Abstract
Population studies have shown that plasma HDL levels correlate inversely with cardiovascular disease risk. In recent years there has been intense interest in developing strategies for exploiting these cardioprotective properties by increasing HDL levels. While this approach has considerable merit, it is important to recognize that HDL are structurally and functionally diverse and consist of numerous, highly dynamic subpopulations of particles that do not all inhibit atherosclerosis to the same extent. For this reason it is essential to assess HDL subpopulation distribution and functionality when considering therapeutic interventions that raise HDL levels. This review documents what is known about the relationship between the metabolism and function of HDL subpopulations and how this affects their cardioprotective properties.
Keywords: HDL remodelling, HDL subpopulations, HDL function
HDL, the smallest and most dense of all plasma lipoproteins, consist of several distinct subpopulations of particles that vary in size, shape, density, surface charge, and composition. An inverse relationship between HDL levels and premature cardiovascular disease has been observed in many large-scale prospective studies (1, 2). This relationship is also evident in animal studies (3, 4).
HDL have several potentially anti-atherogenic properties. The best known of these is their ability to remove cholesterol from cells, such as macrophages in the artery wall, in the first step of the reverse cholesterol transport pathway (5). HDL also inhibit LDL oxidation (6), promote endothelial repair (7), improve endothelial function (8), have anti-thrombotic and anti-inflammatory properties (8, 9), and inhibit the binding of monocytes to the endothelium (10). In addition to preventing atherosclerotic lesion progression, HDL also promote lesion regression in animals (11, 12).
This review presents evidence that several of the aforementioned anti-atherogenic functions of HDL are mediated by specific subpopulations of particles. To appreciate this functional diversity, it is important to understand something of the origins and heterogeneity of HDL subpopulations.
ORIGINS OF HDL
HDL originate as discoidal particles that are either secreted from the liver or assembled in the plasma from the individual constituents. Discoidal HDL consist of two or more apolipoprotein molecules complexed with phospholipids and unesterified cholesterol (Fig. 1A). These particles are excellent substrates for LCAT, the enzyme that generates most of the cholesteryl esters in plasma (13). Cholesteryl esters are extremely hydrophobic and partition into the center of the particles as they are formed. This converts discoidal HDL into the large spherical HDL particles that predominate in normal human plasma. It also depletes the HDL surface of cholesterol and establishes a concentration gradient down which cholesterol from other lipoproteins and cell membranes moves into the HDL fraction, thus ensuring a continual supply of unesterified cholesterol for the LCAT reaction.
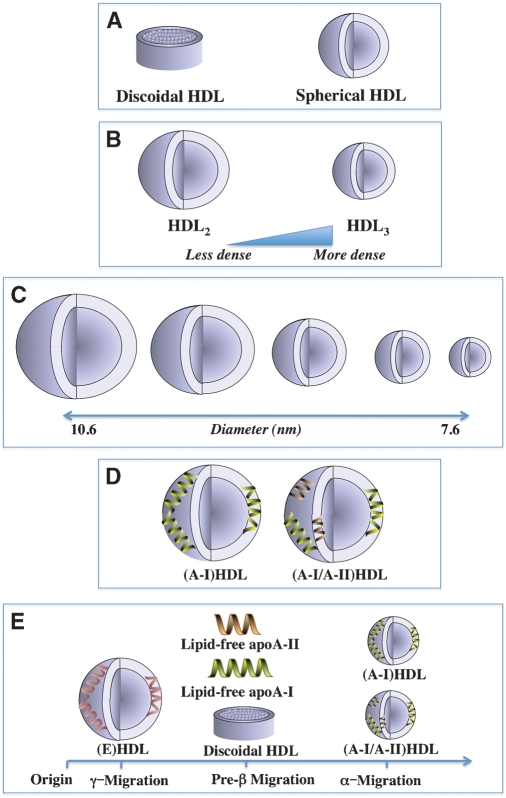
Fig. 1.
HDL heterogeneity. The HDL in human plasma consist of several subpopulations of particles that vary widely in shape (A), density (B), size (C), composition (D), and surface charge (E).
Spherical HDL contain a core of neutral lipids (cholesteryl esters and some triglyceride) surrounded by a surface monolayer of phospholipids, unesterified cholesterol, and apolipoproteins (Fig. 1A). They can be separated by ultracentrifugation on the basis of density into two major subfractions: HDL2 and HDL3, with HDL2 being larger and less dense than HDL3 (Fig. 1B). HDL can also be resolved by nondenaturing gradient gel electrophoresis into five distinct subpopulations of particles 7.6–10.6 nm in diameter (Fig. 1C) (14).
The HDL in human plasma are classified on the basis of their main apolipoproteins, apoA-I and apoA-II, into two populations of particles: those containing apoA-I, but not apoA-II, (A-I)HDL, and those that contain apoA-I and apoA-II, (A-I/A-II)HDL (Fig. 1D) (15). In normal human plasma, apoA-I is distributed approximately equally between (A-I)HDL and (A-I/A-II)HDL, while most of the apoA-II is associated with (A-I/A-II)HDL. When separated by agarose gel electrophoresis on the basis of surface charge, HDL migrate to a γ-, α-or preβ- position (Fig. 1E) (16). Most spherical HDL are α-migrating, while discoidal HDL, lipid-free apoA-I, and lipid-free apoA-II migrate to a preβ-position. A minor subpopulation of large, spherical HDL containing apoE as the only apolipoprotein migrate to a γ-position (17).
REMODELLING AND HDL SUBPOPULATION HETEROGENEITY
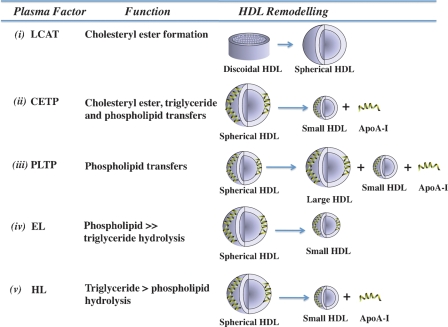
Several plasma factors alter the size, shape, surface charge, and composition of HDL in processes that are collectively termed remodelling. These plasma factors include LCAT, cholesteryl ester transfer protein (CETP), phospholipid transfer protein (PLTP), hepatic lipase (HL), and endothelial lipase (EL) (Fig. 2).
Fig. 2.
HDL Remodelling. Influence of plasma factors on the subpopulation distribution of HDL. LCAT generates cholesteryl esters and remodels discoidal HDL into spherical HDL (i); cholesteryl ester transfer protein (CETP) transfers cholesteryl esters and triglycerides between HDL, LDL, and VLDL; remodels HDL into small particles; and generates lipid-free/lipid-poor apoA-I (ii); phospholipid transfer protein (PLTP) transfers phospholipids between HDL and VLDL and between individual HDL particles; remodels HDL into large and small particles; and generates lipid-free/lipid-poor apoA-I (iii); endothelial lipase (EL) hydrolyses phospholipids and remodels HDL into small particles(iv); and hepatic lipase (HL) hydrolyses phospholipids and triglycerides, remodels HDL into small particles, and generates lipid-free/lipid-poor apoA-I (v).
One of the key events in remodelling is the dissociation of lipid-free or lipid-poor apoA-I from spherical HDL by CETP, PLTP, and HL (18). Lipid-free/lipid-poor apoA-I accounts for up to 5% of the total plasma apoA-I and accepts the cholesterol and phospholipids that efflux from cell membranes via the ATP binding cassette transporter A1 (ABCA1). Progressive lipidation of apoA-I via this pathway generates discoidal HDL and recycles apoA-I back into the HDL fraction. This reduces the rate at which apoA-I is cleared from the circulation and helps to maintain circulating HDL levels.
CETP is a member of the lipopolysaccharide-binding/lipid transfer protein family that transfers cholesteryl esters and triglycerides and, to a lesser extent phospholipids, between HDL, VLDL, and LDL (19). As CETP-mediated transfers of cholesteryl esters between HDL and LDL are rapid relative to the rate at which the lipoproteins are catabolised, these cholesteryl ester pools are in equilibrium in vivo. This is not necessarily the case for CETP-mediated transfers of cholesteryl esters and triglycerides between HDL and VLDL. When VLDL levels are elevated, CETP-mediated transfers of core lipids from HDL to VLDL exceed those from VLDL to HDL, generating core lipid-depleted, triglyceride-enriched HDL that have an excess of surface constituents and are structurally labile. This imbalance is rectified by the dissociation of lipid-free/lipid-poor apoA-I and a reduction in HDL size (Fig. 2). Triglyceride-enriched HDL are also excellent substrates for HL, which further reduces HDL size and enhances the dissociation of lipid-free/lipid-poor apoA-I (Fig. 2) (20). CETP can also remodel HDL into small particles by a fusion process that does not involve the dissociation of lipid-free/lipid-poor apoA-I (21). Inhibiting CETP activity as a therapeutic strategy for increasing HDL levels is under investigation. Although this decreases atherosclerosis in animals (3), there is, as yet, no evidence that it reduces cardiovascular events in humans.
PLTP is a member of the same protein family as CETP. It transfers phospholipids between HDL and VLDL, as well as between different HDL particles. PLTP remodels HDL into large and small particles by particle fusion and the dissociation of lipid-free/lipid-poor apoA-I (Fig. 2) (22). The role of PLTP in atherogenesis is controversial, with reports that its expression in macrophages both enhances and inhibits atherosclerosis in mice (23, 24). It would seem, on balance, that PLTP has an unfavorable effect on atherosclerosis.
EL and HL are members of the triglyceride lipase family with strikingly different substrate specificities. EL has high phospholipase and very low triglyceride lipase activity, while HL has high triglyceride lipase activity and low phospholipase activity (25). Although both enzymes remodel HDL into small particles, HL does this more effectively than EL. EL also differs from HL by not dissociating lipid-free/lipid-poor apoA-I from HDL (Fig. 2) (25, 26). Although their influence on atherosclerosis is poorly defined, EL and HL both regulate plasma HDL levels (27, 28). Additional studies of these enzymes are warranted.
Insights into the regulation of HDL remodelling have been obtained from homogeneous preparations of spherical, reconstituted HDL (rHDL) in which the composition is tightly regulated. This approach has established that apoA-II does not dissociate from HDL. ApoA-II also inhibits the CETP-mediated remodelling of HDL and the dissociation of lipid-free/lipid-poor apoA-I (29). The ability of CETP to remodel HDL and mediate the dissociation of apoA-I is also influenced by the phospholipid composition of the particles (30), while triglyceride-enrichment enhances both HDL remodelling by PLTP and the dissociation of apoA-I (22). ApoA-I and apoA-II also regulate the hydrolysis of HDL phospholipids by EL, and the HL-mediated hydrolysis of HDL phospholipids and triglycerides (31, 32).
RELATIONSHIP BETWEEN THE CARDIOPROTECTIVE PROPERTIES AND SUBPOPULATION DISTRIBUTION OF HDL
The results of human population and transgenic animal studies suggest that HDL subpopulations do not all protect against atherosclerosis equally well. However, evidence relating to the relative importance of (A-I)HDL vs. (A-I/A-II)HDL, large vs. small HDL, and preβ-migrating vs. α-migrating HDL is confusing. For example, the suggestion that lipid-free/lipid-poor apoA-I and discoidal HDL, which both exhibit preβ migration, may be more cardioprotective than spherical, α-migrating HDL is based largely on the observation that preβ-migrating lipid-free/lipid-poor apoA-I is preferred over α-migrating HDL as an acceptor of the cholesterol that effluxes from cells via ABCA1 in the first step of reverse cholesterol transport (33).
While superficially appealing, epidemiological evidence supporting a cardioprotective role for preβ-migrating lipid-free/lipid-poor apoA-I is lacking. When this issue was addressed in a recent analysis of the Veterans Affairs HDL Intervention Trial, subjects with new cardiovascular events had significantly lower levels of large, α-migrating spherical HDL than event-free subjects (34). When apoA-I-containing HDL subpopulations from these individuals were quantified by 2-D gel electrophoresis, the cases had lower levels of large α-migrating HDL and significantly higher levels of small, poorly lipidated, preβ-migrating HDL compared with event-free subjects (34). The concentration of large, α-migrating spherical HDL was also the best negative predictor of recurrent cardiovascular events, while the concentration of smaller, α-migrating HDL was a positive predictor of new events (34). This is consistent with a recent report showing that large, spherical HDL are the preferred acceptors of the cholesterol that effluxes from macrophages via the ATP binding cassette transporter G1 (ABCG1) (35).
The possibility that HDL subpopulations are functionally distinct raises the important question as to which subpopulations should be therapeutic targets for raising HDL levels. One intervention that elevates HDL levels is inhibition of CETP activity. This causes cholesteryl esters to accumulate in HDL and selectively increases the level of large HDL2 particles (36). Inhibition of CETP activity in rabbits by torcetrapib is markedly anti-atherogenic (3). In humans, by contrast, torcetrapib does not reduce atherosclerosis (37, 38). It also caused an excess of deaths and cardiovascular events in a large-scale endpoint trial (39). At present it is not known if this deleterious outcome was related directly to the inhibition of CETP; to the generation of large, possibly dysfunctional HDL particles; or to off-target effects of torcetrapib. Whether CETP inhibition is cardioprotective in humans is currently being investigated with other compounds that appear not to share the off-target effects of torcetrapib.
RELATIONSHIP BETWEEN THE CARDIOPROTECTIVE PROPERTIES OF HDL SUBPOPULATIONS AND THEIR FUNCTIONAL HETEROGENEITY
Influence of HDL subpopulations on cholesterol efflux from cells
HDL promote cholesterol efflux from cell membranes by four distinct pathways: i) via ABCA1 to lipid-poor/lipid-free apoA-I; ii) by passive diffusion to a wide range of HDL acceptors; iii) via scavenger receptor-B1 (SR-B1) to various spherical HDL subpopulations; and iv) via ABCG1 to large, spherical HDL.
SR-B1 is involved in the first and the last step of reverse cholesterol transport. Although its ability to mediate cholesterol efflux from cells in the first step of the pathway lacks specificity, this may not be the case for its ability to deplete HDL of cholesteryl esters in the final step (40). While some investigators have reported that SR-B1 removes cholesteryl esters from (A-I/A-II)HDL more effectively than from (A-I)HDL (41), others have found that it preferentially removes cholesteryl esters from (A-I)HDL (42).
Antioxidant properties of HDL subpopulations
Atherosclerosis is an inflammatory disease that is initiated, in part, by the presence of oxidized LDL in the artery wall. The ability of different HDL subpopulations to inhibit LDL lipid and apolipoprotein oxidation is not well understood. While some investigators have found that HDL3 inhibit LDL oxidation better than HDL2 (43, 44), others have reported that the antioxidant capacity of small, dense HDL is impaired, at least in subjects with the metabolic syndrome (45). Other studies have demonstrated that CETP transfers lipid hydroperoxides from LDL to HDL, where they are reduced to lipid hydroxides and cleared by the liver (46). In that study, lipid hydroperoxide reduction was comparable in HDL2 and HDL3.
HDL-associated proteins such as paraoxonase and platelet-activating factor acetyl hydrolase also contribute to the anti-oxidant properties of HDL. A recent report has suggested that the anti-oxidant properties of paraoxonase are enhanced in (A-I/A-II)HDL (47). It is not known if this is also the case for platelet-activating factor acetyl hydrolase.
Anti-inflammatory properties of HDL subpopulations
The results of in vitro studies have shown that HDL3 inhibit endothelial vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in cultured human umbilical vein endothelial cells more effectively than HDL2 (48). This is unlikely to be caused by differences in the apolipoprotein composition of the particles because discoidal rHDL that contain either apoA-I or apoA-II as the sole apolipoprotein inhibit adhesion molecule expression in activated human umbilical vein endothelial cells equally well. However, the finding that apoA-I-containing discoidal rHDL prepared with phospholipids of varying sn-2 acyl chain length and unsaturation differ markedly in their ability to inhibit inflammation (49) suggests that the varying anti-inflammatory properties of HDL2 and HDL3 is related to their phospholipid composition.
This specificity is not, however, evident in vivo. Irrespective of their phospholipid composition, infusions of apoA-I-containing discoidal rHDL inhibit endothelial expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, as well as neutrophil infiltration into acutely inflamed rabbit carotid arteries equally well (50). This apparent lack of specificity most likely reflects a rapid post-infusion equilibration of the rHDL phospholipids with phospholipids from other lipoproteins and cell membranes.
FUTURE DIRECTIONS AND CONCLUSIONS
Although considerable progress has been made toward understanding the functionality of HDL subpopulations and their impact on cardiovascular disease, much remains unknown. As new strategies for increasing HDL levels are identified, it will be important to determine how they affect HDL subpopulation distribution and function. This approach will not only enhance our understanding of HDL subpopulation functionality, but also identify specific populations of particles that are therapeutic targets for reducing cardiovascular risk.
Abbreviations
ABCA1, ATP binding cassette transporter A1
ABCG1, ATP binding cassette transporter G1
CETP, cholesteryl ester transfer protein
EL, endothelial lipase
HL, hepatic lipase
PLTP, phospholipid transfer protein
rHDL, reconstituted HDL
SR-B1, scavenger receptor-B1
This work was funded by the NHMRC Grant Numbers 222722 and 482800. CAB is supported by a NHF Career Development Award and the Philip Bushell Foundation.
Published, JLR Papers in Press, November 24, 2008.
References
- 1.Barter P., A. M. Gotto, J. C. LaRosa, J. Maroni, M. Szarek, S. M. Grundy, J. J. Kastelein, V. Bittner, and J. C. Fruchart. 2007. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 357 1301–1310. [DOI] [PubMed] [Google Scholar]
- 2.Gordon D. J., J. L. Probstfield, R. J. Garrison, J. D. Neaton, W. P. Castelli, J. D. Knoke, D. R. Jacobs, Jr., S. Bangdiwala, and H. A. Tyroler. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79 8–15. [DOI] [PubMed] [Google Scholar]
- 3.Morehouse L. A., E. D. Sugarman, P. A. Bourassa, T. M. Sand, F. Zimetti, F. Gao, G. H. Rothblat, and A. J. Milici. 2007. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J. Lipid Res. 48 1263–1272. [DOI] [PubMed] [Google Scholar]
- 4.Belalcazar L. M., A. Merched, B. Carr, K. Oka, K. H. Chen, L. Pastore, A. Beaudet, and L. Chan. 2003. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 107 2726–2732. [DOI] [PubMed] [Google Scholar]
- 5.Lewis G. F., and D. J. Rader. 2005. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96 1221–1232. [DOI] [PubMed] [Google Scholar]
- 6.Negre-Salvayre A., N. Dousset, G. Ferretti, T. Bacchetti, G. Curatola, and R. Salvayre. 2006. Antioxidant and cytoprotective properties of high-density lipoproteins in vascular cells. Free Radic. Biol. Med. 41 1031–1040. [DOI] [PubMed] [Google Scholar]
- 7.Tso C., G. Martinic, W. H. Fan, C. Rogers, K. A. Rye, and P. J. Barter. 2006. High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler. Thromb. Vasc. Biol. 26 1144–1149. [DOI] [PubMed] [Google Scholar]
- 8.Mineo C., H. Deguchi, J. H. Griffin, and P. W. Shaul. 2006. Endothelial and antithrombotic actions of HDL. Circ. Res. 98 1352–1364. [DOI] [PubMed] [Google Scholar]
- 9.Cockerill G. W., K. A. Rye, J. R. Gamble, M. A. Vadas, and P. J. Barter. 1995. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 15 1987–1994. [DOI] [PubMed] [Google Scholar]
- 10.Murphy, A. J., K. J. Woollard, A. Hoang, N. Mukhamedova, R. A. Stirzaker, S. P. McCormick, A. T. Remaley, D. Sviridov, and J. Chin-Dusting. 2008. High-Density Lipoprotein Reduces the Human Monocyte Inflammatory Response. Arterioscler Thromb Vasc Biol. [DOI] [PubMed]
- 11.Tangirala R. K., K. Tsukamoto, S. H. Chun, D. Usher, E. Pure, and D. J. Rader. 1999. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 100 1816–1822. [DOI] [PubMed] [Google Scholar]
- 12.Badimon J. J., L. Badimon, and V. Fuster. 1990. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 85 1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonas A. 2000. Lecithin cholesterol acyltransferase. Biochim. Biophys. Acta. 1529 245–256. [DOI] [PubMed] [Google Scholar]
- 14.Blanche P. J., E. L. Gong, T. M. Forte, and A. V. Nichols. 1981. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim. Biophys. Acta. 665 408–419. [DOI] [PubMed] [Google Scholar]
- 15.Cheung M. C., and J. J. Albers. 1984. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. Particles containing A-I and A-II and particles containing A-I but no A-II. J. Biol. Chem. 259 12201–12209. [PubMed] [Google Scholar]
- 16.Castro G. R., and C. J. Fielding. 1988. Early incorporation of cell-derived cholesterol into pre-beta-migrating high-density lipoprotein. Biochemistry. 27 25–29. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y., A. von Eckardstein, S. Wu, N. Maeda, and G. Assmann. 1994. A plasma lipoprotein containing only apolipoprotein E and with gamma mobility on electrophoresis releases cholesterol from cells. Proc. Natl. Acad. Sci. USA. 91 1834–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rye K. A., and P. J. Barter. 2004. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24 421–428. [DOI] [PubMed] [Google Scholar]
- 19.Qiu X., A. Mistry, M. J. Ammirati, B. A. Chrunyk, R. W. Clark, Y. Cong, J. S. Culp, D. E. Danley, T. B. Freeman, K. F. Geoghegan, et al. 2007. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat. Struct. Mol. Biol. 14 106–113. [DOI] [PubMed] [Google Scholar]
- 20.Clay M. A., H. H. Newnham, and P. J. Barter. 1991. Hepatic lipase promotes a loss of apolipoprotein A-I from triglyceride-enriched human high density lipoproteins during incubation in vitro. Arterioscler. Thromb. 11 415–422. [DOI] [PubMed] [Google Scholar]
- 21.Rye K. A., N. J. Hime, and P. J. Barter. 1997. Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion. J. Biol. Chem. 272 3953–3960. [DOI] [PubMed] [Google Scholar]
- 22.Settasatian N., M. Duong, L. K. Curtiss, C. Ehnholm, M. Jauhiainen, J. Huuskonen, and K. A. Rye. 2001. The mechanism of the remodeling of high density lipoproteins by phospholipid transfer protein. J. Biol. Chem. 276 26898–26905. [DOI] [PubMed] [Google Scholar]
- 23.van Haperen R., H. Samyn, M. Moerland, T. van Gent, M. Peeters, F. Grosveld, A. van Tol, and R. de Crom. 2008. Elevated expression of phospholipid transfer protein in bone marrow derived cells causes atherosclerosis. PLoS One. 3 e2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valenta D. T., N. Ogier, G. Bradshaw, A. S. Black, D. J. Bonnet, L. Lagrost, L. K. Curtiss, and C. M. Desrumaux. 2006. Atheroprotective potential of macrophage-derived phospholipid transfer protein in low-density lipoprotein receptor-deficient mice is overcome by apolipoprotein AI overexpression. Arterioscler. Thromb. Vasc. Biol. 26 1572–1578. [DOI] [PubMed] [Google Scholar]
- 25.Jaye M., K. J. Lynch, J. Krawiec, D. Marchadier, C. Maugeais, K. Doan, V. South, D. Amin, M. Perrone, and D. J. Rader. 1999. A novel endothelial-derived lipase that modulates HDL metabolism. Nat. Genet. 21 424–428. [DOI] [PubMed] [Google Scholar]
- 26.Jahangiri A., D. J. Rader, D. Marchadier, L. K. Curtiss, D. J. Bonnet, and K. A. Rye. 2005. Evidence that endothelial lipase remodels high density lipoproteins without mediating the dissociation of apolipoprotein A-I. J. Lipid Res. 46 896–903. [DOI] [PubMed] [Google Scholar]
- 27.Ma K., M. Cilingiroglu, J. D. Otvos, C. M. Ballantyne, A. J. Marian, and L. Chan. 2003. Endothelial lipase is a major genetic determinant for high-density lipoprotein concentration, structure, and metabolism. Proc. Natl. Acad. Sci. USA. 100 2748–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen H. 2004. Hepatic lipase: friend or foe and under what circumstances? Curr. Atheroscler. Rep. 6 343–347. [DOI] [PubMed] [Google Scholar]
- 29.Rye K. A., K. Wee, L. K. Curtiss, D. J. Bonnet, and P. J. Barter. 2003. Apolipoprotein A-II inhibits high density lipoprotein remodeling and lipid-poor apolipoprotein A-I formation. J. Biol. Chem. 278 22530–22536. [DOI] [PubMed] [Google Scholar]
- 30.Rye K. A., M. Duong, M. K. Psaltis, L. K. Curtiss, D. J. Bonnet, R. Stocker, and P. J. Barter. 2002. Evidence that phospholipids play a key role in pre-beta apoA-I formation and high-density lipoprotein remodeling. Biochemistry. 41 12538–12545. [DOI] [PubMed] [Google Scholar]
- 31.Caiazza D., A. Jahangiri, D. J. Rader, D. Marchadier, and K. A. Rye. 2004. Apolipoproteins regulate the kinetics of endothelial lipase-mediated hydrolysis of phospholipids in reconstituted high-density lipoproteins. Biochemistry. 43 11898–11905. [DOI] [PubMed] [Google Scholar]
- 32.Hime N. J., P. J. Barter, and K. A. Rye. 1998. The influence of apolipoproteins on the hepatic lipase-mediated hydrolysis of high density lipoprotein phospholipid and triacylglycerol. J. Biol. Chem. 273 27191–27198. [DOI] [PubMed] [Google Scholar]
- 33.Oram J. F., and A. M. Vaughan. 2006. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 99 1031–1043. [DOI] [PubMed] [Google Scholar]
- 34.Asztalos B. F., D. Collins, K. V. Horvath, H. E. Bloomfield, S. J. Robins, and E. J. Schaefer. 2008. Relation of gemfibrozil treatment and high-density lipoprotein subpopulation profile with cardiovascular events in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Metabolism. 57 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuura F., N. Wang, W. Chen, X. C. Jiang, and A. R. Tall. 2006. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J. Clin. Invest. 116 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brousseau M. E., E. J. Schaefer, M. L. Wolfe, L. T. Bloedon, A. G. Digenio, R. W. Clark, J. P. Mancuso, and D. J. Rader. 2004. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 350 1505–1515. [DOI] [PubMed] [Google Scholar]
- 37.Nissen S. E., J. C. Tardif, S. J. Nicholls, J. H. Revkin, C. L. Shear, W. T. Duggan, W. Ruzyllo, W. B. Bachinsky, G. P. Lasala, and E. M. Tuzcu. 2007. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 356 1304–1316. [DOI] [PubMed] [Google Scholar]
- 38.Bots M. L., F. L. Visseren, G. W. Evans, W. A. Riley, J. H. Revkin, C. H. Tegeler, C. L. Shear, W. T. Duggan, R. M. Vicari, D. E. Grobbee, et al. 2007. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 370 153–160. [DOI] [PubMed] [Google Scholar]
- 39.Barter P. J., M. Caulfield, M. Eriksson, S. M. Grundy, J. J. Kastelein, M. Komajda, J. Lopez-Sendon, L. Mosca, J. C. Tardif, D. D. Waters, et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357 2109–2122. [DOI] [PubMed] [Google Scholar]
- 40.Acton S., A. Rigotti, K. T. Landschulz, S. Xu, H. H. Hobbs, and M. Krieger. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 271 518–520. [DOI] [PubMed] [Google Scholar]
- 41.de Beer M. C., D. M. Durbin, L. Cai, N. Mirocha, A. Jonas, N. R. Webb, F. C. de Beer, and D. R. van Der Westhuyzen. 2001. Apolipoprotein A-II modulates the binding and selective lipid uptake of reconstituted high density lipoprotein by scavenger receptor BI. J. Biol. Chem. 276 15832–15839. [DOI] [PubMed] [Google Scholar]
- 42.Rinninger F., M. Brundert, R. M. Budzinski, J. C. Fruchart, H. Greten, and G. R. Castro. 2003. Scavenger receptor BI (SR-BI) mediates a higher selective cholesteryl ester uptake from LpA-I compared with LpA-I:A-II lipoprotein particles. Atherosclerosis. 166 31–40. [DOI] [PubMed] [Google Scholar]
- 43.Yoshikawa M., N. Sakuma, T. Hibino, T. Sato, and T. Fujinami. 1997. HDL3 exerts more powerful anti-oxidative, protective effects against copper-catalyzed LDL oxidation than HDL2. Clin. Biochem. 30 221–225. [DOI] [PubMed] [Google Scholar]
- 44.Kontush A., S. Chantepie, and M. J. Chapman. 2003. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23 1881–1888. [DOI] [PubMed] [Google Scholar]
- 45.Hansel B., P. Giral, E. Nobecourt, S. Chantepie, E. Bruckert, M. J. Chapman, and A. Kontush. 2004. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 89 4963–4971. [DOI] [PubMed] [Google Scholar]
- 46.Garner B., A. R. Waldeck, P. K. Witting, K. A. Rye, and R. Stocker. 1998. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 273 6088–6095. [DOI] [PubMed] [Google Scholar]
- 47.Moren X., S. Deakin, M. L. Liu, M. R. Taskinen, and R. W. James. 2008. HDL subfraction distribution of paraoxonase-1 and its relevance to enzyme activity and resistance to oxidative stress. J. Lipid Res. 49 1246–1253. [DOI] [PubMed] [Google Scholar]
- 48.Ashby D. T., K. A. Rye, M. A. Clay, M. A. Vadas, J. R. Gamble, and P. J. Barter. 1998. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 18 1450–1455. [DOI] [PubMed] [Google Scholar]
- 49.Baker P. W., K. A. Rye, J. R. Gamble, M. A. Vadas, and P. J. Barter. 2000. Phospholipid composition of reconstituted high density lipoproteins influences their ability to inhibit endothelial cell adhesion molecule expression. J. Lipid Res. 41 1261–1267. [PubMed] [Google Scholar]
- 50.Nicholls S. J., G. J. Dusting, B. Cutri, S. Bao, G. R. Drummond, K. A. Rye, and P. J. Barter. 2005. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 111 1543–1550. [DOI] [PubMed] [Google Scholar]