Abstract
This review presents an overview of mammalian phospholipid synthesis and the cellular locations of the biochemical activities that produce membrane lipid molecular species. The generalized endoplasmic reticulum compartment is a central site for membrane lipid biogenesis, and examples of the emerging relationships between alterations in lipid composition, regulation of membrane lipid biogenesis, and cellular secretory function are discussed.
Keywords: phospholipids, sphingolipids, plasmalogens, unfolded protein response
BIOLOGICAL MEMBRANES
Biological membranes are composed of lipids and proteins that together form hydrophobic barriers that limit the distribution of aqueous macromolecules and metabolites. Cells use membranes for a number of different purposes, including segregation and protection from the environment, compartmentalization of functions, energy production, storage, protein synthesis and secretion, phagocytosis, movement, and cell-cell interaction. Eukaryotic cells contain ordered infrastructures, called organelles, to organize and carry out complex processes and to enable distinct reactions that require a hydrophobic environment. The level and complexity of compartmentalization varies among organisms and among mammalian cells. Some cells also change in size and organelle complexity after biological stimulation. An example of induced membrane biogenesis occurs in naïve B-lymphocytes that are converted to plasma cells (1), and an example of membrane redistribution occurs in macrophages in which the Golgi apparatus is reoriented during transient cytokine synthesis and secretion (2). The versatility of biological membranes is dependent on their structures and biophysical properties, which are dictated by the types of lipids and proteins that compose the membranes. The functions of membranes require a fluid plasticity that is accomplished through alteration in lipid composition. Lipid composition is diverse, not only among different organisms, but also among different compartments within the same cells and between the two leaflets of the same membrane. Lipid composition is determined through regulation of de novo synthesis at designated cellular sites, selective distribution or trafficking to new sites, and by localized remodeling reactions. Understanding the relationships between the dynamic changes in membrane lipid composition and specific cellular events is our current challenge. This review is focused on membrane phospholipid biogenesis in mammalian cells with a particular emphasis on the role played by the endoplasmic reticulum (ER). The ER, together with the Golgi apparatus, is a major site of de novo bulk membrane lipid synthesis, and recent experiments demonstrate a link between phospholipid synthesis and secretion from this compartment.
THE ARCHITECTURE OF THE ER
The ER and Golgi apparatus together constitute the endomembrane compartment in the cytoplasm of eukaryotic cells. The endomembrane compartment is a major site of lipid synthesis, and the ER is where not only lipids are synthesized, but membrane-bound proteins and secretory proteins are also made. The ER is organized into a labyrinthine membrane-bound network of branching tubules and flattened sacs that extends throughout the cytosol. The tubules and sacs interconnect, and their membrane is continuous with the outer nuclear membrane (3). ER and nuclear membranes form a continuous sheet enclosing a single internal space, called the lumen. The ER can be divided into subdomains in relation to their function or location. The nuclear envelope is the domain that separates the genetic material from the cytosol. The ribosomes that synthesize ER-associated proteins are attached to the cytoplasmic aspect of the ER membrane, and these regions are designated as rough ER. The transitional ER is characterized by two domains, namely, a domain associated with ribosomes at a low density and a region that lacks attached ribosomes, called smooth ER. The ER region in close proximity with the mitochondrium is the mitochondrium-associated membrane. Finally, the region in close proximity to the Golgi apparatus, rich in vesicles and tubules, is the ER-Golgi intermediate compartment (ERGIC) (4). The ERGIC domain represents a continuum of the ER and Golgi apparatus where the lipids and lumenal proteins destined for transport to the cell surface or other organelles are transferred and biochemically modified. The cis-Golgi structure is in close proximity to the ERGIC, and the trans-Golgi network is the site for the formation of budding vesicles that distribute the lumenal protein contents. The ER interacts closely with the cytoskeleton, mostly with microtubules. This interaction allows the ER to maintain its position within the cell and facilitates intracellular trafficking, particularly from the smooth ER (4).
THE ER AND THE GOLGI APPARATUS ARE MAJOR SITES OF MEMBRANE LIPID SYNTHESIS
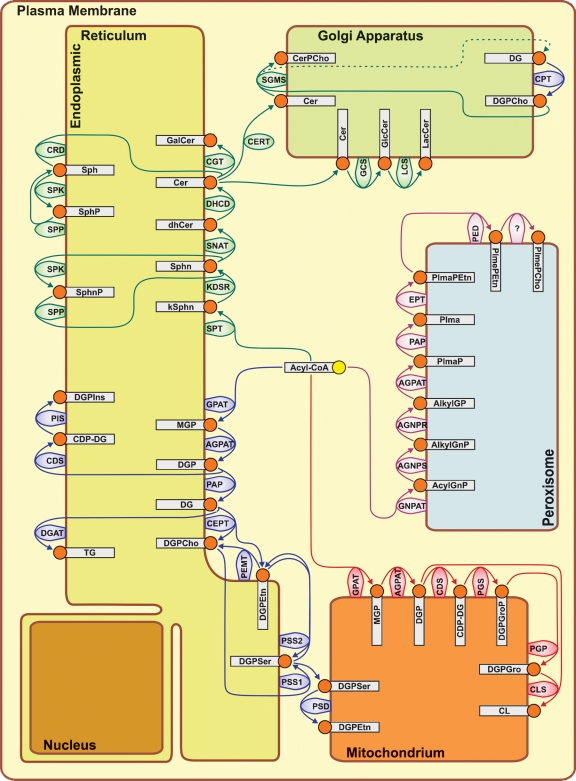
Phospholipids, including glycerophospholipids and sphingolipids, constitute the bulk lipid components of all mammalian membranes. Based on the information contained in several previous reviews (5–19), the phospholipid biosynthetic enzymes that produce membrane lipid products have been assigned to the different organelles in Fig. 1 according to where the majority of protein or activity for each has been measured. Enzymes involved in phospholipid degradation or remodeling are not addressed. Enzymes that synthesize unique glycerophospholipids, called plasmalogens, are included. Water soluble intermediates are not shown in the scheme, with the exception of fatty acyl-CoA, which is used as substrate by acyltransferase enzymes for the synthesis of both glycerolipids and sphingolipids in the ER, cardiolipin (CL) and phosphatidylglycerol (DGPGro) in mitochondria, and for the synthesis of plasmalogens (PlmePEtn and PlmePCho) in the peroxisomes. The family of acyltransferases is quite extended, and only a few isoforms are directly involved in the de novo synthesis of membrane lipids, while others are involved in the remodeling of acyl chains of the different lipid classes (5, 6). The acronyms for the different lipids are those proposed by the Lipid Maps project (20), and the corresponding nomenclature for the lipid biosynthetic enzymes are defined in Table 1. Different isoforms of the lipid biosynthetic enzymes are not pointed out unless they are known to have alternate substrate specificities. Rather, the goal is to obtain a holistic view of the overall process of membrane lipid biogenesis.
Fig. 1.
Compartmentalization of phospholipid biosynthetic activities. The abbreviations are listed in Table 1.
TABLE 1.
Enzyme and lipid abbreviations
| Enzymes (Symbol and Name) | Lipids (Symbol and Name) |
|---|---|
| AGPAT, 1-acyl-sn-glycerol-3-phosphate O-acyltransferase | AcylGnP, 1-acyl-glyceronephosphate |
| AGNPR, acyl/alkylglycerone-phosphate reductase | AlkylGnP, 1-alkyl-glyceronephosphate |
| AGNPS, alkylglycerone-phosphate synthase | AlkylGP, 1-alkyl-glycerophosphate |
| CDS, phosphatidate cytidylyltransferase | CDP-DG, CDP-diacylglycerol |
| CEPT, diacylglycerol choline/ethanolaminephosphotransferase | Cer, N-acylsphingosine (ceramide) |
| CERT, ceramide transfer protein | CerPCho, ceramide phosphocholine (sphingomyelin) |
| CGT, N-acylsphingosine galactosyltransferase | CL, diacylglycerophosphoglycerophosphodiradylglycerol |
| CPT, diacylglycerol cholinephosphotransferase | DG, diacylglycerol |
| CLS, cardiolipin synthase | dhCer, dihydroceramide |
| CRD, ceramidase | GalCer, galactosylceramide |
| DGAT, ciacylglycerol O-acyltransferase | GlcCer, glucosylceramide |
| DHCD, dihydroceramide δ(4)-desaturase | DGP, diacylglycerophosphate |
| EPT, ethanolaminephosphostransferase | DGPCho, diacylglycerophosphocholine |
| GCS, ceramide glucosyltransferase | DGPEtn, diacylglycerophosphoethanolamine |
| GNPAT, glycerone-phosphate O-acyltransferase | DGPGro, diacylglycerophosphoglycerol |
| GPAT, glycerol-3-phosphate O-acyltransferase | DGPGroP, diacylglycerophosphoglycerophosphate |
| KDSR, 3-ketosphinganine reductase | DGPIns, diacylglycerophosphoinositol |
| LCS, polypeptide N-acetylgalactosaminyltransferase | GPSer, diacylglycerophosphoserine |
| PAP, phosphatidic acid phosphatase | kSphn, 3-ketosphinganine |
| PED, plasmanylethanolamine desaturase | LacCer, lactosylceramide |
| PEMT, phosphatidylethanolamine N-methyltransferase | MGP, monoacylglycerophosphate |
| PGP, phosphatidylglycerophosphatase | PlmaH, 1-alkyl,2-acylglycerol |
| PGS, CDP-diacylglycerol-glycerol-3-phosphate 3-phosphatidyltransferase | PlmaP, 1-alkyl,2-acyl-glycerophosphate |
| PIS, CDP-diacylglycerol-inositol 3-phosphatidyltransferase | PlmaPEtn, 1-alkyl,2-acylglycerophosphosphoethanolamine (plasmanylethanolamine) |
| PSD, phostatidylserine decarboxylase | PlmePCho, 1Z-Alkenyl-2-acyl-glycerophosphocholine (plasmenylcholine) |
| PSS1, phosphatidylserine synthase 1 | PlmePEtn, 1Z-Alkenyl,2-acylglycerophosphosphoethanolamine (plasmenylethanolamine) |
| PSS2, phosphatidylserine synthase 2 | Sph, sphingosine |
| SGMS, ceramide cholinephosphotransferase | Sphn, sphinganine |
| SNAT, sphingosine N-acyltransferase | SphnP, sphinganine-1-phosphate |
| SPK, sphinganine kinase | SphP, sphingosine-1-phosphate |
| SPP, sphingosine-1-phosphate phosphatase | TG, triacylglycerol |
| SPT, serine C-palmitoyltransferase |
GPAT and AGPAT activities begin the process of glycerolipid synthesis by attaching the fatty acyl moieties to the 1-position and then the 2-position of glycerol-3-phosphate, respectively. GPAT and AGPAT activities are associated with the ER and the mitochondria, providing the diacylglycerol phosphate (DGP) precursor for phospholipids in both locations. In the ER compartment, the DGP is dephosphorylated by the phosphatidic acid phosphatase enzymes to yield diacylglycerol (DG), which is incorporated into phosphatidylcholine (DGPCho) and phosphatidylethanolamine (DGPEtn). The GPAT and AGPAT association with the mitochondria suggests that these activities provide the DGP precursor for the synthesis of phosphatidylglycerol (DGPGro) and CL located at the same site.
DGPCho is the most abundant glycerophospholipid species in mammalian cells, and it is synthesized in the ER and Golgi apparatus. Two biosynthetic pathways are available for DGPCho synthesis and are located in different endomembrane domains. The Kennedy pathway is the predominant route to DGPCho in most cells, and the final step is catalyzed by the bifunctional CEPT, which is located in the ER, or alternatively by the CPT, which is located in the Golgi apparatus (8, 9). Both the CEPT and the CPT use DG to form DGPCho. The PEMT activity, which converts DGPEtn to DGPCho, is restricted to the mitochondrium-associated membrane.
DGPEtn is the second most abundant glycerophospholipid species, and its de novo synthesis can be catalyzed by the CEPT, located in the ER, or the EPT, a recently described isoform with strict specificity for DGPEtn production (21). An EPT activity has been described that is associated with peroxisomes (17). DGPEtn can also arise from head-group exchange with phosphatidylserine (DGPSer) in the ER as mediated by PSS2 or in the mitochondria by DGPSer decarboxylation, mediated by PSD (10). Differently from DGPCho and DGPEtn, DGPSer is synthesized in the ER through head-group exchange of already-made DGPCho and DGPEtn as catalyzed by PSS1 and PSS2, respectively (10). Triacylglycerol has no structural role but serves primarily as a storage lipid and is also synthesized in the ER (7). Phosphatidylinositol (DGPIns) is synthesized in the ER by the PIS, and, apart from phosphatidylinositol-4-phosphate, its conversion into the highly phosphorylated forms, which play critical roles in signaling and membrane vesicle trafficking, occurs outside the ER (11, 12, 15, 22). CL is present only in the mitochondria, where it is absolutely required for energy production, and its synthesis is restricted to the inner mitochondrial membrane (13, 14, 16). Plasmalogens, which are vinyl-ether linked at the 1-position of the glycerophospholipid, are an important class of lipids, and they contribute almost 18% to the total lipid mass in humans. Among plasmalogens, plasmenylethanolamine (PlmePEtn) is the most abundant and it is also the precursor to plasmenylcholine (PlmePCho). Plasmalogen synthesis occurs in the peroxisomes, where the key enzyme GNPAT initiates formation using a fatty alcohol to yield AlkylGnP (17).
Sphingolipid synthesis spans from the ER, where it begins, to the Golgi complex, where it ends (18, 19). Synthesis of the sphingosine and ceramide (Cer) intermediates occurs in the ER. Cer is then transferred to the Golgi apparatus in two manners, and each mode determines whether Cer is converted into either sphingomyelin (CerPCho) or glucosylceramide (GlcCer) and lactosylceramide (LacCer). Lipids such as DGPCho and DGPIns can be synthesized in the nuclear matrix apart from the nuclear envelope, but information about the exact location of the nuclear enzymes is limited (23).
Although different lipids are synthesized in different organelles, they are widely distributed within the cell and the membrane composition of the different organelles does not necessarily reflect their lipid biosynthetic capacity. DGPCho is synthesized in the endomembrane compartment and in the nuclear compartment in immortalized cells, but it is present everywhere in the cell. DGPSer and CerPCho are synthesized in the ER and Golgi, but they are highly abundant in the plasma membrane. PlmePEtn is synthesized in the peroxisomes but does not accumulate as it is primarily secreted. Within the same membrane, lipids are transported to or segregated into one of the two leaflets of the membrane by virtue of their chemical structure or by the action of enzymes called flippases, whose function is to favor or force the movement of specific lipids between the two leaflets of the membrane (24, 25). The transport of lipids to different membranes can occur through the vesicular pathways, which allow the transport of membrane to even distant cellular locations or by lipid-transfer proteins, a process that is particularly active and fast within membrane contact sites (MCS), where membrane regions from different organelles come in close proximity (within 10 nm) to one another (24). For example, the ER is known to generate MCS structures with mitochondria, plasma membrane, the Golgi apparatus, endosomes, and other organelles. The means by which DGPCho and DGPEtn are transported to the peroxisomes is still unknown, and MCS structures as well as vesicles may be responsible for mediating the process.
REGULATION OF MEMBRANE PHOSPHOLIPID SYNTHESIS AT THE ER
A number of proteins that play a determining role in membrane phospholipid biogenesis are localized to the endomembrane compartment. The rate-limiting enzyme in DGPCho synthesis is the phosphocholine cytidylyltransferase (CCT) (26). The CCT is predominantly located in the nucleus of immortalized cells, but in primary cells, the CCT is almost exclusively found outside the nucleus and in association with the endomembrane compartment (1, 2, 27–29). CCT is not included in Fig. 1 because its substrates and products are water soluble, but the enzyme controls the rate of DGPCho formation through its peripheral association with the cytoplasmic aspect of the endomembrane compartment (26). The CCT activity responds to changes in membrane lipid composition and governs whether more or less DGPCho is made as a function of the proximal accumulation of membrane lipid metabolic products (30).
The ER stress response is a complex signal transduction pathway emanating from the ER membrane that is activated by the perturbation of normal ER metabolism. Lumenal proteins that are properly folded and assembled can leave the ER. Those that are misfolded or incompletely assembled remain in the ER where they disrupt ER homeostasis and cause ER stress. Thus, the ER stress response is oftentimes called the unfolded protein response (UPR) (31). The UPR relieves ER stress by repressing translation, increasing expression of ER chaperones and folding enzymes, and enhancing ER-associated degradation. The UPR has been most extensively studied as it relates to protein quality control in the ER, but membrane lipid metabolism appears to be equally important. Inhibition of DGPCho synthesis leads to DGPCho depletion, activation of some components of the ER stress response, and cell death in cultured fibroblasts (32). Alteration of the ER lipid composition by accumulation of free cholesterol initiates ER stress in macrophages (33, 34), and deficient DGPCho synthesis renders macrophages more sensitive to free cholesterol overload (35). Thus, disruption of membrane lipid homeostasis is a trigger, either directly or indirectly, and mechanisms to reestablish ER lipid composition are components of the response.
Upon induction of the UPR, the mRNA encoding the X-box binding protein 1 (XBP-1) is spliced to form XBP-1(S), which encodes a transcription factor (36). The site of splicing is at the ER membrane, where a transmembrane kinase/endonuclease called IRE1α modifies XBP1 transcripts upon accumulation of misfolded lumenal proteins. XBP-1(S) stimulates the expression of a host of genes, including those that encode the enzymes of the protein translational machinery and vesicular trafficking, and also stimulates membrane phospholipid synthesis (37, 38). The role of XBP-1(S) in initiating the program of membrane biogenesis was discovered in the context of the terminal differentiation of B lymphocytes into antibody-secreting plasma cells (39). Stimulated lymphocytes greatly increase the size of the endomembrane compartment, which is accomplished by a program of lipid biosynthetic gene expression. Elevated gene expression, in turn, increases the abundance of membrane glycerophospholipid precursors that also allosterically activate the CCT (1).
SECRETORY CELLS AND SECRETION
Secretion is a major function of the endomembrane compartment. Most cells have scanty regions of ER, but in certain specialized secretory cells, the ER is abundant. The rough ER is highly developed in pancreatic acinar cells that secrete copious amounts of digestive enzymes (40) as well as in hepatocytes, the principal site of production of lipoprotein particles, which carry lipids via the bloodstream to other parts of the body. XBP-1(S) is a regulator of DGPCho synthesis and ER membrane development, and constitutive expression of XBP-1 is essential for the proper development of professional secretory cells, such as pancreatis islets (41) and hepatocytes (42).
The class of professional secretory cells includes macrophages that have extensive biosynthetic capabilities that, upon stimulation, result in the synthesis and secretion of complement components and cytokines. The generation of polyphosphorylated DGPIns, DG, and DGP can regulate secretion events (43–48). The DGPIns and DG play signaling roles, and DGP is suggested to change local membrane curvature and promote vesicle formation. In addition, CCT and DGPCho synthesis play an essential role in vesicle budding from the Golgi apparatus and bulk secretion of selected macrophage proteins (2). The CCT enzyme is localized to the trans-Golgi network in the primary macrophages where it responds to local changes in membrane composition during secretion. On the other hand, inactivation of CCT stimulates secretion from proliferating immortalized HeLa cells with insufficient DG (49). These results point out a possible interplay between DG supply and DGPCho synthesis that needs to be balanced to support secretion.
SUMMARY
Bulk membrane lipid biogenesis in primary cells largely occurs in the endomembrane compartment, which includes the domains of the ER and Golgi apparatus. Specialized phospholipids are synthesized in mitochondria or perosixomes. Two proteins involved in the regulation of membrane phospholipid biogenesis are associated with the endomembrane compartment, namely, the CCT enzyme in the pathway for DGPCho, and the XBP-1(S) transcription factor. Activation of either protein occurs in response to changes in ER lipid or protein composition, respectively. ER membrane biogenesis can occur during developmental differentiation of secretory cells or in immune cells during the response to stimulation. De novo membrane phospholipid synthesis in differentiated cells is linked with secretion from the Golgi apparatus. The future challenge will be to sort out the relative importance of lipid synthesis or composition in the regulation of cellular events.
Abbreviations
CCT, cytidylyltransferase
Cer, ceramide
CL, cardiolipin
DG, diacylglycerol
DGP, diacylglycerol phosphate
ER, endoplasmic reticulum
ERGIC, ER-Golgi intermediate compartment
MCS, membrane contact site
UPR, unfolded protein response
XBP-1, X-box binding protein 1
This work was supported by National Institutes of Health Grant GM-45737, by Cancer Center Support Grant CA21765, and by the American Lebanese Syrian Associated Charities.
Published, JLR Papers in Press, October 23, 2008.
References
- 1.Fagone P., R. Sriburi, C. Ward-Chapman, M. Frank, J. Wang, C. Gunter, J. W. Brewer, and S. Jackowski. 2007. Phospholipid biosynthesis program underlying membrane expansion during B lymphocyte differentiation. J. Biol. Chem. 282 7591–7605. [DOI] [PubMed] [Google Scholar]
- 2.Tian Y., C. Pate, A. Andreolotti, L. Wang, E. Tuomanen, K. Boyd, E. Claro, and S. Jackowski. 2008. Cytokine secretion requires phosphatidylcholine synthesis. J. Cell Biol. 181 945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuura S., R. Masuda, O. Sakai, and Y. Tashiro. 1983. Immunoelectron microscopy of the outer membrane of rat hepatocyte nuclear envelopes in relation to the rough endoplasmic reticulum. Cell Struct. Funct. 8 1–9. [DOI] [PubMed] [Google Scholar]
- 4.Lavoie C., and J. Paiement. 2008. Topology of molecular machines of the endoplasmic reticulum: a compilation of proteomics and cytological data. Histochem. Cell Biol. 129 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shindou H., and T. Shimizu. 2008. Acyl-CoA: lysophospholipid acyltransferases. J. Biol. Chem. 284 1–5. [DOI] [PubMed] [Google Scholar]
- 6.Yen C. L., S. J. Stone, S. Koliwad, C. Harris, and R. V. Farese, Jr. 2008. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 49 2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman R. A., and D. P. Lee. 2004. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 43 134–176. [DOI] [PubMed] [Google Scholar]
- 8.Henneberry A. L., M. M. Wright, and C. R. McMaster. 2002. The major sites of cellular phospholipid synthesis and molecular determinants of fatty acid and lipid head group specificity. Mol. Biol. Cell. 13 3148–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vance D. E., C. J. Walkey, and Z. Cui. 1997. Phosphatidylethanolamine N-methyltransferase from liver. Biochim. Biophys. Acta. 1348 142–150. [DOI] [PubMed] [Google Scholar]
- 10.Vance J. E. 2008. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J. Lipid Res. 49 1377–1387. [DOI] [PubMed] [Google Scholar]
- 11.Antonsson B. 1997. Phosphatidylinositol synthase from mammalian tissues. Biochim. Biophys. Acta. 1348 179–186. [DOI] [PubMed] [Google Scholar]
- 12.Heacock A. M., and B. W. Agranoff. 1997. CDP-diacylglycerol synthase from mammalian tissues. Biochim. Biophys. Acta. 1348 166–172. [DOI] [PubMed] [Google Scholar]
- 13.Schlame M., D. Rua, and M. L. Greenberg. 2000. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 39 257–288. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki K., O. Kuge, Y. Yamakawa, and M. Nishijima. 2001. Purification of phosphatidylglycerophosphate synthase from Chinese hamster ovary cells. Biochem. J. 354 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inglis-Broadgate S. L., L. Ocaka, R. Banerjee, M. Gaasenbeek, J. P. Chapple, M. E. Cheetham, B. J. Clark, D. M. Hunt, and S. Halford. 2005. Isolation and characterization of murine Cds (CDP-diacylglycerol synthase) 1 and 2. Gene. 356 19–31. [DOI] [PubMed] [Google Scholar]
- 16.Chen D., X. Y. Zhang, and Y. Shi. 2006. Identification and functional characterization of hCLS1, a human cardiolipin synthase localized in mitochondria. Biochem. J. 398 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagan N., and R. A. Zoeller. 2001. Plasmalogens: biosynthesis and functions. Prog. Lipid Res. 40 199–229. [DOI] [PubMed] [Google Scholar]
- 18.Merrill A. H., Jr. 2002. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277 25843–25846. [DOI] [PubMed] [Google Scholar]
- 19.Futerman A. H., and H. Riezman. 2005. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 15 312–318. [DOI] [PubMed] [Google Scholar]
- 20.Fahy E., S. Subramaniam, H. A. Brown, C. K. Glass, A. H. Merrill, Jr., R. C. Murphy, C. R. Raetz, D. W. Russell, Y. Seyama, W. Shaw, et al. 2005. A comprehensive classification system for lipids. J. Lipid Res. 46 839–861. [DOI] [PubMed] [Google Scholar]
- 21.Horibata Y., and Y. Hirabayashi. 2006. Identification and characterization of a human ethanolaminephosphotransferase1 (hEPT1). J. Lipid Res. 48 503–508. [DOI] [PubMed] [Google Scholar]
- 22.Di Paolo G., and P. De Camilli. 2006. Phosphoinositides in cell regulation and membrane dynamics. Nature. 443 651–657. [DOI] [PubMed] [Google Scholar]
- 23.Hunt A. N. 2006. Dynamic lipidomics of the nucleus. J. Cell. Biochem. 97 244–251. [DOI] [PubMed] [Google Scholar]
- 24.Holthuis J. C., and T. P. Levine. 2005. Lipid traffic: floppy drives and a superhighway. Nat. Rev. Mol. Cell Biol. 6 209–220. [DOI] [PubMed] [Google Scholar]
- 25.van Meer G., D. R. Voelker, and G. W. Feigenson. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackowski S., and P. Fagone. 2005. CTP: Phosphocholine cytidylyltransferase: paving the way from gene to membrane. J. Biol. Chem. 280 853–856. [DOI] [PubMed] [Google Scholar]
- 27.Ridsdale R., I. Tseu, J. Wang, and M. Post. 2001. CTP:phosphocholine cytidylyltransferase alpha is a cytosolic protein in pulmonary epithelial cells and tissues. J. Biol. Chem. 276 49148–49155. [DOI] [PubMed] [Google Scholar]
- 28.Tian Y., R. Zhou, J. E. Rehg, and S. Jackowski. 2007. Role of phosphocholine cytidylyltransferase a in lung development. Mol. Cell. Biol. 27 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houweling M., Z. Cui, C. D. Anfuso, M. Bussière, M. H. Chen, and D. E. Vance. 1996. CTP:phosphocholine cytidylyltransferase is both a nuclear and cytoplasmic protein in primary hepatocytes. Eur. J. Cell Biol. 69 55–63. [PubMed] [Google Scholar]
- 30.Attard G. S., R. H. Templer, W. S. Smith, A. N. Hunt, and S. Jackowski. 2000. Modulation of CTP:phosphocholine cytidylyltransferase by membrane curvature elastic stress. Proc. Natl. Acad. Sci. USA. 97 9032–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroder M., and R. J. Kaufman. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74 739–789. [DOI] [PubMed] [Google Scholar]
- 32.Van Der Sanden M. H., M. Houweling, L. M. van Golde, and A. B. Vaandrager. 2003. Inhibition of phosphatidylcholine synthesis induces expression of the endoplasmic reticulum stress and apoptosis-related protein CCAAT/enhancer-binding protein-homologous protein (CHOP/GADD153). Biochem. J. 369 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devries-Seimon T., Y. Li, P. M. Yao, E. Stone, Y. Wang, R. J. Davis, R. Flavell, and I. Tabas. 2005. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 171 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng B., P. M. Yao, Y. Li, C. M. Devlin, D. Zhang, H. P. Harding, M. Sweeney, J. X. Rong, G. Kuriakose, E. A. Fisher, et al. 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5 781–792. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D., W. Tang, P. M. Yao, C. Yang, B. Xie, S. Jackowski, and I. Tabas. 2000. Macrophages deficient in CTP:phosphocholine cytidylyltransferase-a are viable under normal culture conditions but are highly susceptible to free cholesterol-induced death. Molecular genetic evidence that the induction of phosphatidylcholine biosynthesis in free cholesterol-loaded macrophages is an adaptive response. J. Biol. Chem. 275 35368–35376. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto K., T. Sato, T. Matsui, M. Sato, T. Okada, H. Yoshida, A. Harada, and K. Mori. 2007. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6a and XBP1. Dev. Cell. 13 365–376. [DOI] [PubMed] [Google Scholar]
- 37.Sriburi R., S. Jackowski, K. Mori, and J. W. Brewer. 2004. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 167 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sriburi R., H. Bommiasamy, G. L. Buldak, G. R. Robbins, M. Frank, S. Jackowski, and J. W. Brewer. 2007. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J. Biol. Chem. 282 7024–7034. [DOI] [PubMed] [Google Scholar]
- 39.Shaffer A. L., M. Shapiro-Shelef, N. N. Iwakoshi, A. H. Lee, S. B. Qian, H. Zhao, X. Yu, L. Yang, B. K. Tan, A. Rosenwald, et al. 2004. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 21 81–93. [DOI] [PubMed] [Google Scholar]
- 40.Bolender R. P. 1974. Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J. Cell Biol. 61 269–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee A. H., G. C. Chu, N. N. Iwakoshi, and L. H. Glimcher. 2005. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24 4368–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee A. H., E. F. Scapa, D. E. Cohen, and L. H. Glimcher. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 320 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roth M. G. 1999. Lipid regulators of membrane traffic through the Golgi complex. Trends Cell Biol. 9 174–179. [DOI] [PubMed] [Google Scholar]
- 44.Huijbregts R. P., L. Topalof, and V. A. Bankaitis. 2000. Lipid metabolism and regulation of membrane trafficking. Traffic. 1 195–202. [DOI] [PubMed] [Google Scholar]
- 45.Freyberg Z., A. Siddhanta, and D. Shields. 2003. “Slip, sliding away”: phospholipase D and the Golgi apparatus. Trends Cell Biol. 13 540–546. [DOI] [PubMed] [Google Scholar]
- 46.Wenk M. R., and C. P. De. 2004. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. USA. 101 8262–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munro S. 2005. The Golgi apparatus: defining the identity of Golgi membranes. Curr. Opin. Cell Biol. 17 395–401. [DOI] [PubMed] [Google Scholar]
- 48.Lev S. 2006. Lipid homoeostasis and Golgi secretory function. Biochem. Soc. Trans. 34 363–366. [DOI] [PubMed] [Google Scholar]
- 49.Litvak V., N. Dahan, S. Ramachandran, H. Sabanay, and S. Lev. 2005. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat. Cell Biol. 7 225–234. [DOI] [PubMed] [Google Scholar]