Abstract
Fatty acids are a major energy source and important constituents of membrane lipids, and they serve as cellular signaling molecules that play an important role in the etiology of the metabolic syndrome. Acetyl-CoA carboxylases 1 and 2 (ACC1 and ACC2) catalyze the synthesis of malonyl-CoA, the substrate for fatty acid synthesis and the regulator of fatty acid oxidation. They are highly regulated and play important roles in the energy metabolism of fatty acids in animals, including humans. They are presently considered as an attractive target to regulate the human diseases of obesity, diabetes, cancer, and cardiovascular complications. In this review we discuss the role of fatty acid metabolism and its key players, ACC1 and ACC2, in animal evolution and physiology, as related to health and disease.
Keywords: acetyl-coenzyme A carboxylases 1 and 2, ACC1 and ACC2, fatty acid synthase, FAS, carnitine/palmitoyl-transferase 1, CPT1, acyl-CoA, AMP-activated kinase, AMPK
Long-chain fatty acids are major sources of energy and important components of the lipids that comprise the cellular membrane. They are either derived from food or are synthesized from acetyl-coenzyme A (acetyl-CoA) through complex sets of reactions; that includes glycolysis and the citric acid cycle, which collectively lead to the formation of the backbone carbons of fatty acids and glycerols for the synthesis of lipids (1). Acetyl-CoA is also a product of the β-oxidation of fatty acids. Hence, acetyl-CoA stands out as the key intermediate in carbohydrate, amino acid, and lipid metabolism. The synthesis of fatty acids by fatty acid synthase (FAS) requires acetyl-CoA, malonyl-CoA, and NADPH. Malonyl-CoA is the C2 donor in the de novo synthesis of fatty acids, and it plays an important role as an inhibitor of the carnitine/palmitoyl shuttle system for fatty acid oxidation (2). To facilitate these two different roles, fatty acid synthesis and oxidation, two distinct enzymes have evolved: acetyl-CoA carboxylase 1 (ACC1) and acetyl-CoA carboxylase 2 (ACC2).
ACC1, with its biotin prosthetic group, was first discovered in our laboratory in 1958 (3). In prokaryotes, ACC1 is composed of three distinct proteins: biotin carboxylase, biotin carboxyl carrier protein, and transcarboxylase. In the presence of ATP, biotin carboxylase transfers CO2 from bicarbonate to the biotin carboxyl carrier protein, forming the carboxybiotin derivative. The transcarboxylase catalyzes the transfer of the carboxyl group to acetyl-CoA, forming malonyl-CoA. In eukaryotes, however, these proteins are contained within a single multifunctional protein (Mr 262,000) that is encoded by a single gene. While ACC1 is generally expressed in all tissues, it is expressed more in lipogenic tissues: liver, adipose, and lactating mammary gland. In contrast, ACC2 (Mr 282,000) is highly expressed in heart and muscle and to a lesser extent in liver (4). ACC1 and ACC2 are encoded by separate genes localized at chromosomes 17q12 and 12q23, respectively (4, 5). The amino-acid sequences of ACC1 and ACC2 are approximately 80% identical; the first 218 amino acids of ACC2 and its highly hydrophobic N-terminus 20 amino acids account for their molecular weight differences and their distinct cellular localization (5–7). The ACC1-generated malonyl-CoA is utilized by FAS for the synthesis of fatty acids in the cytosol. In contrast, the ACC2-generated malonyl-CoA functions as inhibitor of the carnitine/palmitoyl-transferase 1 (CPT1) activity and the transfer of the fatty acyl group through the carnitine/palmitoyl shuttle system to inside the mitochondria for β-oxidation (Fig. 1). The malonyl-CoA generated by ACC1 and ACC2 within the cell do not mix and are highly segregated.
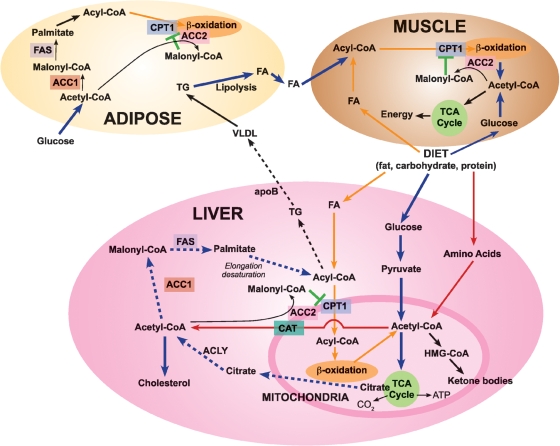
Fig. 1.
Acetyl-coenzyme A carboxylase 1 (ACC1) and acetyl-coenzyme A carboxylase 2 (ACC2) play distinct roles in lipid metabolism in animal tissues. Diet fat, carbohydrate, and protein are digested, and the fatty acids (FA), glucose, and amino acids are transported to various tissues, including liver, adipose, and muscle. In liver, FA are converted to acyl-CoA; glucose undergoes glycolysis and generates pyruvate, which is oxidized in the mitochondria through pyruvate dehydrogenase to acetyl-coenzyme A (acetyl-CoA). Acetyl-CoA is also produced through amino acid metabolism. The acyl-CoA are shuttled into the mitochondria through carnitine/palmitoyl-transferase 1 (CPT1) for β-oxidation and generation of acetyl-CoA. Acetyl-CoA is oxidized through the citric acid cycle to yield energy, H2O, and CO2 or it is converted to (1) citrate, which exits to the cytosol and generates acetyl-CoA through ATP citrate lyase (ACLY), or to (2) ketone bodies, through the hydroxymethylglutaryl-CoA (HMG-CoA) system, or to (3) carnitine/acetyl-CoA (CAT), which exits from the mitochondria to the cytosol. In the cytosol acetyl-CoA is carboxylated to malonyl-CoA by ACC1 and utilized through fatty acid synthase (FAS) reactions to generate palmitate, which is utilized in the synthesis of triglycerides (TG) and VLDL. Also, acetyl-CoA is carboxylated by ACC2 at the mitochondrial membrane to form malonyl-CoA, which inhibits the CPT1 and reduces acyl-CoA transfer to mitochondria for β-oxidation. Basically comparable reactions, with appropriate modifications, occur in adipose and muscle tissues. See the text for a discussion of the impact of ACC2 knockout on fatty acid metabolism.
The ACC1 and ACC2 genes and their products must have played significant roles in the evolutionary development of animals, including humans. Survival of animals requires the availability of adequate food sources; in nature, this is highly variable in quality and quantity. Once they locate the right meal, however, the animals that eat as much as possible, because they do not know when they will eat their next meal, will survive best. As animals consume their meals of carbohydrates, proteins, and fats, through digestion and assimilation, glucose, amino acids, and fatty acids, respectively, are generated. These metabolites provide the substrates for immediate and future energy needs in the forms of glycogen, muscle proteins, and fats, respectively. When no food is available stored glycogen, some muscle proteins and fats are mobilized, degraded, and consumed within the first few days. Fat stores, however, can provide energy to animals for much longer times, in humans for 60 days, in polar bears for 6 months, and in migratory birds flying thousands of miles. For animals to exist during such long periods of starvation, the needed synthesis and saving of the acquired fat require special mechanisms that involve ACC1, ACC2, and CPT1.
Through the active regulation of CPT1 by malonyl-CoA, it became possible to interrelate fatty acid synthesis and oxidation with glucose utilization. As acetyl-CoA is generated in the mitochondria through pyruvate dehydrogenase, it condenses with oxaloacetate to form citrate, which undergoes either oxidation through the citric cycle to generate energy or transfer to the cytosol to generate acetyl-CoA through the ATP citrate lyase (ACLY) reaction (Fig. 1). At the mitochondrial membrane acetyl-CoA is carboxylated by ACC2 to malonyl-CoA, which inhibits CPT1 and reduces the passage of acyl-CoA into the inner mitochondrial vortex for β-oxidation (Fig. 1). The net result is reduced fatty acid oxidation and increased fatty acid and triglyceride (TG) synthesis, at the expense of glucose utilization.
REGULATION OF ACC ISOFORMS
Both ACC genes are under the control of multiple promoters, which are regulated by diet and hormones. Diet, especially a fat-free diet, induces the synthesis of ACC1 and ACC2 and increases their activities. Starvation, or diabetes, represses the expression of the carboxylase genes and decreases the activities of the ACC enzymes (8). Insulin up-regulates the ACC1 promoter, while glucagon down-regulates it (8). A diet rich in carbohydrates induces the transcription of FAS, ACC1, and ACC2 (8). Key transcription factors that regulate these lipogenic genes in response to glucose and insulin are sterol response-elements binding proteins (SREBP-1 and SREBP-2), liver X receptors, and carbohydrate-responsive element-binding protein (ChREBP) (9, 10). Glucose induces the dephosphorylation of ChREBP, which increases its rate of nuclear entry and the stimulation of lipogenic genes (11). In contrast, inhibition of the expression of ChREBP, using adenovirus-mediated RNA interference in cultured hepatocytes, or liver-specific deletion of ChREBP results in the down-regulation of ACCs, FAS, and stearoyl-CoA desaturase 1 (12, 13). Three promoters regulate ACC1 in humans: promoter I is a constitutive promoter and highly active; promoters II and III are subject to regulation by various hormones, they are stimulated by triiodothyronine and repressed by cholesterol, and they are highly expressed in the lactating mammary gland (14). Moreover, in humans, the untranslated exons 1 to 4 of ACC1 are alternately spliced; this generates heterogeneity at the 5′-end of the ACC1 mRNA, which may play a role in transcriptional and translational regulation (8, 14). ACC2 is under the control of two promoters in human: promoter I regulates tissue-specific expression, and promoter II regulation is mediated by myogenic factors such as Myo D and muscle regulatory factor 4 (15).
Activities of both ACC isoforms are highly regulated by other physiological factors in a similar manner. The enzymes are allosterically activated by citrate, and they are inhibited by long-chain saturated fatty acyl-CoA. Their activities are covalently modified by phosphorylation/dephosphorylation mechanisms. Protein kinases such as cAMP-dependent kinase, which phosphorylates ACC1 at Ser 1200, and AMP-activated kinase (AMPK), which phosphorylates ACC1 at Ser 80 and 81 and ACC2 on Ser 219 and 220, reduce their activities (16, 17). The most notable of these kinases is AMPK, a master monitor of energy levels in cells. In tissues, AMPK is activated by a high level of AMP concurrent with a low level of ATP, through mechanisms involving the regulation of its phosphorylation by AMPK-kinase in a cascade; this cascade is activated by exercise, by hormones, and by cellular stressors that deplete ATP (17). Insulin activates ACC by dephosphorylation, whereas glucagon and epinephrine inactivate the enzyme by phosphorylation (18). It has recently been suggested that leptin stimulates fatty acid oxidation through the action of AMPK, which leads to phosphorylation and the inhibition of ACC1 and ACC2 activities in muscles (19). When metabolic fuel is low and ATP is needed, ACC1 and ACC2 are turned off by phosphorylation and the consequential reduction in the levels of malonyl-CoA; this leads to the generation of ATP through increased fatty acid oxidation and the decreased consumption of ATP for fatty acid synthesis.
De novo fatty acid synthesis plays an essential role in animal development, as shown by genetic deletion of ACC, FAS, and ACLY. In mice, a null Acc1 mutation causes embryonic lethality in the homozygous state (7). Timed mating of Acc1+/− mice revealed that the mutant Acc1−/− did not develop beyond the egg cylinder stage (7). In our studies of the knockout of FAS, we found that Fasn−/− mice also die in utero at an early stage of embryonic development (20). Consistent with the essential role of cytosolic ACC1 in mice, knockout studies of cytosolic ACLY showed that Acly−/− mice also died early in embryonic development (21). Together, these studies demonstrate that de novo fatty acid synthesis catalyzed by FAS is vital for viable embryonic development.
ROLE OF LIPOGENESIS IN METABOLIC SYNDROME
That accumulation of fat in tissues, such as muscle and liver, is associated with insulin resistance throughout the whole animal is widely accepted (22). ACC, FAS, and stearoyl-CoA desaturase 1 play important roles in the development of hepatic steatosis and insulin resistance. The most direct evidence for the role of ACC1 in mouse liver, and thus in mouse physiology, came from studies of liver ACC1 knockout mice (LACC1KO) (23). Under normal feeding conditions LACC1KO mice have no obvious health problems, notwithstanding the 70–75% decrease in ACC activity and, thus, in malonyl-CoA level. LACC1KO mice fed a diet inducing obesity developed glucose intolerance and insulin resistance. When fed a fat-free diet, however, there was significant up-regulation of PPARγ and several enzymes in the lipogenic pathway in the LACC1KO mouse livers compared with wild-type (WT) mouse livers. Despite a greater than 2-fold increase in FAS mRNA, protein, and activity, there was a significant decrease in de novo fatty acid synthesis and TG accumulation in the liver. These results suggest that lowering cytosolic malonyl-CoA signals to cells that fatty acids are needed; the cells respond by up-regulating FAS and down-regulating the fatty acid oxidation pathway, partly through the up-regulation of ACC2, the amount of which increased approximately 2-fold in the mouse livers (23). It is interesting that the latest study using intraperitoneal injection of antisense oligonucleotide inhibitors of ACC1 and ACC2 reversed diet-induced hepatic steatosis and hepatic insulin resistance (24). The antisense oligonucleotide inhibitors of ACC1 significantly reduced the mRNA level of ACC1 without significantly altering liver malonyl-CoA levels. Based on our earlier observation that in Acc1+/− heterozygotes the malonyl-CoA levels were essentially unchanged, this result is expected (7). It was surprising, however, that antisense oligonucleotide against ACC1 reduced both fatty acid oxidation and fatty acid synthesis; because the malonyl-CoA level was basically unchanged, the exact effects of these inhibitors remain to be explored (24). An et al. (25) used adenoviral vectors to express a cytosolic form of malonyl-CoA decarboxylase (MCD), which converts malonyl-CoA to acetyl-CoA. These studies showed that, when rats were fed a high-fat diet, the reduction of malonyl-CoA levels in liver resulted in a significant reduction in the plasma levels of fatty acid (FA) and insulin and reversed insulin resistance in muscle and liver. However, while there was a 7-fold increase in cytosolic MCD activity in liver, there was only a modest effect on fatty acid oxidation; plasma ketone bodies were unchanged. The difference in findings between the studies of LACC1KO mice and the studies using recombinant adenovirus expressing MCD could be caused either by the mechanism by which malonyl-CoA levels were lowered or by the different diets that the mice were fed. In addition, because MCD is also localized in the peroxisomes, its high expression may affect the oxidation of very-long-chain fatty acids. A FAS knockout in liver (FASKOL) resulted in mice mutants that are essentially similar to WT mice when fed normal diets (26). When FASKOL mice were fed a zero-fat diet for 28 days, however, they developed fatty liver, hypoglycemia, and hypoinsulinemia, and elevated blood ketone bodies. These phenotypes, which are similar to fasted PPARα-deficient mice, could be reversed by using a PPARα agonist; this suggested that synthesis of “new fat” in the liver is required for the activation of PPARα. Unlike FASKOL mice, when fed a fat-free diet for 28 days LACC1KO mice accumulated less TG in their livers. One major difference between LACC1KO and FASKOL mice is that malonyl-CoA levels in liver were three times higher in the FASKOL mice than in their WT cohorts (26), while in the LACC1KO mice, malonyl-CoA levels were reduced to approximately 75% of those in their WT cohorts (24).
ACC2 IS MAJOR PLAYER IN ENERGY HOMEOSTASIS
Malonyl-CoA inhibition of CPT1 provides a simple but important mechanism for the control of two opposing pathways: fatty acid synthesis and fatty acid oxidation. The subcellular localization of ACC2 at the mitochondrial membrane, and its dominant expression in tissues with a high rate of fatty acid oxidation, strongly suggest that malonyl-CoA produced by ACC2 is a major regulator of fatty acid oxidation. Further support for this hypothesis was derived from studies of Acc2−/− mutant mice: they live and breed well, continuously oxidize fatty acids, eat more food, and gain less weight than their WT cohorts (27). Because ACC1 is the predominant isoform in liver and adipose tissue, the malonyl-CoA levels in both tissues of the WT mice and the Acc2−/− mutant mice were similar. In heart and soleus muscle, where ACC2 is highly expressed, the levels of malonyl-CoA in Acc2−/− mice were 10- and 30-fold lower, respectively, than in the WT mice tissues. Fatty acid oxidation rates in the Acc2−/− mutant mice were significantly higher than in the WT mice, suggesting that only ACC2-produced malonyl-CoA is involved in the regulation of fatty acid oxidation. When challenged with diets that induce obesity and diabetes the Acc2−/− mice maintained normal levels of insulin and glucose, suggesting that increased fatty acid oxidation lowers intracellular fatty acid accumulation which, because of the higher GLUT4 activity, improves insulin sensitivity and increases glucose uptake (Fig. 2) (27–30).
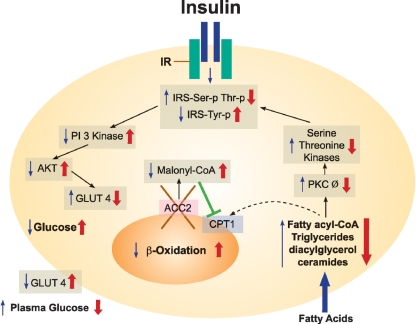
Fig. 2.
Lack of ACC2 improves insulin signaling in animal tissues. Obese tissues lead to insulin resistance. High acyl-CoA activates PKC Ø, resulting in a cascade of serine and threonine kinases and increasing serine and threonine phosphorylation of insulin receptor substrates, IRS-1 and IRS-2. These substrates generate IRS-Ser-p Thr-p, which decreases the activity of PI 3 kinase, down-regulates AKT, and decreases the translocation of GLUT4 to the plasma membrane, leading to a decrease in glucose uptake (black arrows). As a result of ACC2 deletion (red X), tissues continuously oxidize FA in the mitochondria; this leads to a decrease of FA levels, the down-regulation of PKC Ø, a decrease in the Ser-p Thr-p of IRS1 and IRS2, an increase in IRS-Tyr-p, and the up-regulation of insulin signaling, thereby increasing glucose uptake and lowering the plasma blood glucose level.
AMPK, a negative regulator of ACC activities, is a major therapeutic target against insulin resistance and type 2 diabetes (31). Activating AMPK in rat hind limb muscle by perfusion with AICAR led to an increase in fatty acid and glucose oxidation, an inhibition of ACC2 activity, and a decrease in the level of its malonyl-CoA (32). This simultaneous increase in glucose and fatty acid oxidation suggests that activating AMPK leads to a bypassing of the glucose/fatty acid cycle that was proposed by Randle et al. (33). A similar effect was observed in the white adipocytes of Acc2−/− mutant mice, which also exhibited increased levels of glucose and fatty acid oxidation; this strongly suggests a link between AMPK and its target ACC2 (29). Hence, the activation of AMPK by AICAR in skeletal muscle and the absence of ACC2 in adipose increased the cell-surface GLUT4 content and the up-regulation of GLUT4 mRNA, respectively (29, 32). As a result of continuous fatty acid oxidation, there was a significant reduction in lean body mass, including a heart that was smaller in size but normal in function (30, 34). When Acc2−/− mice were fed a high-fat diet, their peripheral and hepatic insulin sensitivities were increased. These improvements in insulin-stimulated glucose metabolism were associated with increases in insulin-stimulated glucose uptake in lipogenic tissues and in heart and skeletal muscle, reduced lipid accumulation in muscle and liver, decreased PKC Ø activity, and increased insulin-stimulated AKT activity in all of these tissues (Fig. 2).
Recent advances in the study of fatty acid metabolism involve several important attempts to develop and test ACC inhibitors. Selective inhibitors against ACC1 and ACC2, and specific ACC2 inhibitors, have been reported (35). However, when used in animals, the inhibitors should reduce fatty acid synthesis and body weight and increase both fatty acid oxidation and insulin sensitivity. Because some of the inhibitors may not be specific against ACCs, and because they may target other pathways as well, their efficacy as potential drugs to target the metabolic syndrome remains to be determined. Nevertheless, all of the findings reported in this review affirmed that the inhibition of ACC2 can be a promising and valuable approach for the future treatment of obesity and type 2 diabetes in humans.
Abbreviations
ACC1, acetyl-coenzyme A carboxylase 1
ACC2, acetyl-coenzyme A carboxylase 2
acetyl-CoA, acetyl-coenzyme A
ACLY, ATP citrate lyase
AMPK, AMP-activated kinase
CAT, carnitine/acetyl-CoA
ChREBP, carbohydrate-responsive element-binding protein
CPT1, carnitine/palmitoyl-transferase 1
FA, fatty acid(s)
FAS, fatty acid synthase
FASKOL, FAS knockout in liver
IRS, insulin receptor substrates
MCD, malonyl-CoA decarboxylase
SREBP, sterol response-elements binding protein
TG, triglyceride(s)
WT, wild-type
This work is supported by grants from the National Institute of Health (GM-63115), the Hefni Technical Training Foundation, and the Medallion Foundation.
Published, JLR Papers in Press, December 1, 2008.
References
- 1.Wakil S. J. 1989. The fatty acid synthase: a proficient multifunctional enzyme. Biochemistry. 28 4523–4530. [DOI] [PubMed] [Google Scholar]
- 2.McGarry J. D., G. P. Mannaerts, and D. W. Foster. 1977. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J. Clin. Invest. 60 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakil S. J., E. R. Titchener, and D. M. Gibson. 1958. Evidence for the participation of biotin in the enzymic synthesis of fatty acids. Biochim. Biophys. Acta. 29 225–226. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Elheiga L., D. B. Almarza-Ortega, A. Baldini, and S. J. Wakil. 1997. Human acetyl-CoA carboxylase 2: molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. J. Biol. Chem. 272 10669–10677. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Elheiga L., A. Jayakumar, A. Baldini, S. S. Chirala, and S. J. Wakil. 1995. Human acetyl-CoA carboxylase: characterization, molecular cloning, and evidence for two isoforms. Proc. Natl. Acad. Sci. USA. 92 4011–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Elheiga L., W. R. Brinkley, L. Zhong, S. S. Chirala, G. Woldegiorgis, and S. J. Wakil. 2000. The subcellular localization of acetyl-CoA carboxylase 2. Proc. Natl. Acad. Sci. USA. 97 1444–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Elheiga L., M. Matzuk, P. Kordari, W. Oh, T. Shaikenov, Z-W. Gu, and S. J. Wakil. 2005. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc. Natl. Acad. Sci. USA. 102 12011–12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim K-H. 1997. Regulation of mammalian acetyl coenzyme A carboxylase. Annu. Rev. Nutr. 17 77–99. [DOI] [PubMed] [Google Scholar]
- 9.Brown M. S., and J. L. Goldstein. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 89 331–340. [DOI] [PubMed] [Google Scholar]
- 10.Pegorier J-P., C. Le May, and J. Girard. 2004. Control of gene expression by fatty acids. J. Nutr. 134 2444S–2449. [DOI] [PubMed] [Google Scholar]
- 11.Davies M. N., B. L. O'Callaghan, and H. C. Towle. 2008. Glucose activates ChREBP by increasing its rate of nuclear entry and relieving repression of its transcriptional activity. J. Biol. Chem. 283 24029–24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dentin R., F. Benhamed, I. Hainault, V. Fauveau, F. Foufelle, J. R. Dyck, J. Girard, and C. Postic. 2006. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 55 2159–2170. [DOI] [PubMed] [Google Scholar]
- 13.Iizuka K., R. K. Bruick, G. Liang, J. D. Horton, and K. Uyeda. 2004. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA. 101 7281–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao J., S. S. Chirala, and S. J. Wakil. 2003. Human acetyl-CoA carboxylase 1 gene: presence of three promoters and heterogeneity at the 5′-untranslated mRNA region. Proc. Natl. Acad. Sci. USA. 100 7517–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J. Y., J. J. Lee, and K. S. Kim. 2003. Acetyl-CoA carboxylase beta expression mediated by MyoD and muscle regulatory factor 4 is differentially affected by retinoic acid receptor and retinoid X receptor. Exp. Mol. Med. 35 23–29. [DOI] [PubMed] [Google Scholar]
- 16.Hardie D. G., and D. A. Pan. 2002. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 30 1064–1070. [DOI] [PubMed] [Google Scholar]
- 17.Hardie D. G. 2008. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. (Lond.). 4 (Suppl): S7–S12. [DOI] [PubMed] [Google Scholar]
- 18.Mabrouk G. M., I. M. Helmy, K. G. Thampy, and S. J. Wakil. 1990. Acute hormonal control of acetyl-CoA carboxylase: the roles of insulin, glucagon, and epinephrine. J. Biol. Chem. 265 6330–6338. [PubMed] [Google Scholar]
- 19.Janovská A., G. Hatzinikolas, V. Staikopoulos, J. McInerney, M. Mano, and G. A. Wittert. 2008. AMPK and ACC phosphorylation: effect of leptin, muscle fibre type and obesity. Mol. Cell. Endocrinol. 284 1–10. [DOI] [PubMed] [Google Scholar]
- 20.Chirala S. S., H. Chang, M. Matzuk, L. Abu-Elheiga, J. Mao, K. Mahon, M. Finegold, and S. J. Wakil. 2003. Fatty acid synthesis is essential in embryonic development: Fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc. Natl. Acad. Sci. USA. 100 6558–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigneux A. P., C. Kosinski, B. Gavino, J. D. Horton, W. C. Skarnes, and S. G. Young. 2004. ATP-citrate lyase deficiency in the mouse. J. Biol. Chem. 279 9557–9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen K. F., and G. I. Shulman. 2006. Etiology of insulin resistance. Am. J. Med. 119 (Supplement 1): S10–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao J., F. J. DeMayo, H. Li, L. Abu-Elheiga, Z. Gu, T. E. Shaikenov, P. Kordari, S. S. Chirala, W. C. Heird, and S. J. Wakil. 2006. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc. Natl. Acad. Sci. USA. 103 8552–8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savage D. B., C. S. Choi, V. T. Samuel, Z. X. Liu, D. Zhang, A. Wang, X. M. Zhang, G. W. Cline, X. X. Yu, J. G. Geisler, et al. 2006. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J. Clin. Invest. 116 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An J., D. M. Muoio, M. Shiota, Y. Fujimoto, G. W. Cline, G. I. Shulman, T. R. Koves, R. Stevens, D. Millington, and C. B. Newgard. 2004. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat. Med. 10 268–274. [DOI] [PubMed] [Google Scholar]
- 26.Chakravarthy M. V., Z. Pan, Y. Zhu, K. Tordjman, J. G. Schneider, T. Coleman, J. Turk, and C. F. Semenkovich. 2005. New hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 1 309–322. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Elheiga L., M. M. Matzuk, K. A. H. Abo-Hashema, and S. J. Wakil. 2001. Continuous fatty acid oxidation in mice lacking acetyl-CoA carboxylase 2. Science. 291 2613–2616. [DOI] [PubMed] [Google Scholar]
- 28.Abu-Elheiga L., W. Oh, P. Kordari, and S. J. Wakil. 2003. ACC2 mutant mice are protected against obesity and diabetes induced by high fat high carbohydrate diets. Proc. Natl. Acad. Sci. USA. 100 10207–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh W., L. Abu-Elheiga, P. Kordari, Z. Gu, T. Shaikenove, S. S. Chirala, and S. J. Wakil. 2005. Glucose and fat metabolism in adipose tissue of acetyl-CoA carboxylase 2 knockout mice. Proc. Natl. Acad. Sci. USA. 102 1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi C. S., D. B. Savage, L. Abu-Elheiga, Z-X. Liu, S. Kim, A. Kulkarni, A. Distefano, Y-J. Hwang, R. M. Reznick, R. Codella, et al. 2007. Continuous fat oxidation in Acetyl-CoA carboxylase 2 mutant mice increases total energy expenditure, reduces fat mass and improves insulin sensitivity. Proc. Natl. Acad. Sci. USA. 104 16480–16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruderman N., and M. Prentki. 2004. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 3 340–351. [DOI] [PubMed] [Google Scholar]
- 32.Koistinen H. A., D. Galuska, A. V. Chibalin, J. Yang, J. R. Zierath, G. D. Holman, and H. Wallberg-Henriksson. 2003. 5-Amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 52 1066–1072. [DOI] [PubMed] [Google Scholar]
- 33.Randle P. J., P. B. Garland, E. A. Newsholme, and C. N. Hales. 1965. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann. N. Y. Acad. Sci. 1 324–333. [DOI] [PubMed] [Google Scholar]
- 34.Essop M. F., H. S. Camp, C. S. Choi, S. Sharma, R. M. Fryer, G. A. Reinhart, P. H. Guthrie, A. Bentebibel, Z. Gu, G. I. Shulman, et al. 2008. Reduced heart size and increased myocardial fuel substrate oxidation in ACC2 mutant mice. Am. J. Physiol. Heart Circ. Physiol. 295 H256–H265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong L., and H. J. Harwood, Jr. 2006. Acetyl-coenzyme A carboxylases: versatile targets for drug discovery. J. Cell. Biochem. 99 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]