Abstract
Since the discovery of the phosphoinositide/phospholipase C (PI/PLC) system in animal systems, we know that phospholipids are much more then just structural components of biological membranes. In the beginning, this idea was fairly straightforward. Receptor stimulation activates PLC, which hydrolyses phosphatidylinositol4,5-bisphosphate [PtdIns(4,5)P2] into two second messengers: inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol (DG). While InsP3 difuses into the cytosol and triggers the release of calcium from an internal store via ligand-gated calcium channels, DG remains in the membrane where it recruits and activates members of the PKC family. The increase in calcium, together with the change in phosphorylation status, (in)activates a variety of protein targets, leading to a massive reprogramming, allowing the cell to appropriately respond to the extracellular stimulus. Later, it became obvious that not just PLC, but a variety of other phospholipid-metabolizing enzymes were activated, including phospholipase A, phospholipase D, and PI 3-kinase. More recently, it has become apparent that PtdIns4P and PtdIns(4,5)P2 are not just signal precursors but can also function as signaling molecules themselves. While plants contain most of the components described above, and evidence for their role in cell signaling is progressively increasing, major differences between plants and the mammalian paradigms exist. Below, these are described “in a nutshell.”
Keywords: phosphatidic acid, phosphoinositide, phospholipase
PLC SIGNALING
If we did not know about the existence of the PI/PLC system from animals and only had the data from plants today (1–5), then we would have never come up with a signaling system as depicted in Fig. 1. The reasons for this are briefly summarized below.
Fig. 1.
Plant PI/PLC signaling: Differences and similarities to the mammalian paradigm. Higher plants lack both InsP3 receptor, a ligand-gated Ca2+ channel, and PKC; hence, these are striked-out (X). Instead, plants seem to use their phosphorylated products, InsP6 and PA, as signaling molecules. PA can also be generated by PLD and is attenuated by PA kinase (PAK), a novel lipid kinase that is absent from mammalian cells. PAK generates diacylglycerolpyrophosphate (DGPP), which might function as a signaling molecule itself (19). Plant PLCs belong to the PLCζ subfamily. It is not known how they are regulated but not through heterotrimeric G-proteins (G), which is therefore striked out. Plant PtdIns(4,5)P2 (PIP2) quantities are extremely low, and plant PLCs lack the PH domain (Fig. 2). Instead, PtdIns4P (PIP) is a better candidate to be the in vivo PLC substrate. The resulting InsP2 can be phosphoryated to InsP6 via two dual-specificity inositolpolyphosphate kinases (IPK), while DG is phosphorylated to PA via DGK. Evidence is also emerging that PtdIns4P and PtdIns(4,5)P2 themselves function as signaling molecules, involving membrane trafficking, organization of the cytoskeleton, and regulation of ion channels. In such a scenario, PLC would function as an attenuator of PIP and PIP2 signaling. Solid arrows indicate metabolic conversions. Dashed arrows represent mechanisms of regulation.
No inositol 1,4,5-trisphosphate receptor nor PKC
Several plant genomes have been sequenced nowadays, including Arabidopsis, rice, and poplar, and many expressed sequence tag libraries of various higher plant species are available, but none of them seem to encode an InsP3 receptor (6). An exception is Chlamydomonas, a unicellular green algae with two flagella, where an InsP3 receptor has been identified. Other ciliated organisms, such as Paramecium, also contain an InsP3 receptor. Apparently, higher plants have lost this in evolution (6). Similarly, the most important diacylglycerol (DG) target, PKC, is lacking from plant genomes, including lower plants. There are numerous articles describing an effect of “PKC-specific” inhibitors, but these most likely reflect protein kinases that are not present in mammalians. These include calcium/calmodulin-dependent protein kinase (CDPK), calcineurin B-like proteins that interact specifically with a group of CBL-interacting protein kinases (CIPK), and AGC kinases (with similarities to PKA, PKC, and PKG) (7). CDPKs and CIPKs respond to calcium, while some members of the CDPK and AGC family may be regulated by phosphoinositides and/or phosphatidic acid (PA; see below). Although we were hopeful before the genomic era (3), we have to accept now that plants lack PKC.
Low on PtdInsP2
Labeling experiments using 32Pi or 3H-Inositol, but also recent PtdInsP2 biosensor (GFPPHPLCδ1) studies, have shown that plant cells contain extremely low amounts of PtdInsP2 (8–10). In contrast, PtdIns4P levels seem to be normal, i.e., similar to those observed in animals. Typical 32P-ratios between PtdInsP and PtdInsP2, measured in many different plant cells and tissues, reveal 30- to 100-fold lower PtdInsP2 levels than PtdInsP. In animals and Chlamydomonas, the PtdInsP:PtdInsP2 ratio is usually close to 1 (1–3).
Plant PLCs belong to the PLCζ class
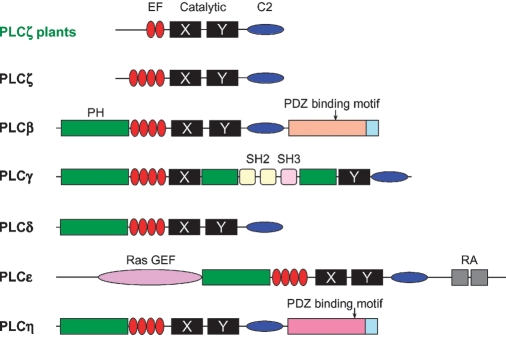
Eukaryotic PI/PLCs have been classified into β, γ, δ, ɛ, η, and ζ subfamilies (Fig. 2). While mammalian cells contain all six isoforms (13 in total), plants exhibit only one class of PLCs. Originally, these were classified as PLCδ isoforms (4); however, after the recent discovery of the sperm-specific mammalian PLCζ, which lacks the typical Pleckstrin homology (PH) domain, it became clear that plant PLCs belong to the PLCζ class (Fig. 2) (12).
Fig. 2.
Domain structure and organization of PI/PLC isozymes. Plant PLCs belong to the most simple group, the PLCζs. PLCη undergoes alternative splicing, generating variable C termini with a PDZ binding motif being only present in the longer forms. Adapted from (11).
The ζ-isoform represents the most simple PI/PLC, consisting of the minimal core structure: the catalytic X- and Y-domain, an EF-hand domain, and a C2 lipid binding domain (Fig. 2). Additional subfamilies contain the PH domain and various other conserved sequence regions, allowing PLCβs to be regulated by trimeric G-proteins, PLCγs by tyrosine kinases, and PLCɛs by trimeric G-proteins and Ras. It is still not clear how PLCδ, -η, and -ζ isoforms are regulated, but this may involve calcium, especially for PLCη (Fig. 2) (11).
Plant PLC activity indeed requires calcium. At low micromolar Ca2+ concentrations, both PtdIns4P and PtdIns(4,5)P2 are hydrolyzed and at millimolar concentrations, PLC also uses PtdIns as substrate (3). In vivo, PLC has always been assumed to hydrolyze PtdIns(4,5)P2. However, plants have no, or very little, PtdInsP2 in their membranes, and an InsP3 receptor is lacking, so wouldn't it make more sense to propose PtdIns4P as the in vivo substrate? In vitro, PtdIns4P is equally well hydrolyzed as PtdIns(4,5)P2, and in vivo, PtdIns4P turnover and quantities are much more in agreement with the PA responses resulting from DG phosphorylation than PtdIns(4,5)P2. Moreover, plant PLCs lack the PH domain and are thus unlikely to find the few molecules of PtdIns(4,5)P2. Recent studies using a PtdIns4P biosensor indicate that there is plenty of PtdIns4P in the plasma membrane, with at least one additional pool occurring at the Golgi (8).
InsP6 rather than InsP3
When microinjected, or released via photoactivation of a caged variant, InsP3 was shown to release Ca2+ from an intracellular store in the early 90s. Obviously, this fitted the paradigm, so the plant PI system was a fact, even though Robin Irvine was still skeptical (13). It now seems he was right! Recent work from Brearley's lab indicates that the Ca2+ release is actually caused by InsP6 (14). InsP6 was shown to release Ca2+ at a 10-fold lower concentration than InsP3, and when InsP3 was microinjected, it was rapidly converted into InsP6. Also, the hormonal stimulation via abscisic acid (ABA) (to which it was linked) was shown to generate an InsP6 response rather than InsP3 (14).
In yeast, InsP6 is not related to Ca2+ signaling but directly regulates gene transcription and mRNA export from the nucleus. This pathway involves a PLC and two inositol polyphosphate multikinases, which can stepwise phosphorylate InsP3 to InsP6 (15). Could this reflect the pathway that is operational in plants too? PLCζ could hydrolyze PtdIns4P to produce Ins(1,4)P2, which would then be sequentially phosphorylated by similar inositol dual-specificity polyphosphate multikinases (IPK). Two such Arabidopsis IPK genes, AtIPKβ1 and AtIPKβ2, have recently been identified (Table 1) (16).
TABLE 1.
Phospholipid signaling KO mutants in Arabidopsis
| Class | Arabidopsis Gene | Enzymatic Activity (in Vitro) | Phenotype | Refs. |
|---|---|---|---|---|
| PLA | AtPLAI | Acylhydrolase (sn-1 and sn-2) preferring galactolipids over phospholipids | Less resistant to necrotrophic fungus Botrytis cinerea | (35) |
| AtDAD1 | sn1-acylhydrolase in JA pathway | Anther dehiscence, pollen maturation, and flower opening | (41) | |
| PLC | AtPLC | PI/PLC | Lateral root growth | –a |
| PLD | AtPLDα1 | Phospholipase D | Reduced ABA responses; enhanced seed quality | (55, 56) |
| AtPLDα3 | Phospholipase D | Reduced salt tolerance | (57) | |
| AtPLDδ | Phospholipase D | Increased sensitivity to oxidative stress; reduced freezing tolerance | (58, 59) | |
| AtPLDα1/PLDδ | Phospholipase D | Reduced salt and osmotic stress tolerance | (60) | |
| AtPLDζ2 | Phospholipase D | Reduced auxin sensitivity; increased sensitivity to Pi starvation | (61, 62) | |
| AtPLDζ1/2 | Phospholipase D | Increased sensitivity to Pi starvation | (63) | |
| PI3K | AtVPS34 | PI 3-kinase | Lethal; antisense plants are severely affected in growth and development | (43–45) |
| PI4K | AtPI4Kβ1 | PI 4-kinase | AtPI4Kβ1/β2 double mutants display distorted root hair development | (24) |
| AtPI4Kβ2 | PI 4-kinase | |||
| PIP5K | AtPIP5K3 | PI4P 5 kinase | Shorter root hairs | (22, 23) |
| 3PTase | AtPTEN1 | Dual specificity 3-PTase and Tyr phosphatase | Pollen development (RNA interference) (pollen cell death after mitogenesis) | (54) |
| 4PTase | RHD4, AtSAC7 | PtdIns4P 4-phosphatase | Bulging root hairs | (25) |
| 5PTase | FRA7 AtSAC1 | PtdIns(3,5)P2 5-phosphatase | Alterations in actin cytoskeleton organization and reduced cell wall thickening | (53) |
| AtSAC9 | Ins(1,4,5)P3- and PtdIns(4,5)P2 5-phosphatase | Reduced growth, hyponastic, purple stress leaves | (64) | |
| At5Ptase1 and 2 | Type I inositol polyphosphate 5-phosphatase toward Ins(1,4,5)P3 | Germination and seedling development in double mutant | (65) | |
| FRA3 | Type II inositol poly-phosphate 5-phosphatase; PtdIns(4,5)P2 > Ins(1,4,5)P3 | Reduction in secondary wall thickness and stem strength, alterated actin deposition in fiber cells | (66) | |
| At5Ptase13 | Type I inositol polyphosphate 5-phosphatase toward Ins(1,4,5)P3 | Altered auxin levels, blue light signaling | (67, 68) | |
| MRH3 (At5Ptase5) | Inositol polyphosphate 5-phosphatase | Root hair initiation | (69) | |
| CVP2 (At5Ptase6) | Inositol polyphosphate 5-phosphatase | Vascular patterning cotyledon | (70) | |
| IPK | AtIPK1/AtIPK2β | Inositolpolyphospate kinase | InsP6 levels down, Pi sensing, and root hair growth | (16) |
Unpublished observations.
Plants are well known for their phytate (InsP6) content in seeds, which mainly reflects the mechanism to store huge amounts of phosphate and inositol required for germination. Nonetheless, InsP6 may play a completely different role during plant development and in response to agonists, similar to the one that is emerging in mammalian and yeast fields (15). An exciting example of such may be the unexpected discovery of InsP6 in the crystal structure of the auxin (a plant hormone) receptor, TIR1 where it is structurally required for auxin binding and receptor function (17). The major question now is, does this reflect InsP6 signaling and does it require a PLC-mediated pathway?
PA signaling rather than DG
A convincing role for DG as a plant signaling molecule has never really been reported. Though it cannot be excluded, the lack of evidence over the last 20 years, together with the absence of its primary target PKC, leaves very little ground to put it into the plant PI/PLC model today (Fig. 1). As a precursor for glycolipids, storage lipids, and the major structural phospholipids, together accounting for ∼90% of all plant lipids, DG does not seem to be the most favorable molecule as a membrane-localized-second messenger either. Instead, we see that the PLC-generated DG is rapidly phosphorylated to PA by DG kinase (DGK) and that PA has typically emerged as the plant's second messenger (5, 18, 28). Over the years, a number of biotic (pathogens) and abiotic (e.g., temperature, osmotic) stress signals have been shown to activate this pathway; meanwhile, a number of plant PA targets have been identified (5). Arabidopsis contains seven DGK genes, which are differentially expressed throughout the plant and in response to stress, but knockout mutants have not resulted in a phenotype yet (unpublished observations), indicating a high degree of redundancy. PA can also be generated via the PLD pathway. More about PLD, PA signaling, and downstream targets can be found below.
PAK and DGPP
What is also different from mammalian systems is that PA can be phosphorylated into DGPP by a PA kinase (PAK) (Fig. 1). The gene encoding this novel lipid kinase is still unknown, but the enzyme seems to be present in every tissue and is enriched in plasma membrane fractions. The idea is that PAK attenuates PA signaling, but DGPP could also be a signaling molecule itself (19).
Inositol lipids and phosphates
Apart from being signaling precursors, it is evident that PtdIns4P and PtdIns(4,5)P2 can also function as signaling molecules themselves. Both lipids are involved in polar growth of pollen tubes and root hairs and during cell plate formation, as shown by lipid biosensors, but also judged from the recent phenotypes of PI 4-kinase, PtdInsP 5-kinase and PtdIns4P phosphatase mutants (Table 1) (8, 20–25). Similarly, several cell wall assembly mutants have been identified that may reflect defects in vesicular trafficking (fragile fibers; FRA mutants) and vascular patterning (Table 1). These include several 5-phosphatase mutants, of which it is not always clear whether this affects the lipid or the inositolphosphate, nonetheless emphasizing their importance.
Transgenic plants constitutively overexpressing a (human) InsP3 5-phosphatase have been reported to have various phenotypes. While these have been interpreted as “attenuation of InsP3 signaling” (26), they might very well reflect defects in InsP6 or raffinose family oligosacharide metabolism. The latter requires inositol as a precursor and functions to protect cellular structures during desiccation and as carbon reserves for early germination (27).
PHOSPHOLIPASE D
As mentioned above, PA is emerging as an important plant lipid second messenger. PA is rapidly and transiently generated in response to a variety of biotic and abiotic stresses, either via the PLC/DGK pathway as discussed above, or directly via PLD.
This enzyme catalyzes the hydrolysis of structural lipids, like PC and PE, to produce PA and the respective headgroup. Using differential 32P1-labeling techniques and PLD-specific transphosphatidylation assays, it is possible to distinguish between both PA-generating pathways (28). In this way, a variety of environmental cues have been shown to activate the PLD pathway, including plant defense elicitors, cold, wounding, heat, oxidative stress, and osmotic stress (5, 29, 30).
Plants are true PLD champions. While humans only contain 2 PLD genes, and yeast 1 (SPO14), Arabidopsis contains 12 PLD genes and rice even 17 (29–31). Plant PLDs can be classified into 2 groups based on their lipid binding domains. Those with a combined PX and PH domain belong to the PLDζ class and are homologous to the mammalian and yeast PLDs. The others, representing the majority of plant PLDs, belong to the C2 class, containing a C2 (calcium and lipid binding) domain. Arabidopsis has 10 C2-PLDs, PLDα1-3, β1-2, γ1-3, δ, and ɛ, and two PX-PH-PLDs, PLDζ1-2.
Using T-DNA insertion knockout mutants, individual PLDs have been linked to specific plant responses, including ABA signaling, osmotic stress, reactive oxygen species (ROS), freezing, auxin, Pi starvation, and root and root hair development (Table 1).
PA properties and its targets
PA formation has a profound effect on membrane curvature and surface charge. Its small anionic phosphomonoester headgroup resides very close to the hydrophobic interior of the lipid bilayer, which is different from other anionic phospholipids. Moreover, hydrogen bonding increases the negative charge of PA, explaining why it can form strong interactions with target proteins, which has recently been proposed as the electrostatic/hydrogen bond switch model (32). The combined effects are likely to be crucial for specific PA responses (33). Major progress has been made in identifying molecular PA targets. Like in mammalian cells, these include protein kinases, phosphatases, and proteins involved in membrane trafficking and the organization of the cytoskeleton. Both activation of positive regulators and inhibition of negative regulators have been reported (5, 18). Examples include PDK1, mediating responses to ROS in root hair development and pathogens, CTR1, a crucial protein kinase in ethylene signaling, ABI1, a protein phosphatase in ABA signaling, AtCP, an actin capping protein, and AGD7, an ArfGAP (32). Though several PA binding motifs have been recognized (5, 34), a general PA binding domain still remains obscure.
PHOSPHOLIPASE A
PLA catalyzes the hydrolysis of phospholipids into lysophospholipids and free fatty acids, either at the sn-1 (PLA1) or 2-position (PLA2) of the glycerol backbone, or both (PLB). Plants contain numerous PLAs. In Arabidopsis, three different families can be distinguished: four small secretory sPLAs, 10 patatin-like pPLAs, and 14 lipase-like PLA1s. For most, it is not clear what their substrate is or which position they hydrolyze; some exhibit acyltransferase or acylhydrolyse activity, sometimes even toward nonphospholipids, such as galactolipids (35). As such, it is not always clear whether effects reflect general lipid metabolism or signaling. Nonetheless, evidence is increasing for PLA's involvement in disease resistance, auxin, and light (Table 1; 35–39).
Similar to the eicosanoid (C20) pathway in animals, plants exhibit an octadecanoid (C18) pathway, playing an important role in, for example, the plant's defense against pathogens and herbivores, in particular, jasmonic acid (JA) and its volatile, methyl JA (40). JA is also important in flower development. The latter involves DAD (for defective in anther dehishence), a gene encoding a PLA1 (41).
Lysophopholipids have also been also implicated in cell signaling (3). In particular, lysophosphatidylcholine, which is proposed to activate a vacuolar H+/Na+ antiporter to regulate the cytosolic pH in response to a pathogenic elicitor (36, 42).
PI3 KINASE
Arabidopsis only contains one PI3K, which is Vps34p-like (type III). Silencing AtVPS43 causes severe defects in development, while knockout mutants are lethal, indicating an important function (43–45). About 5–15% of the PtdInsP pool in plants is PtdIns3P, with the majority (∼80%) being PtdIns4P and containing a few percent of PtdIns5P (46). PtdIns3P has been imaged in living cells by expressing the PtdIns3P biosensor YFP-2xFYVE, revealing predominant labeling of late endosomes, multivesicular bodies, and prevacuolar membranes (9). Only ∼20% of the early endosomes were labeled. The YFP-2xFYVE labeling pattern is sensitive to wortmannin, as all labeled structures disappeared within 20 min of treatment, with all fluorescence reappearing in the nucleus. HPLC-headgroup analyses revealed that the FYVE-overexpressing cells contained double amounts of PtdIns3P. Apparently, cells sense free PtdIns3P levels, and since overexpression of FYVE leads to preoccupation of PtdIns3P, competing with endogenous targets, cells simply make more. This probably also explains why there is no apparent phenotype in cell suspensions or Arabidopsis seedlings that constitutively express the sensor (9), although overexpression behind a root-hair-specific promotor did have a dose-dependent effect on root hair elongation (47). Wortmannin and LY294002 inhibit root hair growth. The same inhibitors have been used to imply the involvement of PtdIns3P in the production of ROS and actin dynamics (43, 47, 48). In Arabidopsis, 11 proteins with a PX and 16 with a FYVE domain have been predicted (49).
Other D3-PPI
Plants contain small amounts of PtdIns(3,5)P2 but lack PtdInsP3. Earlier, the presence of PtdIns(3,4)P2 had been reported, but this was before the discovery of PtdIns(3,5)P2 and has not been reproduced so far (50). Like yeast, plants make PtdIns(3,5)P2 in response to osmotic stress (50). Yeast PtdIns(3,5)P2 is involved in the retrograde trafficking between organelles and the endocytic/lysosomal system and made by a PtdIns3P 5-kinase called Fab1p (51). FAB1 mutants (for formation of aploid and binucleate cells) have enlarged vacuoles that do not acidify correctly and have nuclear segregation defects. Arabidopsis contains 4 putative FAB genes (1). Proposed effectors for PtdIns(3,5)P2 include the PROPPIN family of seven-bladed β-propellers (51) of which Arabidopsis homologs are present.
Degradation of PtdIns(3,5)P2 occurs through 3- or 5-phosphatases. In vitro, At5PTase11 and AtSAC1/FRA7 have been shown to dephosphorylate PtdIns(3,5)P2 at the 5-position (52). AtSAC1/FRA7 mutants have defects in the organization of the actin cytoskeleton and exhibit reduced cell wall thickening (53). Whether PtdIns(3,5)P2 is the (only) substrate in vivo remains to be shown. In total, Arabidopsis contains 15 At5PTase and 9 SAC genes (80–89). The SAC (for suppressor of actin) family of phosphatases contains both 4- and 5-specific phosphatases (Table 1).
3-Phosphatases
Arabidopsis contains three homologs of PTEN (54) and two potential MTM (myotubularin-type phosphatase) genes that are predicted to be catalytically active. AtPTEN1 is a dual-specificity phosphatase which, in vitro, has phosphatase activity toward phosphotyrosine and PtdInsP3. It is exclusively found in pollen grains and expressed during the later stages of development. Knockout mutants are lethal, and RNA interference suppression results in cell death after mitosis, indicating that this gene is essential for pollen tube development (54).
Acknowledgments
The authors apologize to those whose original work could not be cited due to heavy restrictions in the number of references.
Abbreviations
ABA, abscisic acid
CDPK, calcium/calmodulin-dependent protein kinase
DG, diacylglycerol
DGK, diacylglycerol kinase
DGPP, diacylglycerolpyrophosphate
InsP3, inositol 1,4,5-trisphosphate
IPK, inositol dual-specificity polyphosphate multikinase
JA, jasmonic acid
PA, phosphatidic acid
PAK, phosphatidic acid kinase
PH, Pleckstrin homology domain
PI/PLC, phosphoinositide/phospholipase C
PLA, phospholipase A
PLD, phospholipase D
PtdInsP2, phosphatidylinositol4,5-bisphosphate
ROS, reactive oxygen species
The authors gratefully acknowledge financial support from the Netherlands Organization for Scientific Research (VIDI 864.05.001, VIDI 700.56.429, and ECHO 700.56.007) and the European Union (COST FA0605).
Published, JLR Papers in Press, December 20, 2008.
References
- 1.Meijer H. J., and T. Munnik. 2003. Phospholipid-based signaling in plants. Annu. Rev. Plant Biol. 54 265–306. [DOI] [PubMed] [Google Scholar]
- 2.Boss W. F., A. J. Davis, Y. J. Im, R. M. Galvao, and I. Y. Perera. 2006. Phosphoinositide metabolism: towards an understanding of subcellular signaling. Subcell. Biochem. 39 181–205. [DOI] [PubMed] [Google Scholar]
- 3.Munnik T., R. F. Irvine, and A. Musgrave. 1998. Phospholipid signalling in plants. Biochim. Biophys. Acta. 1389 222–272. [DOI] [PubMed] [Google Scholar]
- 4.Mueller-Roeber B., and C. Pical. 2002. Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol. 130 22–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testerink C., and T. Munnik. 2005. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci. 10 368–375. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler G. L., and C. Brownlee. 2008. Ca2+ signalling in plants and green algae - changing channels. Trends Plant Sci. 13 506–514. [DOI] [PubMed] [Google Scholar]
- 7.Bogre L., L. Okresz, R. Henriques, and R. G. Anthony. 2003. Growth signalling pathways in Arabidopsis and the AGC protein kinases. Trends Plant Sci. 8 424–431. [DOI] [PubMed] [Google Scholar]
- 8.Vermeer, J. E., J. M. Thole, J. Goedhart, E. Nielsen, T. Munnik, and T. W. Gadella, Jr. 2008. Imaging phosphatidylinositol 4-phosphate dynamics in living plant cells. Plant J. 10.1111/j.1365–313X.2008.03679.x. [DOI] [PubMed]
- 9.Vermeer J. E., W. van Leeuwen, R. Tobena-Santamaria, A. M. Laxalt, D. R. Jones, N. Divecha, T. W. Gadella, Jr., and T. Munnik. 2006. Visualization of PtdIns3P dynamics in living plant cells. Plant J. 47 687–700. [DOI] [PubMed] [Google Scholar]
- 10.van Leeuwen W., J. E. Vermeer, T. W. Gadella, Jr., and T. Munnik. 2007. Visualization of phosphatidylinositol 4,5-bisphosphate in the plasma membrane of suspension-cultured tobacco BY-2 cells and whole Arabidopsis seedlings. Plant J. 52 1014–1026. [DOI] [PubMed] [Google Scholar]
- 11.Cockcroft S. 2006. The latest phospholipase C, PLCɛ, is implicated in neuronal function. Trends Biochem. Sci. 31 4–7. [DOI] [PubMed] [Google Scholar]
- 12.Tasma I. M., V. Brendel, S. A. Whitham, and M. K. Bhattacharyya. 2008. Expression and evolution of the phosphoinositide-specific phospholipase C gene family in Arabidopsis thaliana. Plant Physiol. Biochem. 46 627–637. [DOI] [PubMed] [Google Scholar]
- 13.Irvine R. 1990. Cell physiology. Messenger gets the green light. Nature. 346 700–701. [DOI] [PubMed] [Google Scholar]
- 14.Lemtiri-Chlieh F., E. A. MacRobbie, A. A. Webb, N. F. Manison, C. Brownlee, J. N. Skepper, J. Chen, G. D. Prestwich, and C. A. Brearley. 2003. Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc. Natl. Acad. Sci. USA. 100 10091–10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michell R. H. 2008. Inositol derivatives: evolution and functions. Nat. Rev. Mol. Cell Biol. 9 151–161. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson-Paulik J., R. J. Bastidas, S. T. Chiou, R. A. Frye, and J. D. York. 2005. Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc. Natl. Acad. Sci. USA. 102 12612–12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan X., L. I. Calderon-Villalobos, M. Sharon, C. Zheng, C. V. Robinson, M. Estelle, and N. Zheng. 2007. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 446 640–645. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., S. P. Devaiah, W. Zhang, and R. Welti. 2006. Signaling functions of phosphatidic acid. Prog. Lipid Res. 45 250–278. [DOI] [PubMed] [Google Scholar]
- 19.van Schooten B., C. Testerink, and T. Munnik. 2006. Signalling diacylglycerol pyrophosphate, a new phosphatidic acid metabolite. Biochim. Biophys. Acta. 1761 151–159. [DOI] [PubMed] [Google Scholar]
- 20.Dowd P. E., S. Coursol, A. L. Skirpan, T. H. Kao, and S. Gilroy. 2006. Petunia phospholipase C1 is involved in pollen tube growth. Plant Cell. 18 1438–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helling D., A. Possart, S. Cottier, U. Klahre, and B. Kost. 2006. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell. 18 3519–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenzel I., T. Ischebeck, S. Konig, A. Holubowska, M. Sporysz, B. Hause, and I. Heilmann. 2008. The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell. 20 124–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusano H., C. Testerink, J. E. Vermeer, T. Tsuge, H. Shimada, A. Oka, T. Munnik, and T. Aoyama. 2008. The Arabidopsis Phosphatidylinositol Phosphate 5-Kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell. 20 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preuss M. L., A. J. Schmitz, J. M. Thole, H. K. Bonner, M. S. Otegui, and E. Nielsen. 2006. A role for the RabA4b effector protein PI-4Kβ1 in polarized expansion of root hair cells in Arabidopsis thaliana. J. Cell Biol. 172 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thole J. M., J. E. Vermeer, Y. Zhang, T. W. Gadella, Jr., and E. Nielsen. 2008. Root hair defective4 encodes a phosphatidylinositol-4-phosphate phosphatase required for proper root hair development in Arabidopsis thaliana. Plant Cell. 20 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera I. Y., C. Y. Hung, C. D. Moore, J. Stevenson-Paulik, and W. F. Boss. 2008. Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase exhibit increased drought tolerance and altered abscisic acid signaling. Plant Cell. 20 2876–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karner U., T. Peterbauer, V. Raboy, D. A. Jones, C. L. Hedley, and A. Richter. 2004. myo-Inositol and sucrose concentrations affect the accumulation of raffinose family oligosaccharides in seeds. J. Exp. Bot. 55 1981–1987. [DOI] [PubMed] [Google Scholar]
- 28.Munnik T. 2001. Phosphatidic acid: an emerging plant lipid second messenger. Trends Plant Sci. 6 227–233. [DOI] [PubMed] [Google Scholar]
- 29.Wang X. 2005. Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 139 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bargmann B. O., and T. Munnik. 2006. The role of phospholipase D in plant stress responses. Curr. Opin. Plant Biol. 9 515–522. [DOI] [PubMed] [Google Scholar]
- 31.Li G., F. Lin, and H. W. Xue. 2007. Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLDβ1 in seed germination. Cell Res. 17 881–894. [DOI] [PubMed] [Google Scholar]
- 32.Kooijman, E. E., and C. Testerink. 2009. Phosphatidic acid - an electrostatic/hydrogen bond switch? In Plant Lipid Signaling. T. Munnik, editor. Springer-Verlag, Heidelberg. In press
- 33.Roth M. G. 2008. Molecular mechanisms of PLD function in membrane traffic. Traffic. 9 1233–1239. [DOI] [PubMed] [Google Scholar]
- 34.Stace C. L., and N. T. Ktistakis. 2006. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim. Biophys. Acta. 1761 913–926. [DOI] [PubMed] [Google Scholar]
- 35.Yang W., S. P. Devaiah, X. Pan, G. Isaac, R. Welti, and X. Wang. 2007. AtPLAI is an acyl hydrolase involved in basal jasmonic acid production and Arabidopsis resistance to Botrytis cinerea. J. Biol. Chem. 282 18116–18128. [DOI] [PubMed] [Google Scholar]
- 36.Viehweger K., W. Schwartze, B. Schumann, W. Lein, and W. Roos. 2006. The Galpha protein controls a pH-dependent signal path to the induction of phytoalexin biosynthesis in Eschscholzia californica. Plant Cell. 18 1510–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo J., H. Y. Lee, H. Choi, Y. Choi, Y. Lee, Y. W. Kim, S. B. Ryu, and Y. Lee. 2008. Phospholipase A2β mediates light-induced stomatal opening in Arabidopsis. J. Exp. Bot. 59 3587–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherer G. F., M. Zahn, J. Callis, and A. M. Jones. 2007. A role for phospholipase A in auxin-regulated gene expression. FEBS Lett. 581 4205–4211. [DOI] [PubMed] [Google Scholar]
- 39.Holk A., S. Rietz, M. Zahn, H. Quader, and G. F. Scherer. 2002. Molecular identification of cytosolic, patatin-related phospholipases A from Arabidopsis with potential functions in plant signal transduction. Plant Physiol. 130 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsir L., H. S. Chung, A. J. Koo, and G. A. Howe. 2008. Jasmonate signaling: a conserved mechanism of hormone sensing. Curr. Opin. Plant Biol. 11 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishiguro S., A. Kawai-Oda, J. Ueda, I. Nishida, and K. Okada. 2001. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell. 13 2191–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viehweger K., B. Dordschbal, and W. Roos. 2002. Elicitor-activated phospholipase A2 generates lysophosphatidylcholines that mobilize the vacuolar H+ pool for pH signaling via the activation of Na+-dependent proton fluxes. Plant Cell. 14 1509–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leshem Y., L. Seri, and A. Levine. 2007. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 51 185–197. [DOI] [PubMed] [Google Scholar]
- 44.Lee Y., E. S. Kim, Y. Choi, I. Hwang, C. J. Staiger, Y. Y. Chung, and Y. Lee. 2008. The Arabidopsis phosphatidylinositol 3-kinase is important for pollen development. Plant Physiol. 147 1886–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welters P., K. Takegawa, S. D. Emr, and M. J. Chrispeels. 1994. AtVPS34, a phosphatidylinositol 3-kinase of Arabidopsis thaliana, is an essential protein with homology to a calcium-dependent lipid binding domain. Proc. Natl. Acad. Sci. USA. 91 11398–11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meijer H. J., C. P. Berrie, C. Iurisci, N. Divecha, A. Musgrave, and T. Munnik. 2001. Identification of a new polyphosphoinositide in plants, phosphatidylinositol 5-monophosphate (PtdIns5P), and its accumulation upon osmotic stress. Biochem. J. 360 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee Y., G. Bak, Y. Choi, W. I. Chuang, H. T. Cho, and Y. Lee. 2008. Roles of phosphatidylinositol 3-kinase in root hair growth. Plant Physiol. 147 624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi Y., Y. Lee, B. W. Jeon, C. J. Staiger, and Y. Lee. 2008. Phosphatidylinositol 3- and 4-phosphate modulate actin filament reorganization in guard cells of day flower. Plant Cell Environ. 31 366–377. [DOI] [PubMed] [Google Scholar]
- 49.van Leeuwen W., L. Okresz, L. Bogre, and T. Munnik. 2004. Learning the lipid language of plant signalling. Trends Plant Sci. 9 378–384. [DOI] [PubMed] [Google Scholar]
- 50.Meijer H. J. G., N. Divecha, H. van den Ende, A. Musgrave, and T. Munnik. 1999. Hyperosmotic stress induces rapid synthesis of phosphatidyl-D-inositol 3,5-bisphosphate in plant cells. Planta. 208 294–298. [Google Scholar]
- 51.Dove S. K., and Z. E. Johnson. 2007. Our FABulous VACation: a decade of phosphatidylinositol 3,5-bisphosphate. Biochem. Soc. Symp. 74 129–139. [DOI] [PubMed] [Google Scholar]
- 52.Ercetin M. E., and G. E. Gillaspy. 2004. Molecular characterization of an Arabidopsis gene encoding a phospholipid-specific inositol polyphosphate 5-phosphatase. Plant Physiol. 135 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong R., D. H. Burk, C. J. Nairn, A. Wood-Jones, W. H. Morrison 3rd, and Z. H. Ye. 2005. Mutation of SAC1, an Arabidopsis SAC domain phosphoinositide phosphatase, causes alterations in cell morphogenesis, cell wall synthesis, and actin organization. Plant Cell. 17 1449–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta R., J. T. Ting, L. N. Sokolov, S. A. Johnson, and S. Luan. 2002. A tumor suppressor homolog, AtPTEN1, is essential for pollen development in Arabidopsis. Plant Cell. 14 2495–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W., C. Qin, J. Zhao, and X. Wang. 2004. Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA. 101 9508–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devaiah S. P., X. Pan, Y. Hong, M. Roth, R. Welti, and X. Wang. 2007. Enhancing seed quality and viability by suppressing phospholipase D in Arabidopsis. Plant J. 50 950–957. [DOI] [PubMed] [Google Scholar]
- 57.Hong Y., X. Pan, R. Welti, and X. Wang. 2008. Phospholipase Dα3 is involved in the hyperosmotic response in Arabidopsis. Plant Cell. 20 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W., M. Li, W. Zhang, R. Welti, and X. Wang. 2004. The plasma membrane-bound phospholipase Dδ enhances freezing tolerance in Arabidopsis thaliana. Nat. Biotechnol. 22 427–433. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W., C. Wang, C. Qin, T. Wood, G. Olafsdottir, R. Welti, and X. Wang. 2003. The oleate-stimulated phospholipase D, PLDδ, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell. 15 2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bargmann, B. O., A. M. Laxalt, B. T. Riet, B. van Schooten, E. Merquiol, C. Testerink, M. A. Haring, D. Bartels, and T. Munnik. Multiple Plds required for high salinity and water-deficit tolerance in plants. Plant Cell Physiol. Epub ahead of print. November 18, 2008; doi:10.1093/pcp/pcn173. [DOI] [PMC free article] [PubMed]
- 61.Cruz-Ramirez A., A. Oropeza-Aburto, F. Razo-Hernandez, E. Ramirez-Chavez, and L. Herrera-Estrella. 2006. Phospholipase Dζ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc. Natl. Acad. Sci. USA. 103 6765–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li G., and H. W. Xue. 2007. Arabidopsis PLDζ2 regulates vesicle trafficking and is required for auxin response. Plant Cell. 19 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li M., C. Qin, R. Welti, and X. Wang. 2005. Double knockouts of phospholipase Dζ1 and ζ2 in Arabidopsis affect root elongation during phosphate-limited growth, but do not affect root hair patterning. Plant Physiol. 140 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams M. E., J. Torabinejad, E. Cohick, K. Parker, E. J. Drake, J. E. Thompson, M. Hortter, and D. B. Dewald. 2005. Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress-response pathway. Plant Physiol. 138 686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunesekera B., J. Torabinejad, J. Robinson, and G. E. Gillaspy. 2007. Inositol polyphosphate 5-phosphatases 1 and 2 are required for regulating seedling growth. Plant Physiol. 143 1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong R., D. H. Burk, W. H. Morrison III, and Z. H. Ye. 2004. FRAGILE FIBER3, an Arabidopsis gene encoding a type II inositol polyphosphate 5-phosphatase, is required for secondary wall synthesis and actin organization in fiber cells. Plant Cell. 16 3242–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin W. H., Y. Wang, B. Mueller-Roeber, C. A. Brearley, Z. H. Xu, and H. W. Xue. 2005. At5PTase13 modulates cotyledon vein development through regulating auxin homeostasis. Plant Physiol. 139 1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen X., W. H. Lin, Y. Wang, S. Luan, and H. W. Xue. 2008. An inositol polyphosphate 5-phosphatase functions in PHOTOTROPIN1 signaling in Arabidopis by altering cytosolic Ca2+. Plant Cell. 20 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones M. A., M. J. Raymond, and N. Smirnoff. 2006. Analysis of the root-hair morphogenesis transcriptome reveals the molecular identity of six genes with roles in root-hair development in Arabidopsis. Plant J. 45 83–100. [DOI] [PubMed] [Google Scholar]
- 70.Carland F. M., and T. Nelson. 2004. Cotyledon vascular pattern2-mediated inositol (1, 4, 5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs. Plant Cell. 16 1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]