Abstract
Phospholipid synthesis in the yeast Saccharomyces cerevisiae is a complex process that involves regulation by both genetic and biochemical mechanisms. The activity levels of phospholipid synthesis enzymes are controlled by gene expression (e.g., transcription) and by factors (lipids, water-soluble phospholipid precursors and products, and covalent modification of phosphorylation) that modulate catalysis. Phosphatidic acid, whose levels are controlled by the biochemical regulation of key phospholipid synthesis enzymes, plays a central role in the regulation of phospholipid synthesis gene expression.
Keywords: phospholipid synthesis, gene regulation, enzyme regulation, phosphatidic acid, yeast
The budding yeast Saccharomyces cerevisiae, with its full complement of organelles, synthesizes membrane phospholipids by pathways that are generally common to those found in higher eukaryotic organisms (Fig. 1) (1, 2). Its tractable genetics has facilitated the identification and characterization of nearly all of the structural and regulatory genes that are involved in de novo phospholipid synthesis (2, 3). Moreover, the purification and characterization of several key phospholipid synthesis enzymes have led to an understanding of the biochemical regulation of phospholipid synthesis (1, 4, 5). In S. cerevisiae, phospholipid synthesis is a complex process that is regulated by both genetic and biochemical mechanisms (1, 3–9). The expression of phospholipid synthesis genes is controlled at the levels of transcription and mRNA stability (1, 3, 10). The activities of key phospholipid synthesis enzymes are regulated by lipids, water-soluble phospholipid precursors and products, and by the covalent modification of phosphorylation (1, 4, 8). Moreover, the regulation of phospholipid synthesis is interrelated with the synthesis of other major lipid classes [e.g., fatty acids, triacylglycerol (TAG), sterols, sphingolipids] (1, 11–15). In this review, we focus on how genetic and biochemical mechanisms work together to regulate phospholipid synthesis.
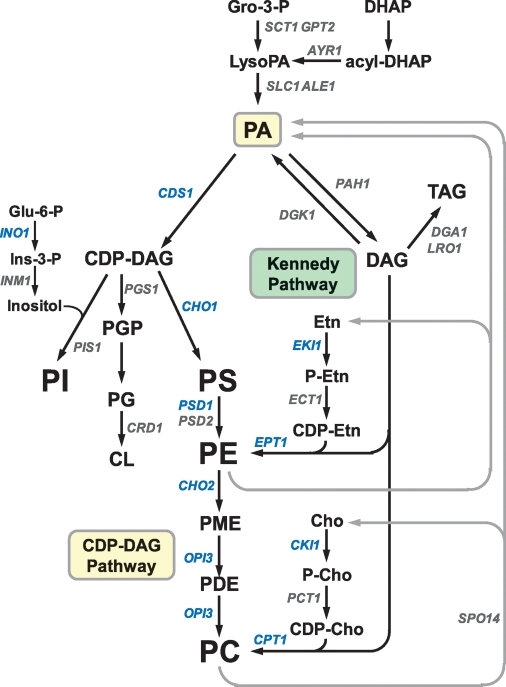
Fig. 1.
Phospholipid synthesis pathways in S. cerevisiae. The pathways shown for the synthesis of phospholipids include the relevant steps discussed in this review. The synthesis of PE and PC from lysoPE and lysoPC, respectively, is not shown in the figure. The genes that are known to encode enzymes catalyzing individual steps in the lipid synthesis pathways are indicated. The UASINO-containing genes that are subject to regulation by the Ino2-Ino4 activation complex and the Opi1 repressor are blue. Gro, glycerol; DHAP, dihydroxyacetone phosphate; Glu, glucose; Ins, inositol; PME, phosphatidylmonomethylethanolamine; PDE, phosphatidyldimethylethanolamine; Etn, ethanolamine; Cho, choline.
PHOSPHOLIPID SYNTHETIC PATHWAYS
The de novo pathways for the synthesis of phospholipids in S. cerevisiae are shown in Fig. 1. All major phospholipids are derived from phosphatidate (PA), which is partitioned between CDP-diacylglycerol (CDP-DAG) and diacylglycerol (DAG). The major phospholipids phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylserine (PS) are primarily synthesized from PA via CDP-DAG (i.e., CDP-DAG pathway) (1, 3). CDP-DAG is also used for the synthesis of phosphatidylglycerol and cardiolipin, phospholipids that are confined to mitochondrial membranes (16). As in higher eukaryotes, DAG is used for the synthesis of the storage lipid TAG and for the synthesis of PE and PC via the CDP-ethanolamine and CDP-choline branches, respectively, of the Kennedy pathway (1, 11). The ethanolamine or choline required for the Kennedy pathway is obtained from growth medium supplementation or from the phospholipase D-mediated turnover of the PE or PC synthesized via CDP-DAG (Fig. 1) (17–19).
Mutants (e.g., cho1, psd1 psd2, cho2, and opi3) defective in the CDP-DAG pathway can synthesize PE or PC if they are supplemented with ethanolamine, choline, lysoPE, lysoPC, or PC with short acyl chains (1, 20–23). Ethanolamine and choline are converted to PE and PC via the Kennedy pathway (Fig. 1). lysoPE and lysoPC are acylated to PE and PC, respectively, by the ALE1-encoded lysophospholipid acyltransferase (21, 22, 24, 25). Short acyl chain PC, which is not incorporated into membranes, is remolded with 16- and 18-carbon acyl chains (26) through the activities of phospholipase B and lysophospholipid acyltransferase (27–29). Kennedy pathway mutants (e.g., cki1 eki1 and cpt1 ept1) defective in both the CDP-choline and CDP-ethanolamine branches synthesize PC only via the CDP-DAG pathway (19, 30, 31). These mutants, unlike those defective in the CDP-DAG pathway (1), do not exhibit any auxotrophic requirements (19, 31).
GENETIC AND BIOCHEMICAL MECHANISMS FOR THE REGULATION OF PHOSPHOLIPID SYNTHESIS
The synthesis of phospholipids is regulated by mechanisms that affect the expression of enzymes and the modulation of their activities. The expression of phospholipid synthesis genes is controlled by multiple factors, including nutrient availability, growth stage, pH, and temperature (1, 2, 9, 16, 28, 32). The mechanisms responsible for the regulation of gene expression include a number of cis- and trans-acting elements (7, 9). In this review, we focus on phospholipid synthesis genes that contain the inositol-responsive element (UASINO) and that are regulated by the transcription factors Ino2, Ino4, and Opi1. As will be discussed below, the transcriptional regulation of UASINO-containing genes is generally triggered by the biochemical regulation of phospholipid synthesis enzymes.
Genes encoding enzymes in both the CDP-DAG (CDS1, CHO1, PSD1, CHO2, OPI3) and Kennedy (EKI1, EPT1, CKI1, CPT1) pathways and for the synthesis of PI (INO1) contain a UASINO element in the promoter. The UASINO element is the binding site for the Ino2-Ino4 heterodimer complex that activates transcription (3, 7). Repression of UASINO-containing gene expression is controlled by Opi1, whose function is governed by its nuclear localization (3, 7, 33). Opi1 binds PA and the Scs2 protein at the nuclear/endoplasmic reticulum (ER) membrane, which blocks its repressor function in the nucleus (33, 34). When PA levels are reduced, Opi1 is released from the nuclear/ER membrane and enters into the nucleus, where it attenuates transcription by binding to Ino2 (3, 7). Thus, PA content at the nuclear/ER membrane is an important factor that controls the Opi1-mediated regulation of UASINO-containing gene expression (3).
Conditions that trigger the regulation of UASINO-containing genes include nutrient availability (e.g., inositol or zinc) and growth stage (1, 7, 9). For example, the expression of UASINO-containing genes is activated when the essential nutrient zinc is supplemented to the growth medium (9, 35). By contrast, the gene expression is repressed by inositol supplementation and requires ongoing PC synthesis (1, 3, 7). In this regulation, ethanolamine or choline supplementation enhances the repressive effect on gene expression (1). Depletion of inositol or zinc from the growth medium has the opposite effect on gene expression (1, 3, 7, 9). The UASINO-containing genes are maximally expressed in the exponential phase of growth, whereas they are repressed in the stationary phase of growth (1, 3, 7). The repression of the UASINO-containing genes by zinc depletion, or when cells enter the stationary phase of growth, is independent of inositol supplementation (1, 9).
Regulation by inositol and zinc
The mechanisms that control PA content and expression of UASINO-containing genes are typified by the inositol- and zinc-mediated regulation of phospholipid synthesis. Upon inositol supplementation, the synthesis of PI is elevated through increased substrate availability for the PIS1-encoded PI synthase (36). In addition, inositol directly inhibits the activity of the CHO1-encoded PS synthase, which favors the utilization of CDP-DAG for PI synthesis (36). This biochemical regulation draws upon PA content through CDP-DAG and causes the translocation of Opi1 into the nucleus for repression of UASINO-containing genes (3, 33). Overall, the repression of UASINO-containing genes leads to a decrease in the synthesis of enzymes used in both the CDP-DAG and Kennedy pathways, and changes in phospholipid composition that include an increase in PI and decreases in PA, PS, and PC (1, 36).
Regulation of UASINO-containing genes by zinc also involves the control of PA content through the activation of PI synthase. This regulation that occurs in the absence of inositol supplementation is mediated by the zinc-sensing and zinc-inducible transcriptional activator Zap1 and the zinc-responsive cis-acting element (UASZRE) of phospholipid synthesis genes (9). Zinc depletion results in an increase in PI synthesis through increased expression of the PIS1-encoded PI synthase (35, 37) that is mediated by the interaction of Zap1 with a UASZRE in the PIS1 promoter (35, 37). As indicated above, the increase in PI synthesis causes a decrease in PA content. The net result is the Opi1-mediated repression of UASINO-containing genes and a decrease in the activities of the CDP-DAG pathway enzymes (35). The major effects of zinc depletion on phospholipid composition include an increase in PI and a decrease in PE (35). Interestingly, the PC content is not significantly affected by zinc depletion, although enzyme activities in the CDP-DAG pathway are repressed (35). Maintenance of a normal PC content has been attributed to the Zap1-mediated activation of CKI1-encoded choline kinase expression for PC synthesis via the Kennedy pathway (38). Any effect that Opi1 would have on CKI1 expression (because it contains a UASINO element) is overcome by the derepression of CKI1 by Zap1 (38).
Regulation by CTP and AdoHcy
CTP and AdoHcy are molecules that regulate UASINO-containing genes as well as the activities of phospholipid synthesis enzymes. CTP is essential for phospholipid synthesis; it is the direct precursor of the activated, energy-rich intermediates CDP-DAG, CDP-choline, and CDP-ethanolamine (6). CTP is also used as the phosphate donor for the synthesis of PA by the DGK1-encoded DAG kinase (39). Because the cellular levels of CTP are primarily controlled by product inhibition of CTP synthetase activity, expression of a mutant enzyme lacking this regulation results in elevated levels of CTP as well as an increased rate of PA synthesis and the derepression of UASINO-containing genes (6, 40). The increase in PA content and the inactivation of Opi1 repressor function (39) may result from the stimulation of DAG kinase activity by increased availability of its substrate CTP. CTP also favors an elevation of PA content by inhibiting PAH1-encoded PA phosphatase2 activity (41).
AdoHcy is a product of the AdoMet-dependent methylation reactions that are catalyzed by the CHO2-encoded PE methyltransferase and OPI3-encoded phospholipid methyltransferase in the CDP-DAG pathway (Fig. 1). AdoHcy, which is removed by the SAH1-encoded AdoHcy hydrolase (13), is a competitive inhibitor of the methyltransferase enzymes (42). Thus, downregulation of the AdoHcy hydrolase causes the accumulation of AdoHcy and the inhibition of PC synthesis, which leads to an increase in PA content and the derepression of UASINO-containing genes (13). Although the effects of AdoHcy on phospholipid composition have not been addressed, its accumulation causes an increase in TAG synthesis and lipid droplet content (13).
ROLES OF PA PHOSPHATASE AND DAG KINASE IN CONTROLLING PA CONTENT
Among the enzymes (e.g., lysoPA acyltransferase, CDP-DAG synthase, PA phosphatase, DAG kinase, phospholipase D) that contribute to the metabolism of PA, the PAH1-encoded PA phosphatase has been identified as a key regulator of PA content. Cells lacking the enzyme activity contain an elevated PA content and exhibit the derepression of UASINO-containing phospholipid synthesis genes (14, 32, 43). In addition, elevated PA content stimulates PS synthase activity (44), which would favor the synthesis of PC via the CDP-DAG pathway. Moreover, the elevation of PA content triggers the anomalous expansion of the nuclear/ER membrane (32, 43), underscoring the importance of phospholipid synthesis to organelle synthesis/structure. In contrast to the loss of PAH1-encoded PA phosphatase activity, overexpression of the enzyme activity causes the repression of INO1 expression and inositol auxotrophy (45).
The DGK1-encoded DAG kinase has recently been identified as an enzyme that counteracts the role that PAH1-encoded PA phosphatase plays in controlling PA content and the transcriptional regulation of UASINO-containing genes (39, 46). The overexpression of the enzyme activity causes an increase in PA content, the derepression of UASINO-containing genes, and the anomalous nuclear/ER membrane expansion (46) like those shown in the pah1Δ mutant (32, 43). In addition, the overexpression of DGK1-encoded DAG kinase activity bypasses the inositol auxotrophy (e.g., repression of INO1 and other UASINO-containing genes) caused by the overexpression of PAH1-encoded PA phosphatase activity (46). Moreover, the dgk1Δ mutation bypasses the phenotypes caused by the pah1Δ mutation (39, 46).
PAH1-encoded PA phosphatase and DGK1-encoded DAG kinase are biochemically regulated by CDP-DAG. CDP-DAG stimulates PA phosphatase activity (47) but inhibits DAG kinase activity (39). Thus, the regulation of these PA metabolic enzymes by an elevated level of CDP-DAG favors a decrease in PA content and the Opi1-mediated repression of UASINO-containing genes. One of the UASINO-containing genes that is repressed by Opi1 is CDS1 (48), which encodes CDP-DAG synthase (49). Thus, the regulation of its expression provides a mechanism for controlling the synthesis of CDP-DAG from PA and the CDP-DAG-dependent synthesis of phospholipids. This notion is supported by genetic evidence that a cds1 mutant defective in CDP-DAG synthase activity exhibits an elevated PA content and the derepression of UASINO-containing genes (50, 51). The increased DAG levels caused by the CDP-DAG-mediated regulation of PA phosphatase and DAG kinase activities would be channeled to phospholipids via the Kennedy pathway or to the storage lipid TAG.
SUMMARY AND PERSPECTIVES
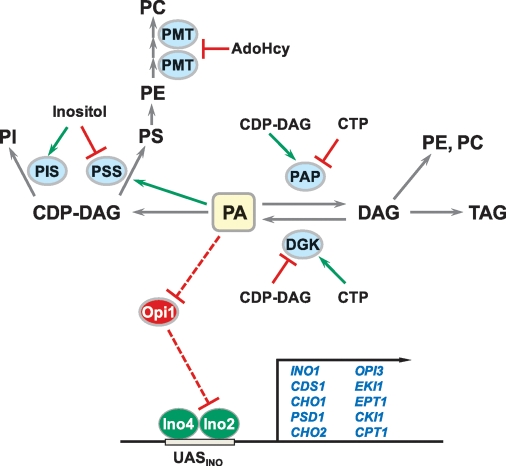
A major theme of this review is that the biochemical regulation of phospholipid synthesis enzymes ultimately controls the cellular content of PA, a lipid precursor and signaling molecule that triggers the transcriptional regulation of UASINO-containing phospholipid synthesis genes. The model shown in Fig. 2 incorporates this theme and highlights the central role that the PAH1-encoded PA phosphatase and DGK1-encoded DAG kinase play in controlling PA content. Interestingly, the effector molecules inositol, CTP, and CDP-DAG play important roles in the regulation of phospholipid synthesis by stimulating or inhibiting key phospholipid synthesis enzymes (Fig. 2).
Fig. 2.
Model for the biochemical regulation of phospholipid synthesis. The upper portion of the diagram shows the major steps in the synthesis of phospholipids. The key enzymes (PMT, CHO2-encoded PE methyltransferase and OPI3-encoded phospholipid methyltransferase; PIS, PIS1-encoded PI synthase; PSS, CHO1-encoded PS synthase; PAP, PAH1-encoded PA phosphatase; DGK, DGK1-encoded DAG kinase) that are biochemically regulated by phospholipid precursors and products are highlighted by the blue ellipses. The bottom portion of the diagram shows the transcriptional activation of UASINO-containing genes (blue) by the Ino2-Ino4 complex. Elevated PA (highlighted in yellow) content prevents the translocation of Opi1 into the nucleus, and thus its repressor function of UASINO-containing phospholipid synthesis genes. The color green designates stimulation, whereas the color red designates inhibition.
As discussed above, the repression of UASINO-containing genes in response to inositol supplementation or zinc depletion is attributed to the regulation of PI synthase activity or PIS1 gene expression, respectively (36, 37). Previous studies have shown that the levels of PA phosphatase activity are elevated in inositol-supplemented (52) and zinc-depleted (53) cells as well as in stationary phase cells (54). Thus, elevated PA phosphatase activity provides another mechanism for controlling PA content under these growth conditions. While it is clear that PAH1-encoded PA phosphatase plays a major role in controlling PA content, its role in the regulation of UASINO-containing gene expression under the growth conditions discussed above has yet to be established.
While specifically not discussed in this review, the activities of phospholipid synthesis enzymes (e.g., PA phosphatase, PS synthase, choline kinase, and CTP synthetase) are also regulated by phosphorylation, affecting the cellular concentrations of phospholipid precursors and products (8, 45). The protein kinases that mediate this regulation include protein kinase A, protein kinase C, and cyclin-dependent protein kinase (8, 32, 45). However, additional studies are needed to make physiological connections between the phosphorylation-mediated regulation of enzyme activities and the transcriptional regulation of UASINO-containing phospholipid synthesis genes.
Abbreviations
CDP-DAG, CDP-diacylglycerol
DAG, diacylglycerol
ER, endoplasmic reticulum
PA, phosphatidate
PC, phosphatidylcholine
PE, phosphatidylethanolamine
PI, phosphatidylinositol
PS, phosphatidylserine
TAG, triacylglycerol
UASINO, upstream activating sequence inositol-responsive element
UASZRE, upstream activating sequence zinc-responsive element
This work was supported in part by the United States Public Health Service, National Institutes of Health Grants GM-28140 and GM-50679.
Published, JLR Papers in Press, October 27, 2008.
Footnotes
The PAH1-encoded PA phosphatase is a Mg2+-dependent enzyme that is involved in de novo lipid synthesis. It is distinct from the DPP1- and LPP1-encoded lipid phosphate phosphatase enzymes that dephosphorylate PA and a host of lipid phosphate molecules by a catalytic mechanism that does not require Mg2+ (5). The DPP1- and LPP1-encoded enzymes play specific roles in controlling lipid phosphate metabolism confined to the vacuole and Golgi membranes, respectively (5).
References
- 1.Carman G. M., and S. A. Henry. 1999. Phospholipid biosynthesis in the yeast Saccharomyces cerevisiae and interrelationship with other metabolic processes. Prog. Lipid Res. 38 361–399. [DOI] [PubMed] [Google Scholar]
- 2.Gaspar M. L., M. A. Aregullin, S. A. Jesch, L. R. Nunez, M. Villa-Garcia, and S. A. Henry. 2007. The emergence of yeast lipidomics. Biochim. Biophys. Acta. 1771 241–254. [DOI] [PubMed] [Google Scholar]
- 3.Carman G. M., and S. A. Henry. 2007. Phosphatidic acid plays a central role in the transcriptional regulation of glycerophospholipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 282 37293–37297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carman G. M., and G. M. Zeimetz. 1996. Regulation of phospholipid biosynthesis in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 271 13293–13296. [DOI] [PubMed] [Google Scholar]
- 5.Carman G. M., and G. S. Han. 2006. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem. Sci. 31 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y-F., and G. M. Carman. 2008. CTP synthetase and its role in phospholipid synthesis in the yeast Saccharomyces cerevisiae. Prog. Lipid Res. 47 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M., L. C. Hancock, and J. M. Lopes. 2007. Transcriptional regulation of yeast phospholipid biosynthetic genes. Biochim. Biophys. Acta. 1771 310–321. [DOI] [PubMed] [Google Scholar]
- 8.Carman G. M., and M. C. Kersting. 2004. Phospholipid synthesis in yeast: regulation by phosphorylation. Biochem. Cell Biol. 82 62–70. [DOI] [PubMed] [Google Scholar]
- 9.Carman G. M., and G. S. Han. 2007. Regulation of phospholipid synthesis in Saccharomyces cerevisiae by zinc depletion. Biochim. Biophys. Acta. 1771 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi H-S., and G. M. Carman. 2007. Respiratory deficiency mediates the regulation of CHO1-encoded phosphatidylserine synthase by mRNA stability in Saccharomyces cerevisiae. J. Biol. Chem. 282 31217–31227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajakumari S., K. Grillitsch, and G. Daum. 2008. Synthesis and turnover of non-polar lipids in yeast. Prog. Lipid Res. 47 157–171. [DOI] [PubMed] [Google Scholar]
- 12.Gaspar M. L., S. A. Jesch, R. Viswanatha, A. L. Antosh, W. J. Brown, S. D. Kohlwein, and S. A. Henry. 2008. A block in endoplasmic reticulum-to-Golgi trafficking inhibits phospholipid synthesis and induces neutral lipid accumulation. J. Biol. Chem. 283 25735–25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malanovic N., I. Streith, H. Wolinski, G. Rechberger, S. D. Kohlwein, and O. Tehlivets. 2008. S-adenosyl-L-homocysteine hydrolase, key enzyme of methylation metabolism, regulates phosphatidylcholine synthesis and triacylglycerol homeostasis in yeast: implications for homocysteine as a risk factor of atherosclerosis. J. Biol. Chem. 283 23989–23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han G-S., W-I. Wu, and G. M. Carman. 2006. The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281 9210–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickson R. C. 2008. Thematic review series: sphingolipids. New insights into sphingolipid metabolism and function in budding yeast. J. Lipid Res. 49 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G., S. Chen, M. N. Thompson, and M. L. Greenberg. 2007. New insights into the regulation of cardiolipin biosynthesis in yeast: implications for Barth syndrome. Biochim. Biophys. Acta. 1771 432–441. [DOI] [PubMed] [Google Scholar]
- 17.Patton-Vogt J. L., P. Griac, A. Sreenivas, V. Bruno, S. Dowd, M. J. Swede, and S. A. Henry. 1997. Role of the yeast phosphatidylinositol/phosphatidylcholine transfer protein (Sec14p) in phosphatidylcholine turnover and INO1 regulation. J. Biol. Chem. 272 20873–20883. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z., M. Fang, M. P. Rivas, A. J. Faulkner, P. C. Sternweis, J. Engebrecht, and V. A. Bankaitis. 1998. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc. Natl. Acad. Sci. USA. 95 12346–12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K., K-H. Kim, M. K. Storey, D. R. Voelker, and G. M. Carman. 1999. Isolation and characterization of the Saccharomyces cerevisiae EKI1 gene encoding ethanolamine kinase. J. Biol. Chem. 274 14857–14866. [DOI] [PubMed] [Google Scholar]
- 20.Riekhof W. R., and D. R. Voelker. 2006. Uptake and utilization of lyso-phosphatidylethanolamine by Saccharomyces cerevisiae. J. Biol. Chem. 281 36588–36596. [DOI] [PubMed] [Google Scholar]
- 21.Riekhof W. R., J. Wu, J. L. Jones, and D. R. Voelker. 2007. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 282 28344–28352. [DOI] [PubMed] [Google Scholar]
- 22.Riekhof W. R., J. Wu, M. A. Gijon, S. Zarini, R. C. Murphy, and D. R. Voelker. 2007. Lysophosphatidylcholine metabolism in Saccharomyces cerevisiae: the role of P-type ATPases in transport and a broad specificity acyltransferase in acylation. J. Biol. Chem. 282 36853–36861. [DOI] [PubMed] [Google Scholar]
- 23.Yon J. O., H. Nakamura, A. Ohta, and M. Takagi. 1998. Incorporation of extracellular phospholipids and their effect on the growth and lipid metabolism of the Saccharomyces cerevisiae cho1/pss mutant. Biochim. Biophys. Acta. 1394 23–32. [DOI] [PubMed] [Google Scholar]
- 24.Jain S., N. Stanford, N. Bhagwat, B. Seiler, M. Costanzo, C. Boone, and P. Oelkers. 2007. Identification of a novel lysophospholipid acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 282 30562–30569. [DOI] [PubMed] [Google Scholar]
- 25.Tamaki H., A. Shimada, Y. Ito, M. Ohya, J. Takase, M. Miyashita, H. Miyagawa, H. Nozaki, R. Nakayama, and H. Kumagai. 2007. LPT1 encodes a membrane-bound O-acyltransferase involved in the acylation of lysophospholipids in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 282 34288–34298. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K., R. Fukuda, Y. Ono, H. Eguchi, S. Nagasawa, Y. Nakatani, H. Watanabe, H. Nakanishi, R. Taguchi, and A. Ohta. 2008. Incorporation and remodeling of extracellular phosphatidylcholine with short acyl residues in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1781 391–399. [DOI] [PubMed] [Google Scholar]
- 27.Stålberg K., A. C. Neal, H. Ronne, and U. Stahl. 2008. Identification of a novel GPCAT activity and a new pathway for phosphatidylcholine biosynthesis in S. cerevisiae. J. Lipid Res. 49 1794–1806. [DOI] [PubMed] [Google Scholar]
- 28.Patton-Vogt J. 2007. Transport and metabolism of glycerophosphodiesters produced through phospholipid deacylation. Biochim. Biophys. Acta. 1771 337–342. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Murray J. P., and C. R. McMaster. 2007. Phosphatidylcholine synthesis and its catabolism by yeast neuropathy target esterase 1. Biochim. Biophys. Acta. 1771 331–336. [DOI] [PubMed] [Google Scholar]
- 30.McMaster C. R., and R. M. Bell. 1994. Phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. Regulatory insights from studies employing null and chimeric sn-1,2-diacylglycerol choline- and ethanolaminephosphotransferases. J. Biol. Chem. 269 28010–28016. [PubMed] [Google Scholar]
- 31.Morash S. C., C. R. McMaster, R. H. Hjelmstad, and R. M. Bell. 1994. Studies employing Saccharomyces cerevisiae cpt1 and ept1 null mutants implicate the CPT1 gene in coordinate regulation of phospholipid biosynthesis. J. Biol. Chem. 269 28769–28776. [PubMed] [Google Scholar]
- 32.Santos-Rosa H., J. Leung, N. Grimsey, S. Peak-Chew, and S. Siniossoglou. 2005. The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24 1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loewen C. J. R., M. L. Gaspar, S. A. Jesch, C. Delon, N. T. Ktistakis, S. A. Henry, and T. P. Levine. 2004. Phospholipid metabolism regulated by a transcription factor sensing phosphatidic acid. Science. 304 1644–1647. [DOI] [PubMed] [Google Scholar]
- 34.Loewen C. J. R., and T. P. Levine. 2005. A highly conserved binding site in vesicle-associated membrane protein-associated protein (VAP) for the FFAT motif of lipid-binding proteins. J. Biol. Chem. 280 14097–14104. [DOI] [PubMed] [Google Scholar]
- 35.Iwanyshyn W. M., G. S. Han, and G. M. Carman. 2004. Regulation of phospholipid synthesis in Saccharomyces cerevisiae by zinc. J. Biol. Chem. 279 21976–21983. [DOI] [PubMed] [Google Scholar]
- 36.Kelley M. J., A. M. Bailis, S. A. Henry, and G. M. Carman. 1988. Regulation of phospholipid biosynthesis in Saccharomyces cerevisiae by inositol. Inositol is an inhibitor of phosphatidylserine synthase activity. J. Biol. Chem. 263 18078–18085. [PubMed] [Google Scholar]
- 37.Han S-H., G-S. Han, W. M. Iwanyshyn, and G. M. Carman. 2005. Regulation of the PIS1-encoded phosphatidylinositol synthase in Saccharomyces cerevisiae by zinc. J. Biol. Chem. 280 29017–29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soto A., and G. M. Carman. 2008. Regulation of the Saccharomyces cerevisiae CKI1-encoded choline kinase by zinc depletion. J. Biol. Chem. 283 10079–10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han G-S., L. O'Hara, S. Siniossoglou, and G. M. Carman. 2008. Characterization of the yeast DGK1-encoded CTP-dependent diacylglycerol kinase. J. Biol. Chem. 283 20443–20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostrander D. B., D. J. O'Brien, J. A. Gorman, and G. M. Carman. 1998. Effect of CTP synthetase regulation by CTP on phospholipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 273 18992–19001. [DOI] [PubMed] [Google Scholar]
- 41.Wu W-I., and G. M. Carman. 1994. Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiae by nucleotides. J. Biol. Chem. 269 29495–29501. [PubMed] [Google Scholar]
- 42.Gaynor P. M., and G. M. Carman. 1990. Phosphatidylethanolamine methyltransferase and phospholipid methyltransferase activities from Saccharomyces cerevisiae. Enzymological and kinetic properties. Biochim. Biophys. Acta. 1045 156–163. [DOI] [PubMed] [Google Scholar]
- 43.Han G. S., S. Siniossoglou, and G. M. Carman. 2007. The cellular functions of the yeast lipin homolog Pah1p are dependent on its phosphatidate phosphatase activity. J. Biol. Chem. 282 37026–37035. [DOI] [PubMed] [Google Scholar]
- 44.Bae-Lee M., and G. M. Carman. 1990. Regulation of yeast phosphatidylserine synthase and phosphatidylinositol synthase activities by phospholipids in Triton X-100/phospholipid mixed micelles. J. Biol. Chem. 265 7221–7226. [PubMed] [Google Scholar]
- 45.O'Hara L., G. S. Han, S. Peak-Chew, N. Grimsey, G. M. Carman, and S. Siniossoglou. 2006. Control of phospholipid synthesis by phosphorylation of the yeast lipin Pah1p/Smp2p Mg2+-dependent phosphatidate phosphatase. J. Biol. Chem. 281 34537–34548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han G-S., L. O'Hara, G. M. Carman, and S. Siniossoglou. 2008. An unconventional diacylglycerol kinase that regulates phospholipid synthesis and nuclear membrane growth. J. Biol. Chem. 283 20433–20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu W-I., and G. M. Carman. 1996. Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiae by phospholipids. Biochemistry. 35 3790–3796. [DOI] [PubMed] [Google Scholar]
- 48.Homann M. J., S. A. Henry, and G. M. Carman. 1985. Regulation of CDP-diacylglycerol synthase activity in Saccharomyces cerevisiae. J. Bacteriol. 163 1265–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen H., P. N. Heacock, C. J. Clancey, and W. Dowhan. 1996. The CDS1 gene encoding CDP-diacylglycerol synthase in Saccharomyces cerevisiae is essential for cell growth. J. Biol. Chem. 271 789–795. [DOI] [PubMed] [Google Scholar]
- 50.Klig L. S., M. J. Homann, S. D. Kohlwein, M. J. Kelley, S. A. Henry, and G. M. Carman. 1988. Saccharomyces cerevisiae mutant with a partial defect in the synthesis of CDP-diacylglycerol and altered regulation of phospholipid biosynthesis. J. Bacteriol. 170 1878–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen H., and W. Dowhan. 1996. Reduction of CDP-diacylglycerol synthase activity results in the excretion of inositol by Saccharomyces cerevisiae. J. Biol. Chem. 271 29043–29048. [DOI] [PubMed] [Google Scholar]
- 52.Morlock K. R., Y-P. Lin, and G. M. Carman. 1988. Regulation of phosphatidate phosphatase activity by inositol in Saccharomyces cerevisiae. J. Bacteriol. 170 3561–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwanyshyn, W. M. 2005. Regulation of Phospholipid Synthesis in Saccharomyces cerevisiae by Zinc. PhD Dissertation. Rutgers University, New Brunswick, NJ. [DOI] [PubMed]
- 54.Hosaka K., and S. Yamashita. 1984. Regulatory role of phosphatidate phosphatase in triacylglycerol synthesis of Saccharomyces cerevisiae. Biochim. Biophys. Acta. 796 110–117. [PubMed] [Google Scholar]