, R. Dacosta, M. MacConmara, J. Lederer, G. Sukhova, J. L. Witztum, A. H. Sharpe, and A. H. Lichtman. 2006. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 114 2047–2055. [DOI] [PubMed] [Google Scholar]
- 35.Ait-Oufella H., B. L. Salomon, S. Potteaux, A. K. Robertson, P. Gourdy, J. Zoll, R. Merval, B. Esposito, J. L. Cohen, S. Fisson, et al. 2006. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12 178–180. [DOI] [PubMed] [Google Scholar]
- 36.Mor A., D. Planer, G. Luboshits, A. Afek, S. Metzger, T. Chajek-Shaul, G. Keren, and J. George. 2007. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27 893–900. [DOI] [PubMed] [Google Scholar]
- 37.Mallat Z., S. Besnard, M. Duriez, V. Deleuze, F. Emmanuel, M. F. Bureau, F. Soubrier, B. Esposito, H. Duez, C. Fievet, et al. 1999. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 85 e17–e24. [DOI] [PubMed] [Google Scholar]
- 38.Caligiuri G., M. Rudling, V. Ollivier, M. P. Jacob, J. B. Michel, G. K. Hansson, and A. Nicoletti. 2003. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol. Med. 9 10–17. [PMC free article] [PubMed] [Google Scholar]
- 39.Potteaux S., B. Esposito, O. Van Oostrom, V. Brun, P. Ardouin, H. Groux, A. Tedgui, and Z. Mallat. 2004. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 24 1474–1478. [DOI] [PubMed] [Google Scholar]
- 40.Von Der Thusen J. H., J. Kuiper, M. L. Fekkes, P. De Vos, T. J. Van Berkel, and E. A. Biessen. 2001. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr−/− mice. FASEB J. 15 2730–2732. [DOI] [PubMed] [Google Scholar]
- 41.Pinderski L. J., M. P. Fischbein, G. Subbanagounder, M. C. Fishbein, N. Kubo, H. Cheroutre, L. K. Curtiss, J. A. Berliner, and W. A. Boisvert. 2002. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 90 1064–1071. [DOI] [PubMed] [Google Scholar]
- 42.Mallat Z., A. Gojova, V. Brun, B. Esposito, N. Fournier, F. Cottrez, A. Tedgui, and H. Groux. 2003. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation. 108 1232–1237. [DOI] [PubMed] [Google Scholar]
- 43.Ait-Oufella H., B. Horvat, Y. Kerdiles, O. Herbin, P. Gourdy, J. Khallou-Laschet, R. Merval, B. Esposito, A. Tedgui, and Z. Mallat. 2007. Measles virus nucleoprotein induces a regulatory immune response and reduces atherosclerosis in mice. Circulation. 116 1707–1713. [DOI] [PubMed] [Google Scholar]
- 44.Steffens S., F. Burger, G. Pelli, Y. Dean, G. Elson, M. Kosco-Vilbois, L. Chatenoud, and F. Mach. 2006. Short-term treatment with anti-CD3 antibody reduces the development and progression of atherosclerosis in mice. Circulation. 114 1977–1984. [DOI] [PubMed] [Google Scholar]
- 45.Taleb S., O. Herbin, H. Ait-Oufella, W. Verreth, P. Gourdy, V. Barateau, R. Merval, B. Esposito, K. Clement, P. Holvoet, et al. 2007. Defective leptin/leptin receptor signaling improves regulatory t cell immune response and protects mice from atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27 2691–2698. [DOI] [PubMed] [Google Scholar]
- 46.van Puijvelde G. H., A. D. Hauer, P. de Vos, R. van den Heuvel, M. J. van Herwijnen, R. van der Zee, W. van Eden, T. J. van Berkel, and J. Kuiper. 2006. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 114 1968–1976. [DOI] [PubMed] [Google Scholar]
- 47.Harats D., N. Yacov, B. Gilburd, Y. Shoenfeld, and J. George. 2002. Oral tolerance with heat shock protein 65 attenuates mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J. Am. Coll. Cardiol. 40 1333–1338. [DOI] [PubMed] [Google Scholar]
- 48.Maron R., G. Sukhova, A. M. Faria, E. Hoffmann, F. Mach, P. Libby, and H. L. Weiner. 2002. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 106 1708–1715. [DOI] [PubMed] [Google Scholar]