Abstract
Cyclooxygenase (COX; prostaglandin G/H synthase, EC 1.14.99.1) catalyzes the first two steps in the biosynthesis of prostaglandins (PGs). The two COX isoforms COX-1 and COX-2 are the targets of the widely used nonsteroidal anti-inflammatory drugs, indicating a role for these enzymes in pain, fever, inflammation, and tumorigenesis. The ubiquitous constitutive expression of COX-1 and inducible expression of COX-2 have led to the widely held belief that COX-1 produces homeostatic PGs, while PGs produced by COX-2 are primarily pathophysiological. However, recent discoveries call this paradigm into question and reveal as yet underappreciated functions for both enzymes. This review focuses on some of these new insights.
Keywords: prostaglandin, endocannabinoid, inflammation, nonsteroidal anti-inflammatory, coxib, hydroperoxide, arachidonic acid, essential fatty acid
The cyclooxygenase isoforms (COX-1 and COX-2) are among the most thoroughly studied and best understood mammalian oxygenases. Possessing two separate but linked active sites, the COXs catalyze the bis-dioxygenation and subsequent reduction of arachidonic acid (AA) to prostaglandin (PG)G2 and PGH2 (Fig. 1A). The mechanism of oxygenation has been well characterized through kinetics, mutagenesis, and X-ray crystallography (1–3). PGH2 is subject to metabolism by downstream enzymes yielding the family of PGs, each member of which exerts a range of physiologic effects through specific G-protein-coupled receptors (Fig. 1B) (4, 5). The discovery that the COXs are the target of the nonsteroidal anti-inflammatory drugs (NSAIDs), which play a primary therapeutic role in the treatment of pain, fever, and inflammation (6), promulgated the first wave of experimentation on the constitutively expressed COX-1 during the 1970s and 1980s. Then, just as interest began to wane, the discovery of the inducible isoform, COX-2, rekindled a massive new effort that ultimately led to new insights about both isoforms. A search of PubMed over the past 2 years indicates that there have been over 70 review articles containing “cyclooxygenase” in their title, leading one to question the need for yet another. However, despite the overwhelming mass of data available on these enzymes, recent discoveries suggest that some original assumptions concerning their roles in physiology and pathophysiology require reexamination. This review will emphasize these issues.
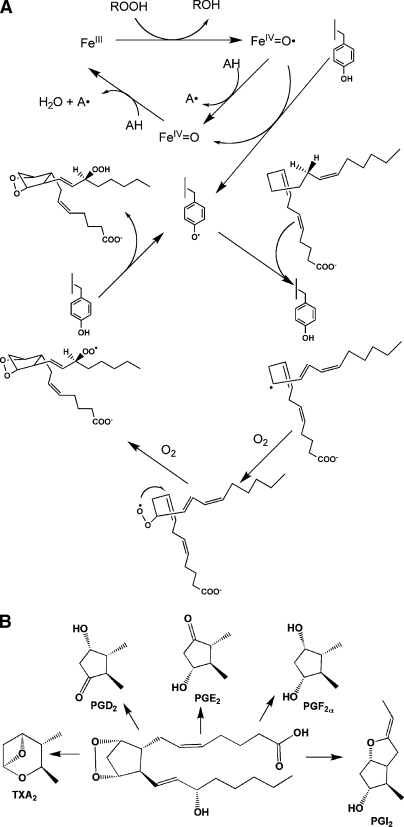
Fig. 1.
The cyclooxygenase reaction. A: The peroxidase cycle leads to abstraction of a hydrogen atom from Tyr-385 forming a tyrosyl radical and activating the cyclooxygenase active site. The tyrosyl radical abstracts the pro-S hydrogen atom from carbon-13 of AA, initiating the cyclooxygenase reaction, the final step of which regenerates the tyrosyl radical. B: Conversion of PGH2 to the biologically active PGs.
STRUCTURE OF THE COX ENZYMES
Human COX-1 and COX-2 are homodimers of 576 and 581 amino acids, respectively. Both enzymes contain three high mannose oligosaccharides, one of which facilitates protein folding. A fourth oligosaccharide, present only in COX-2, regulates its degradation. Considering the 60% identity in sequence between COX-1 and COX-2, it is not surprising that their three-dimensional structures are nearly superimposable. Each subunit of the dimer consists of three domains, the epidermal growth factor domain (residues 34–72), the membrane binding domain (residues 73–116), and the catalytic domain comprising the bulk of the protein, which contains the cyclooxygenase and peroxidase active sites on either side of the heme prosthetic group (1, 2, 7, 8).
On the opposite side of the protein from the membrane binding domain, the peroxidase active site consists of the heme positioned at the bottom of a shallow cleft. This structure provides considerable solvent accessibility to the heme with the exception of a cluster of several hydrophobic amino acids that form a dome over part of the cleft. The structure of the active site helps to explain the promiscuous substrate specificity of the COX peroxidase, which reduces a wide range of primary and secondary organic hydroperoxides (9). Although the hydrophobic dome would appear to explain the preference of the peroxidase for organic peroxides over H2O2, mutation of the dome residues to alanine has little effect on peroxidase activity or substrate specificity (10).
The cyclooxygenase active site lies on the opposite side of the heme from the peroxidase active site at the top of an L-shaped channel that originates in the membrane binding domain. The mouth of the channel consists of the lobby, a large volume that narrows to a constriction that must open before substrates or inhibitors can pass deeper into the channel. Above the constriction, the channel is surrounded by hydrophobic residues, which outline the nearly right angle bend and the narrow terminus. When AA binds in the cyclooxygenase active site, it lies with its carboxyl group at the constriction and its ω-methyl group at the narrow terminus of the channel. This places carbon-13 of AA at the bend in the channel in close proximity to Tyr-385, which is the critical catalytic amino acid for the cyclooxygenase reaction (1, 2, 8).
Mechanism of the cyclooxygenase reaction
The first step in the conversion of AA to the hydroperoxy-endoperoxide, PGG2, is abstraction of the pro-S hydrogen atom from carbon-13. The steps that follow (Fig. 1A) are consistent with the mechanism of nonenzymatic lipid peroxidation, so the main contributions of COX to PGG2 formation are to restrict the options for hydrogen abstraction and dictate reaction stereochemistry. Cyclooxygenase catalysis requires that the enzyme first be activated, a process dependent on the peroxidase activity. Two-electron reduction of a peroxide substrate results in the oxidation of the ferric heme to an oxo-ferryl porphyrin radical cation. Transfer of an electron to the heme from Tyr-385 of the protein generates a tyrosyl radical in the cyclooxygenase active site. This radical, as noted above, is positioned perfectly to abstract the pro-S hydrogen from carbon-13 of AA, initiating the cycloxygenase reaction (Fig. 1A). The final step of the reaction, reduction of the peroxyl radical to the hydroperoxide to form PGG2, regenerates the tyrosyl radical. Thus, activated COX can carry out multiple turnovers without need to repeat the activation step. After initiating the cyclooxygenase reaction, the primary function of the peroxidase is to reduce the 15-hydroperoxy of PGG2 to the corresponding alcohol of PGH2 (1–3).
COX-1 AND COX-2: STRUCTURAL AND FUNCTIONAL DIFFERENCES
COX-1 is widely distributed and constitutively expressed in most tissues where it is found. Its gene, Ptgs-1, codes for a 2.8 kb mRNA, which is relatively stable. By contrast, the gene for COX-2, Ptgs-2, is an immediate early gene that is activated by a wide variety of inflammatory and proliferative stimuli, and the 4 kb COX-2 mRNA turns over rapidly due to the presence of instability sequences in the 3′-untranslated region (1, 2). The difference in the pattern of gene expression provides an obvious explanation for the existence of the two COX isoforms, suggesting that COX-1 provides PGs that are required for homeostatic functions, including gastric cytoprotection and hemostasis, whereas COX-2 plays the predominant role in PG formation during pathophysiologic states, such as inflammation and tumorigenesis. This “COX-2 hypothesis” drove the rapid development of selective COX-2 inhibitors, which were predicted to have anti-inflammatory activity without the gastrointestinal side effects of traditional NSAIDs (11, 12). However, continued studies with mice genetically deficient in COX-1 or COX-2 as well as clinical experience with COX-2 selective inhibitors (see below) have raised questions about this widely accepted paradigm of COX function.
Differential expression of COX-1 and COX-2
Autopsy samples from 20 healthy human trauma victims show relatively uniform COX-1 expression in nearly all tissues, with most of the protein localized to the blood vessels, smooth muscle cells, interstitial cells, platelets, and mesothelial cells. Although more highly variable, COX-2 protein was found in nearly all tissues and was most often localized to parenchymal cells (13). Constitutive COX-2 expression is well recognized in brain, kidney, and the female reproductive tract, and evidence for induction of COX-1 during the lipopolysaccharide (LPS)-mediated inflammatory response and cellular differentiation has been reported (14–18). Although these data generally support the COX-2 hypothesis, they also show that expression of neither enzyme fully fits the paradigm.
Physiologic versus pathophysiologic roles
COX-1 is constitutively expressed in resident inflammatory cells, and results of studies from COX-1 knockout mice and selective inhibitors confirm a role for COX-1 in multiple inflammatory models. Adding complexity are the findings that COX-2 deletion or inhibition may lead to reduction or exacerbation of the inflammatory response, depending on the model. COX-2 appears to play a significant role in resolution of inflammation, a role that is important in the healing of gastric ulcers. This latter finding, combined with the fact that COX-1 knockout mice do not display increased susceptibility to ulceration and that COX-1 selective inhibitors do not induce gastric lesions, calls into question the putative gastric cytoprotective role usually ascribed to COX-1. In fact, ulceration is observed with a combination of COX-1 and COX-2 selective inhibitors, indicating that reduction in the total levels of PGs is more important than inhibition of PGs generated from a particular COX enzyme. Recent studies suggest that the roles of the isoforms are reversed in the brain because COX-1 knockout mice exhibit a reduced inflammatory response to intrathecal LPS, whereas COX-2 knockout mice display exacerbated inflammation (16, 19–21).
Studies with COX-2 knockout mice demonstrate homeostatic functions for this isoform. Genetic deletion of COX-2 produces a severe disruption of postnatal kidney development, and female knockout mice are infertile due to failure of ovulation and embryo implantation (15, 16). As noted below, the cardiotoxicity associated with the prolonged use of COX-2 selective inhibitors confirms a homeostatic role for COX-2 in the cardiovascular system (22, 23). Thus, it is clear that the original COX-2 hypothesis ascribing a homeostatic function to COX-1 and a pathophysiologic function to COX-2 is oversimplified and in some cases completely erroneous.
Differential functions of the COX proteins
If the only basis for the differences between the COX isoforms was their differential gene expression, then replacing the gene for COX-2 with that for COX-1 should produce no noticeable phenotype. However, “knockin” of the COX-1 gene into the COX-2 locus in mice only partially replenishes the deficit in PGI2 synthesis and fails to fully ameliorate defects in reproductive and renal function associated with COX-2 deletion (24). These results clearly indicate that COX-1 and COX-2 are not functionally interchangeable at the protein level.
One basis for the results of the COX-1 knockin study may lie in differential coupling between the two COX proteins and downstream synthases. For example, lack of full restoration of PGI2 synthesis by COX-1 knockin may be due to a failure of coupling between COX-1 with PGI synthase. Numerous studies support selective isoform association (25, 26), but much of this work has been done with cells overexpressing the relevant enzymes, and no basis for the differential coupling has been advanced. Therefore, confirmation of this hypothesis awaits further investigation.
An alternative explanation for the difference in isoform function may be that COX-2 requires lower concentrations of hydroperoxide for activation than does COX-1 (27). Although this difference does not usually affect kinetic parameters measured in vitro, within the reducing environment of the intact cell, it translates into an ability of COX-2 to function at lower AA concentrations than COX-1 (24, 26, 28). The structural and mechanistic bases for the difference in hydroperoxide requirement are not fully understood, but site-directed mutagenesis studies indicate that Thr-383, a residue near the heme in COX-2, is at least partly responsible for its greater hydroperoxide sensitivity (25).
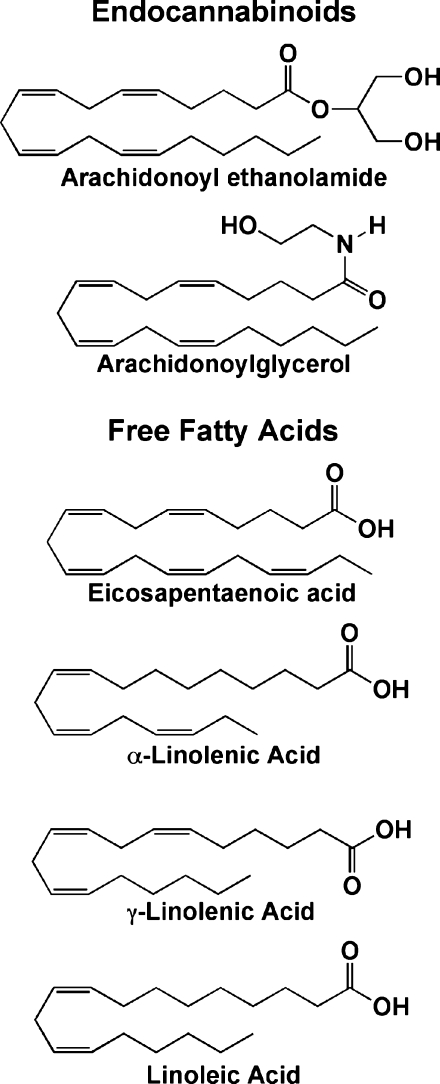
A third explanation for the differences in function between COX-1 and COX-2 may lie in COX-2's wider substrate specificity. For example, COX-2 is capable of metabolizing ester and amide derivatives of AA that are poor substrates for COX-1 (29). Of particular interest are the glyceryl ester and ethanolamide of AA, 2-arachidonoylglycerol (2-AG) and arachidonoyl ethanolamide, respectively, which are endogenous ligands for the CB1 and CB2 cannabinoid receptors (Fig. 2). The products of 2-AG and arachidonoyl ethanolamide metabolism by COX-2 are the glyceryl ester and ethanolamide derivatives of PGH2 (PGH2-G and PGH2-EA, respectively), which are subject to further metabolism by the same enzymes that metabolize PGH2, with the exception of thromboxane synthase. Thus, formation of glyceryl ester or ethanolamide analogs of PGE2, PGF2α, PGD2, and PGI2 is possible, depending on the enzymes present in the environment.
Fig. 2.
Selective COX-2 substrates. The structures of endocannabinoids and free fatty acids that are metabolized more efficiently by COX-2 than by COX-1 are shown.
Prostaglandin gyceryl esters (PG-Gs) are subject to hydrolysis by esterases present in blood and tissues, which confounds efforts to detect them in vivo. Nevertheless, low levels of PG-Gs have been observed in rat paw tissue and in cultures of LPS-pretreated murine resident peritoneal macrophages and RAW264.7 cells stimulated with zymosan and ionomycin, respectively. These results suggest that PG-Gs may be produced under physiological or pathophysiological conditions. A growing number of studies suggest a physiologic role for COX-2-dependent 2-AG oxygenation, including evidence of calcium mobilization through distinct and novel receptors, activation of the peroxisome proliferator-activated receptor δ, and regulation of endocannabinoid tone (29–31).
Differences in substrate specificity between the COX isoforms are not limited to neutral derivatives of 20:4. Indeed, COX-2 has greater capacity to oxygenate a number of polyunsaturated free fatty acids that are poor substrates for COX-1 (Fig. 2) (26, 32). Since these include ω-3 fatty acids, it is possible that the differential use of these lipids by the COX enzymes may help to explain the health benefits of dietary ω-3 fatty acids, a hypothesis that remains an intriguing subject for future work (33).
A final difference in COX isoform function that may be of physiologic significance lies in the ability of the aspirin-treated enzymes to oxygenate AA. Aspirin inhibits PG formation by both isoforms via covalent modification of Ser-530 in the cyclooxygenase active site. However, COX-2 retains the capacity to oxygenate AA, resulting in the formation of 15R-hydroperoxy-eicosatetraenoic acid instead of PGG2 (34). Through this lipoxygenase-type reaction, aspirin-treated COX-2, together with other lipoxygenases, forms polyhydroxylated lipids known as aspirin-triggered lipoxins and resolvins. Since these lipids have anti-inflammatory activity, their production may help to explain some of the clinical benefits of aspirin (35).
COX INHIBITION
COX-2-selective inhibitors: clinical experience
Dating to Egyptian times, the clinical use of NSAIDs far preceded the characterization of COX as their molecular target (6). Aspirin is the only clinically used NSAID that covalently modifies the COX protein. All other NSAIDs act noncovalently and most can be classified as either rapidly reversible, competitive inhibitors or slow, tight binding inhibitors. The kinetics and mechanism of inhibitor binding were recently reviewed in detail (36). Despite the fact that NSAIDs block all PG synthesis, they are efficacious and relatively safe drugs. However, a range of hazardous side effects, of which gastrointestinal toxicity is of primary clinical importance, preclude NSAID use in highly sensitive patients. Based on the COX-2 hypothesis, the obvious solution to the gastrointestinal toxicity of NSAIDS was the development of selective COX-2 inhibitors. The resultant massive effort in the pharmaceutical industry placed new drugs, the coxibs, on the market within 8 years after the discovery of COX-2 (23).
Although more recent data question the basic premise of the COX-2 hypothesis, the coxibs, including celecoxib (Celebrex), rofecoxib (Vioxx), and valdecoxib (Bextra), proved to have good anti-inflammatory activity, and some exhibited reduced gastrointestinal toxicity. Aggressive marketing resulted in widespread use even among patients who did not experience gastrointestinal toxicity with NSAIDs. Reports of increased COX-2 expression in numerous forms of cancer led to several large-scale clinical trials exploring the benefits of chronic celecoxib or rofecoxib to prevent colon polyp recurrence. Those studies confirmed the value of coxibs for this purpose, but also revealed significant cardiovascular toxicity associated with chronic use (2–4% of patients after 3 years). This finding eventually led to the removal of rofecoxib and valdecoxib from the market (23).
The preponderance of data suggests that the cardiotoxicity of COX-2-selective inhibitors is mechanism-based and likely associated with the inhibition of PGI2 synthesis in the blood vessel wall. However, the fact that only a relatively small percentage of patients suffered cardiovascular side effects after 3 years of drug exposure suggests that other factors also contribute. The discovery of the cardiovascular toxicity of COX-2-selective inhibitors refutes the initial hypothesis that COX-2 plays no role in homeostasis. It also has led to concerns that traditional NSAIDs may carry unrecognized cardiovascular side effects. There are no well-controlled studies of the long-term toxicity of NSAIDs, but meta-analysis suggests the potential of some cardiovascular risk with these drugs (22, 23).
COX-2 inhibitors: future prospects
Following the removal of rofecoxib and valdecoxib from the market, COX-2 inhibitors were rejected almost as enthusiastically (and perhaps as irrationally) as they had been embraced. It is arguable that initially use of these drugs should have been restricted to people who suffered gastrointestinal toxicity from NSAIDs. Similarly, the relatively low incidence of cardiovascular events suggests that a large portion of the population can take COX-2-selective inhibitors safely, especially for short courses of therapy. Thus, a careful risk-benefit analysis might identify a population of patients for which COX-2-selective inhibitors should remain the drug of choice.
A possible application of COX-2-selective inhibitors may lie in the detection and/or treatment of cancer (37–39). As noted above, COX-2 expression is elevated in a large number of malignancies, so a COX-2 ligand should accumulate selectively in tumor tissue compared with surrounding normal tissue. Such a ligand, if appropriately labeled, could serve as an imaging agent for cancer diagnosis (40). Similar logic may be applied to the use of COX-2-directed ligands for the development of anti-cancer agents.
Promising results for the development of COX-2-directed imaging agents have been achieved through modification of coxibs to produce probes for PET and SPECT imaging (41–44). However, full realization of the potential of this approach requires the ability to attach a wide variety of imaging or therapeutic moieties to a COX-2 binding nucleus. Such flexibility has been achieved through the discovery that amidation or esterification of carboxylate-containing NSAIDs conveys COX-2 selectivity. Extensive work has shown that a large number of indomethacin primary and secondary amides and esters of great structural diversity are potent and selective COX-2 inhibitors in vitro and in vivo (36, 45). This implies that a large number of possible imaging or antitumor moieties may be easily tethered to indomethacin through amide or ester linkage with a strong likelihood of generating a selective COX-2 inhibitor.
CONCLUSIONS AND FUTURE PROSPECTS
From the perspective of enzymology and protein biochemistry, the study of the COX enzymes may be considered a mature field. There are few enzymes of lipid biochemistry for which there is such a wealth of structural and functional information. Yet, as the story of the discovery of COX-2 with the subsequent rise and fall of the coxibs so clearly illustrates, our most strongly supported paradigms must be subject to constant scrutiny. Ongoing work is presently focused on better defining the functional differences between the COX proteins and rationally evaluating an appropriate use for COX isoform-selective inhibition.
Abbreviations
AA, arachidonic acid
2-AG, 2-arachydonoylglycerol
COX, cyclooxygenase
LPS, lipopolysaccharide
NSAID, nonsteroidal anti-inflammmatory drug
PG, prostaglandin
PG-G, prostaglandin gyceryl ester
Research on COX structure and function in the authors' laboratory is supported by grants from the National Institutes of Health (CA-89450 and GM-15431).
Published, JLR Papers in Press, October 23, 2008.
References
- 1.Smith W. L., D. L. DeWitt, and R. M. Garavito. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69 145–182. [DOI] [PubMed] [Google Scholar]
- 2.Rouzer C. A., and L. J. Marnett. 2003. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 103 2239–2304. [DOI] [PubMed] [Google Scholar]
- 3.van der Donk W. A., A. L. Tsai, and R. J. Kulmacz. 2002. The cyclooxygenase reaction mechanism. Biochemistry. 41 15451–15458. [DOI] [PubMed] [Google Scholar]
- 4.Samuelsson B., M. Goldyne, E. Granstrom, M. Hamberg, S. Hammarstrom, and C. Malmsten. 1978. Prostaglandins and thromboxanes. Annu. Rev. Biochem. 47 997–1029. [DOI] [PubMed] [Google Scholar]
- 5.Hata A. N., and R. M. Breyer. 2004. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 103 147–166. [DOI] [PubMed] [Google Scholar]
- 6.Vane J. R. 1971. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 231 232–235. [DOI] [PubMed] [Google Scholar]
- 7.Mbonye U. R., C. Yuan, C. E. Harris, R. S. Sidhu, I. Song, T. Arakawa, and W. L. Smith. 2008. Two distinct pathways for cyclooxygenase-2 protein degradation. Biol. Chem. 283 8611–8623. [DOI] [PubMed] [Google Scholar]
- 8.Garavito R. M., M. G. Malkowski, and D. L. DeWitt. 2002. The structures of prostaglandin endoperoxide H synthases-1 and -2. Prostaglandins Other Lipid Mediat. 68–69 129–152. [DOI] [PubMed] [Google Scholar]
- 9.Kulmacz R. J., W. A. van der Donk, and A. L. Tsai. 2003. Comparison of the properties of prostaglandin H synthase-1 and -2. Prog. Lipid Res. 42 377–404. [DOI] [PubMed] [Google Scholar]
- 10.Liu J., S. A. Seibold, C. J. Rieke, I. Song, R. I. Cukier, and W. L. Smith. 2007. Prostaglandin endoperoxide H synthases: peroxidase hydroperoxide specificity and cyclooxygenase activation. J. Biol. Chem. 282 18233–18244. [DOI] [PubMed] [Google Scholar]
- 11.DuBois R. N., S. B. Abramson, L. Crofford, R. A. Gupta, L. S. Simon, B. A. Van de Putte, and P. E. Lipsky. 1998. Cyclooxygenase in biology and disease. FASEB J. 12 1063–1073. [PubMed] [Google Scholar]
- 12.Masferrer J. L., B. S. Zweifel, P. T. Manning, S. D. Hauser, K. M. Leahy, W. G. Smith, P. C. Isakson, and K. Seibert. 1994. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. USA. 91 3228–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zidar, N., K. Odar, D. Glavac, M. Jerse, T. Zupanc, and D. Stajer. Cyclooxygenase in normal human tissues - is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. Epub ahead of print. July 24, 2008; doi: 10.1111/j.1582-4934.2008.00430.x. [DOI] [PMC free article] [PubMed]
- 14.McAdam B. F., I. A. Mardini, A. Habib, A. Burke, J. A. Lawson, S. Kapoor, and G. A. FitzGerald. 2000. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J. Clin. Invest. 105 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsky P. E., P. Brooks, L. J. Crofford, R. DuBois, D. Graham, L. S. Simon, L. B. van de Putte, and S. B. Abramson. 2000. Unresolved issues in the role of cyclooxygenase-2 in normal physiologic processes and disease. Arch. Intern. Med. 160 913–920. [DOI] [PubMed] [Google Scholar]
- 16.Langenbach R., C. Loftin, C. Lee, and H. Tiano. 1999. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem. Pharmacol. 58 1237–1246. [DOI] [PubMed] [Google Scholar]
- 17.Wallace J. L., A. Bak, W. McKnight, S. Asfaha, K. A. Sharkey, and W. K. MacNaughton. 1998. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 115 101–109. [DOI] [PubMed] [Google Scholar]
- 18.Gudis K., and C. Sakamoto. 2005. The role of cyclooxygenase in gastric mucosal protection. Dig. Dis. Sci. 50 (Suppl. 1): S16–S23. [DOI] [PubMed] [Google Scholar]
- 19.Wallace J. L., W. McKnight, B. K. Reuter, and N. Vergnolle. 2000. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 119 706–714. [DOI] [PubMed] [Google Scholar]
- 20.Aid S., R. Langenbach, and F. Bosetti. 2008. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J. Neuroinflammation. 5 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi S. H., R. Langenbach, and F. Bosetti. 2008. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 22 1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosser T., S. Fries, and G. A. FitzGerald. 2006. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J. Clin. Invest. 116 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marnett L. J. 2009. The COXIB experience: a look in the rear-view mirror. Annu. Rev. Pharmacol. Toxicol. 49 265–290. [DOI] [PubMed] [Google Scholar]
- 24.Yu Y., J. Fan, Y. Hui, C. A. Rouzer, L. J. Marnett, A. J. Klein-Szanto, G. A. FitzGerald, and C. D. Funk. 2007. Targeted cyclooxygenase gene (ptgs) exchange reveals discriminant isoform functionality. J. Biol. Chem. 282 1498–1506. [DOI] [PubMed] [Google Scholar]
- 25.Ueno N., Y. Takegoshi, D. Kamei, I. Kudo, and M. Murakami. 2005. Coupling between cyclooxygenases and terminal prostanoid synthases. Biochem. Biophys. Res. Commun. 338 70–76. [DOI] [PubMed] [Google Scholar]
- 26.Smith W. L., and R. Langenbach. 2001. Why there are two cyclooxygenase isozymes. J. Clin. Invest. 107 1491–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulmacz R. J. 2005. Regulation of cyclooxygenase catalysis by hydroperoxides. Biochem. Biophys. Res. Commun. 338 25–33. [DOI] [PubMed] [Google Scholar]
- 28.Morita I. 2002. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 68–69 165–175. [DOI] [PubMed] [Google Scholar]
- 29.Kozak K. R., B. C. Crews, L. Ray, H. H. Tai, J. D. Morrow, and L. J. Marnett. 2001. Metabolism of prostaglandin glycerol esters and prostaglandin ethanolamides in vitro and in vivo. J. Biol. Chem. 276 36993–36998. [DOI] [PubMed] [Google Scholar]
- 30.Rouzer C. A., and L. J. Marnett. 2008. Non-redundant functions of cyclooxygenases: oxygenation of endocannabinoids. J. Biol. Chem. 283 8065–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh M., H. Wang, Y. Ai, E. Romeo, J. P. Luyendyk, J. M. Peters, N. Mackman, S. K. Dey, and T. Hla. 2007. COX-2 suppresses tissue factor expression via endocannabinoid-directed PPARdelta activation. J. Exp. Med. 204 2053–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laneuville O., D. K. Breuer, N. Xu, Z. H. Huang, D. A. Gage, J. T. Watson, M. Lagarde, D. L. DeWitt, and W. L. Smith. 1995. Fatty acid substrate specificites of human prostaglandin-endoperoxide H synthase-1 and -2. Formation of 12-hydroxy-(9Z,13E/Z,15Z)-octadecatrienoic acids from a-linolenic acid. J. Biol. Chem. 270 19330–19336. [DOI] [PubMed] [Google Scholar]
- 33.Smith W. L. 2005. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr. Opin. Cell Biol. 17 174–182. [DOI] [PubMed] [Google Scholar]
- 34.Rowlinson S. W., B. C. Crews, D. C. Goodwin, C. Schneider, J. K. Gierse, and L. J. Marnett. 2000. Spatial requirements for 15-(R)-hydroxy-5Z,8Z,11Z, 13E-eicosatetraenoic acid synthesis within the cyclooxygenase active site of murine COX-2: why acetylated COX-1 does not synthesize 15-(R)-HETE. J. Biol. Chem. 274 6586–6591. [DOI] [PubMed] [Google Scholar]
- 35.Serhan C. N., N. Chiang, and T. E. Van Dyke. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blobaum A. L., and L. J. Marnett. 2007. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 50 1425–1441. [DOI] [PubMed] [Google Scholar]
- 37.Subbaramaiah K., and A. J. Dannenberg. 2003. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol. Sci. 24 96–102. [DOI] [PubMed] [Google Scholar]
- 38.Trifan O. C., and T. Hla. 2003. Cyclooxygenase-2 modulates cellular growth and promotes tumorigenesis. J. Cell. Mol. Med. 7 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crosby C. G., and R. N. DuBois. 2003. The cyclooxygenase-2 pathway as a target for treatment or prevention of cancer. Expert Opin. Emerg. Drugs. 8 1–7. [DOI] [PubMed] [Google Scholar]
- 40.Herschman H. R., J. J. Talley, and R. DuBois. 2003. Cyclooxygenase 2 (COX-2) as a target for therapy and noninvasive imaging. Mol. Imaging Biol. 5 286–303. [DOI] [PubMed] [Google Scholar]
- 41.Toyokuni T., J. S. Kumar, J. C. Walsh, A. Shapiro, J. J. Talley, M. E. Phelps, H. R. Herschman, J. R. Barrio, and N. Satyamurthy. 2005. Synthesis of 4-(5-[18F]fluoromethyl-3-phenylisoxazol-4-yl)benzenesulfonamide, a new [18F]fluorinated analogue of valdecoxib, as a potential radiotracer for imaging cyclooxygenase-2 with positron emission tomography. Bioorg. Med. Chem. Lett. 15 4699–4702. [DOI] [PubMed] [Google Scholar]
- 42.Schuller H. M., G. Kabalka, G. Smith, A. Mereddy, M. Akula, and M. Cekanova. 2006. Detection of overexpressed COX-2 in precancerous lesions of hamster pancreas and lungs by molecular imaging: implications for early diagnosis and prevention. ChemMedChem. 1 603–610. [DOI] [PubMed] [Google Scholar]
- 43.de Vries E. F., J. Doorduin, R. A. Dierckx, and A. van Waarde. 2008. Evaluation of [(11)C]rofecoxib as PET tracer for cyclooxygenase 2 overexpression in rat models of inflammation. Nucl. Med. Biol. 35 35–42. [DOI] [PubMed] [Google Scholar]
- 44.Wuest F., T. Kniess, R. Bergmann, and J. Pietzsch. 2008. Synthesis and evaluation in vitro and in vivo of a 11C-labeled cyclooxygenase-2 (COX-2) inhibitor. Bioorg. Med. Chem. 16 7662–7670. [DOI] [PubMed] [Google Scholar]
- 45.Kalgutkar A. S., A. B. Marnett, B. C. Crews, R. P. Remmel, and L. J. Marnett. 2000. Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J. Med. Chem. 43 2860–2870. [DOI] [PubMed] [Google Scholar]