Abstract
Lipid peroxidation is a common event in health and is greatly accelerated in pro-inflammatory settings such as hypercholesterolemia. Consequently, oxidation-specific epitopes are generated, which are pro-inflammatory and immunogenic, leading to both adaptive and innate responses. Because innate immune mechanisms use conserved germline pattern recognition receptors (PRRs) that are preformed and present at birth, it is not obvious why they should bind to such epitopes. In this review, we put forward the hypothesis that because oxidation-specific epitopes are ubiquitous in both health and disease, and because they in essence represent “danger signals,” they constitute a class of pathogen-associated molecular patterns leading to the natural selection of multiple innate PRRs that target such epitopes. We suggest that apoptotic cells, and the blebs and microparticles released from such cells, which are rich in oxidation-specific epitopes and thus pro-inflammatory, constitute an endogenous set of selecting antigens. In turn, natural antibodies, scavenger receptors, and soluble innate proteins, such as pentraxins, all represent PRRs that target such epitopes. We discuss the evidence for this hypothesis and the consequences of such responses in health and disease, such as atherosclerosis.
Keywords: oxidation-specific epitopes, natural antibodies, oxidized lipids, oxidized LDL, scavenger receptors
Hypercholesterolemia not only leads to enhanced binding of apolipoprotein B (apoB) containing lipoproteins in the arterial intima but also initiates the pro-inflammatory and pro-oxidant conditions that play an obligatory role in the perpetuation and progression of the disease. Inflammation is mediated by immune mechanisms, which profoundly impact atherogenesis, accelerating but providing atheroprotective influences as well (1–4). Why should immune mechanisms be involved in the response to hypercholesterolemia? Immunity is most often thought of in the context of responses to infectious pathogens, but evidence to support other than a minor role for direct involvement of bacterial or viral agents in atherosclerosis is lacking. Indeed, atherogenesis can proceed in mice maintained even in a completely sterile environment (5). In this review, we will develop the hypothesis that the enhanced lipid peroxidation that occurs with hypercholesterolemia, and the consequent generation of “oxidation-specific” epitopes, leads to activation of immune responses that impact atherogenesis. Understanding these mechanisms will lead to new insights into atherogenesis and may lead to novel immunoregulatory therapies to modulate disease progression.
Adaptive Immunity and Oxidation-Specific Epitopes
Adaptive immunity is the active response to perceived changes that are outside the realm of “self,” such as the wide variety of epitopes presented by foreign pathogens. Indeed, the essence of adaptive immunity is the generation of an almost limitless number of high-affinity B-cell and T-cell receptors (∼1014 to 1018) through somatic mutations and induced junctional diversity at VDJ gene recombination sites to produce both cellular (via T-cells) and humoral immunity [via secreted antibodies (Abs) from B-cell-derived plasma cells] against selecting pathogens. Although the adaptive response is delayed in time due to the selection and maturation of T- and B-cell responses, it provides a specific and high-affinity response to newly recognized pathogens.
A variety of potential antigens have been described in the atherosclerotic lesion that could serve to activate such adaptive immune responses, including bacterial and viral antigens. However, among these, oxidation-specific epitopes, as occur when LDL is oxidized (OxLDL), are the ones most widely studied to date (4). Multiple oxidative mechanisms are present in the vascular wall that can lead to the generation of highly reactive oxidized lipids. In turn, these can modify proteins and lipids present not only on LDL, for example, but on a wide variety of extracellular and cellular components as well. We have termed these oxidation-specific epitopes to indicate that common modifications could be produced under widely different inflammatory settings and on widely different proteins or even lipids. For example, the common oxidation product, malondialdehyde (MDA), and its many complex condensation products, can modify proteins and lipids leading to common oxidation-specific neo-epitopes that are immunogenic and, importantly, are recognized in a hapten-specific manner. Thus, as we have shown, an MDA adduct formed on apoB of OxLDL could lead to hapten-specific MDA monoclonal antibodies [such as MDA2 and E014 (6, 7)] that not only recognize MDA epitopes in atherosclerotic plaques (8), but MDA modified proteins in diabetic kidneys (9) and Heyman nephritis (10), as well as in Alzheimer plaques (11). Because lipid peroxidation is a common process even in health, and is greatly accelerated in inflammatory diseases, such oxidation-specific epitopes are ubiquitous in both health and disease. Indeed, cells undergoing apoptosis, which are under intense pro-oxidant stress, display a variety of immunogenic oxidation-specific epitopes on their surface (as discussed below) (12). In turn, these “neo-self” epitopes are recognized as “foreign” by adaptive immune surveillance. Evidence for humoral and cell-mediated immunity to a variety of oxidation-specific epitopes, such as autoantibodies to OxLDL and MDA-modified LDL (MDA-LDL), has been documented in both animal models of atherosclerosis and in humans (4, 13). While the originating immunogen that elicited such responses may be varied in a generalized setting of inflammation, as occurs in hypercholesterolemia, the ensuing adaptive immune responses have been shown to profoundly modulate the rate of atherosclerotic lesion progression, where an abundance of such epitopes are found. For example, as first demonstrated in our lab (14) and confirmed and extended by others (15), immunization with homologous MDA-LDL, OxLDL, or mimics of oxidation epitopes (16) can reduce the progression of atherosclerosis in animal models of atherosclerosis, even in the presence of marked hypercholesterolemia.
Lymphocytes in general have been found to modulate murine atherosclerosis (1–3). T-cells, especially interferon-γ-secreting Th1 cells, are considered pro-atherogenic, whereas several subsets of regulatory T-cells confer atheroprotection (see review by Mallat et al. in this issue). By contrast, B-cells, though not found in lesions, are nevertheless now thought to provide an overall protective function, in part through generation of Abs that bind to oxidation-specific epitopes (4).
Innate Immunity and Oxidation-Specific Epitopes
In contrast with adaptive responses, innate immunity uses natural selection of receptors, which play a vital and nonredundant role in the initial defense against invading pathogens and in maintaining homeostasis against a variety of “self-antigens.” Because these receptors are preformed and are present at birth and/or matured via positive selection during the neonatal period or shortly thereafter, they are available for almost immediate defense against a perceived pathogen. Thus, innate receptors of necessity are focused on highly conserved motifs that are present on multiple pathogens as well as many neo-epitopes and even apparent “self-or neo-self epitopes.” These receptors are called pattern recognition receptors (PRRs) and the “conserved” patterns to which they bind are called pathogen-associated molecular patterns (PAMPs).
In this review, we suggest that oxidation-specific epitopes are a major target of many innate PRRs. However, it is not apparent why such preformed PRRs should be involved in a disease process such as atherosclerosis, which would not be expected to exert evolutionary pressure. We put forward the hypothesis that because oxidation-specific epitopes are ubiquitous in both health and disease, and because they in essence represent “danger signals,” they constitute a class of PAMPs that has led to the natural selection of multiple PRRs that target such epitopes. This includes both cellular PRRs, such as scavenger receptors (SRs) and toll-like receptors on macrophages, and soluble PRRs, such as pentraxins and natural antibodies (NAbs). Furthermore, the fact that oxidation-specific epitopes often, if not frequently, share molecular identity with epitopes found on infectious pathogens as well provides further selecting pressure for receptors targeted to such epitopes. Thus, oxidation-specific epitopes are a major target of innate immunity.
NAbs Recognize Oxidation-Specific Epitopes
Our own appreciation that NAbs recognized oxidation-specific epitopes arose from studies in which we cloned a panel of hybridomas from cholesterol-fed apolipoprotein E-deficient mice, which have high IgM titers to such epitopes. We cloned eight hybridomas secreting IgM to OxLDL (7). Each of these, such as the prototypic E06, bound to both the lipid and apoB moiety of OxLDL and specifically to the phosphocholine (PC) headgroup of oxidized phospholipids (OxPLs), such as 1-palmitoyl-2-(5′-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC), but not to the PC of native PL (17–19). In the case of the apoB isolated from OxLDL, we showed that the OxPL was covalently linked to the lysines of apoB via the reactive aldehydes of the oxidized sn2 side chain, leaving the PC headgroup free to bind E06.
E06 became of enormous interest when we found that it inhibited the uptake of OxLDL by macrophage scavenger receptors CD36 and SR-BI (20, 21). Indeed, we demonstrated that both the lipid moiety of OxLDL, as well as the solubilized apoB of OxLDL, could bind to CD36 and that E06 could inhibit the binding of both. Furthermore, we showed that POVPC linked to BSA via its sn2 side chain also blocked the binding of OxLDL. These data strongly supported the interpretation that the PC moiety of OxPC was a sufficient ligand to mediate binding to CD36. This was confirmed by demonstrating the specific binding of the labeled POVPC peptide to CD36-transfected COS-7 cells (22).
NAbs are an essential layer of innate immunity (23, 24). In mice, a special subset of B-cells termed B-1 cells, secrete NAbs, which are predominantly IgM and IgA. In contrast with B-2 cells of adaptive immunity, which are negatively selected in fetal life producing anergy, B-1 cells are positively selected; thus, NAbs are present at birth or shortly thereafter and can be found in gnotobiotic mice reared in the complete absence of external antigenic stimulation. In uninfected mice, most, if not all, IgM in plasma are of B-1 origin, which are predominantly secreted from the spleen even in the absence of antigen. However, established B-1 cell clones can be expanded later in life by antigen exposure leading to increased IgM levels in plasma. B-1 cells have restricted use of VH genes that are minimally edited; thus, the IgM they generate are reflective of germline usage, with minimal to no mutations (25). Because they are conserved by natural selection, the presumption is that, fundamentally, NAbs provide advantageous properties maintaining homeostasis, such as their crucial role in immediate host defenses against “pathogens” (26), which, as we show below, include endogenous pathogens bearing neo-self epitopes, such as oxidation-specific epitopes.
We subsequently showed, by direct sequencing of the VH/VL chains and use of anti-idiotypic Abs, that all the hybridomas were 100% homologous to the classic germline-encoded NAb T15, secreted by a well-characterized B-1 cell clone described more than 30 years ago (18). The T15 clone, which was a spontaneous murine plasmacytoma that secreted an IgA with the T15 idiotype, is considered the classic germline NAb. It was intensively studied because it binds to PC (not as part of a PL) covalently linked to the cell wall polysaccharide of pathogens and confers optimal protection to mice from lethal infection with Streptococcus pneumonia (27). In further studies cited below, we went on to show that such OxPL were greatly increased in apoptotic cells as well (28). Thus, these studies demonstrated molecular (and immunological) mimicry between the PC of OxPL present on OxLDL and apoptotic cells on one hand and the PC moiety present on pneumococcus and many other infections pathogens on the other hand. This dual specificity for an endogenous self- or neo-self-antigen and an exogenous pathogen has been described as a characteristic of NAbs (26).
Because immunization of mice with heat-inactivated PC-containing pneumococci was known to robustly expand T15 B-1 cell clones, and because such IgM blocked the uptake of OxLDL by macrophages, we immunized cholesterol-fed LDLR−/− mice with heat killed pneumococci to see if this would reduce atherosclerosis. Indeed, immunization induced high titers of E06/T15 IgM and significantly reduced atherosclerosis (29). The atheroprotective property of anti-PC IgM was corroborated in a vein graft atherosclerosis model by Faria-Neto et al., in which they infused T15 IgM intravenously (30), and by Caligiuri et al, who demonstrated that immunizing apoE−/− mice with PC-KLH was atheroprotective (16). Furthermore, we (28, 31) and others (32) have shown that E06 can bind to and block pro-inflammatory effects of PC containing OxPL, and this property undoubtedly also contributes importantly to the anti-inflammatory and anti-atherogenic properties of these oxidation-specific IgM.
However, the PC of OxPL is only one example of what are likely to be a wide variety of such oxidation-specific epitopes to which NAbs bind. For example, we cloned a NAb from LDLR−/− mice, termed LRO1, which bound to oxidized cardiolipin but not native cardiolipin (33). LDL contains cardiolipin, and LRO1 also bound to OxLDL, but not native LDL, as well as to atherosclerotic lesions. LRO1 also bound to apoptotic cells but not viable cells. Cardiolipin is a major phospholipid of the inner leaflet of mitochondria, which is oxidized when cells undergo mitochondrial disruption that occurs during apoptosis (34).
There are many oxidation-specific epitopes. Data developing in our laboratory suggest that in normal mice, 20% to 30% of all IgM derived from B-1 cell clones bind to such oxidation-specific epitopes (unpublished observations). Among these, we found a high prevalence of IgM to MDA and the complex structural adducts that occur when MDA is added to proteins. Remarkably, we previously documented a high titer of IgM to MDA-LDL even in wild-type C57BL/6 mice as well as in healthy adult humans (1, 13). Furthermore, in studies of newborn human umbilical cord blood, we found a high titer of IgM to MDA epitopes, and similar to such NAbs in mice, they bind apoptotic cells and atherosclerotic tissues. The role of NAbs in humans is not well defined, but since IgM do not cross the placenta, it is generally considered that such IgM represent the human equivalent of innate NAbs. These data suggest that oxidation-specific epitopes are an important target of innate NAbs in mice and humans.
Macrophage PRRs Recognize Oxidation-Specific Epitopes
“Scavenger receptors” of macrophages were so named because they bound and internalized OxLDL, but not native LDL (35), and to date, eight different classes of such receptors have been identified (36). They are now recognized to be multifunctional receptors that bind and internalize ligands, which include polyanionic ligands and apoptotic cells, as well as mediate adhesion and present antigens. Via their ability to sample their environment and present antigens to T- and B-cells, macrophages and dendritic cells serve as vital links between innate and adaptive immune responses. The first such receptor identified, the “acetyl LDL receptor,” now known as SR-A (37), which comes in several varieties, not only binds modified LDL, including OxLDL, but of relevance to our discussion, also binds both gram positive and gram negative microbial ligands, including live bacteria. Indeed, SR-A-deficient mice have enhanced susceptibility to certain infections, such as Listeria monocytogenes and Staphylococcus aureus (38). However, it is now apparent that a variety of SR recognize various oxidation-specific epitopes present on OxLDL and on apoptotic cells, including SRA-1,2 MARCOS, CD36, SR-BI, LOX-1, PSOX, and others (36). Furthermore, evidence is beginning to accumulate that another important class of innate PRRs, members of the toll-like receptor family and associated proteins, such as MD2, also bind minimally modified LDL, OxLDL, and certain oxidized phospholipids, fatty acids, and cholesteryl esters, perhaps in cooperation with some of the SRs noted above (39–41).
The exact chemical structures of the oxidation-specific epitopes to which they bind are beginning to be defined. Work from our laboratory first showed that the same PC-containing OxPL on OxLDL and apoptotic cells recognized by NAb E06 was also a specific ligand recognized by CD36 and SR-B1 (21, 22). However, the recent elegant studies by Hazen and colleagues have shown that various oxidized moieties on the sn2 side chain of OxPL (in both PC and phosphatidylserine containing PL) are also sufficient ligands to mediate binding of both OxLDL and apoptotic cells to CD36 (42, 43). Indeed, we speculate that the binding of both the PC and the oxidized side chain moieties of OxPL to different sites on CD36 would lead to cooperative high-affinity binding of OxLDL to CD36. These data illustrate what appears to be the common “rule” that such innate PRRs have the capacity to bind to multiple PAMPs. As another example related to binding of modified LDL, OxLDL and acetyl LDL were both shown to bind to SRA, but at different molecular sites (44). Because these PRRs are germline encoded, and thus limited in number, it is likely that those receptors with the capacity to bind multiple ligands, perhaps even related epitopes, such as oxidation-specific epitopes, would be the ones most conserved.
Soluble PRRs Recognize Oxidation-Specific Epitopes
Aside from NAbs, among the known soluble innate proteins, the only one which to date has been shown to bind to an oxidation epitope is the short pentraxin C-Reactive Protein (CRP). CRP, an acute phase protein and a valuable biomarker of the inflammatory state, has became widely known now as an important and independent biomarker of cardiovascular disease. However, this protein was originally noted for its ability to bind to the PC adduct covalently bound to the cell wall polysaccharide of S. pneumonia and for its ability to mediate enhanced clearance of this and other pathogens. However, we have shown that similar to E06, CRP specifically binds to the PC moiety of OxPLs, whether present on OxLDL or on apoptotic cells, but does not bind to the PC of unoxidized phospholipids (45). Furthermore, CRP is found in atherosclerotic lesions and is colocalized with PC containing OxPL, presumably on OxLDL and apoptotic cells (45). Indeed, CRP binds to ischemic myocardium in experimentally induced myocardial infarction (46), and we speculate that the CRP binds directly to the apoptotic and dying cells expressing such OxPLs.
There are a variety of other soluble innate proteins, including other pentraxins and various complement proteins, and we hypothesize that future studies will find that many other highly conserved “innate” soluble proteins will be identified with the capacity to bind such epitopes.
Summary and Functional Roles of Innate PRRs to Oxidation-Specific Epitopes
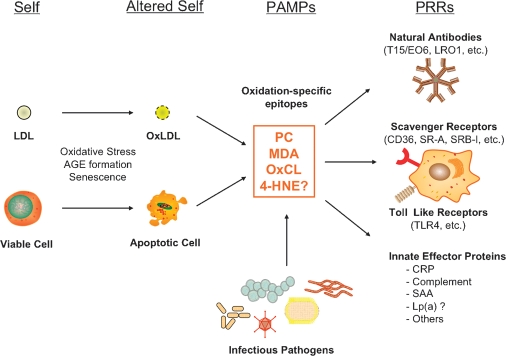
Our general hypothesis that oxidation-specific epitopes are an important target of innate immunity and form a novel set of PAMPs that are recognized by multiple PRRs of innate immunity is summarized in Fig. 1. In the case of PC as a PAMP, published data strongly support this paradigm. Thus, the PC of unoxidized PL, as present in native LDL or viable cells, is “self” and not recognized by innate PRRs. By contrast, the PC of OxPL, present on OxLDL and on apoptotic cells, becomes “altered self” and is recognized by specific NAbs, such as E06; by scavenger receptors, such as CD36 and SR-B1; and by innate soluble proteins, such as CRP. In turn, we suggest that each of these PRRs will also recognize the PC present on the cell wall polysaccharide of pathogens, such as S. pneumoniae.
Fig. 1.
Oxidation-specific epitopes are a class of PAMPs recognized by multiple arcs of innate immunity, including NAbs, SRs, and innate soluble proteins. These epitopes may be derived from oxidation-induced alterations of “self” to generate “altered self,” which become “danger signals” targeted by multiple arcs of innate immunity. In addition, these epitopes often share molecular identity with structures on infectious pathogens. [Modified from Fig. 1 of Chou et al. (51), with permission.]
In a similar manner, there are published and developing data that MDA (and its inclusive set of adducts) defines another equally important class of PAMPs. We predict that in a similar manner, other oxidation-specific epitopes, such as MDA, trans-4-hydroxy-2-nonenal (4-HNE), and oxidized cardiolipin (OxCL), will be PAMPs for a concerted set of innate PRRs. Likely, other types of common posttranscriptional modifications, such as advanced glycation endpoducts, will similarly be found to be targets of these innate PRRs.
Because PRRs are germline encoded, the observation that oxidation-specific epitopes, such as PC, are targeted by multiple innate PRRs strongly implies that these epitopes represent important “danger signals” against which multiple defenses are selected to provide homeostasis. Although many of these NAbs also bind infectious pathogens, because B-1 cells are positively selected in fetal life, or shortly thereafter, and because NAbs are present even in germfree mice, this strongly implies that endogenous antigens must be the primary selecting antigens. A similar argument may be offered for scavenger receptors and even some innate soluble proteins. However, the observation that these PRRs also recognize similar epitopes on infectious pathogens suggests that they are likely to be important activating agents later in life as well.
We also suggest that the fundamentally important altered-self antigens leading to oxidation-specific innate PRRs are apoptotic cells and the apoptotic blebs and cellular debris that result. Cells undergoing programmed cell death develop mitochondrial disruption, leading to generalized enhancement of oxidative events. In turn, a wide variety of lipids are oxidized, including cardiolipin, phosphatidylserine, and phosphatidylcholine. Such epitopes are greatly enriched in the apoptotic blebs that bud off from such cells (12, 31) and are present in the circulation as microparticles. We have shown that syngenic apoptotic cells are highly immunogenic and pro-inflammatory if not promptly cleared (28), and since apoptosis is a universal biological event from the earliest stages of development, we suggest that this provides a strong evolutionary pressure for innate mechanisms to efficiently clear such dying cells. Indeed, the fact that there are apparently multiple and redundant mechanisms to effect clearance of apoptotic cells indicates the biological importance of this process (47). Remarkably, all of the different oxidation-specific monoclonal antibodies we have cloned bind to apoptotic cells and apoptotic bodies, presumably to effect their removal. Indeed, in the presence of another innate protein complement, EO6/T15 has the capacity to enhance apoptotic cell clearance in vivo (48). In a similar manner, it is likely that NAbs to a variety of oxidation-specific epitopes would enhance clearance of apoptotic cells and the blebs and microparticles formed from such dying cells. Furthermore, these same epitopes are ligands mediating apoptotic cell recognition by various scavenger receptors (36). Failure to adequately clear the daily burden of apoptotic cells would likely lead to inflammation and contribute to atherogenesis, and even autoimmunity, which typifies the many murine models of lupus.
There are other potential protective mechanisms for NAbs that can be proposed. As noted above, the NAb E06 can inhibit scavenger-receptor-mediated uptake of OxLDL by macrophages, thereby decreasing atherosclerosis. Of potentially even greater value is the ability of E06 to bind to OxPL and directly inhibit their pro-inflammatory effects on endothelial cells (28, 31) and macrophages (32). Presumably other oxidation-specific NAbs may neutralize other pro-atherogenic and pro-inflammatory effects of other components of OxLDL. Finally, by forming immune complexes with various oxidized lipids, either free or as components of OxLDL or apoptotic cells, they may not only facilitate their enhanced removal, but since these are immunogenic, may block their ability to provoke immunogenic responses.
Therapeutic Applications
Because NAbs have been conserved by natural selection, it is likely that on balance they must have been beneficial to the host. Because we have shown in principle that increasing the titer of such antibodies can be anti-atherogenic, identifying the epitopes to which such NAbs bind should identify antigens that could be used to develop an atheroprotective vaccine. In addition, enhancing such NAb titers, possibly even passively, could be used to limit the pro-inflammatory effects of oxidized lipids in acute situations, such as in acute coronary syndromes (49), or in viral induced respiratory distress syndromes (32). Antibodies to these epitopes could be used to image not only atherosclerotic lesions (50) but a variety of inflammatory settings in which such epitopes are expressed, as well as to target therapeutic molecules to active sites of inflammation. It can be envisioned that a better understanding of this important compartment of innate immunity will ultimately identify novel therapeutic targets that can be exploited to interfere with atherogenesis and inflammatory states in general.
Abbreviations
Ab, antibody
apoB, apolipoprotein B
CRP, C-Reactive Protein
MDA, malondialdehyde
NAb, natural antibody
OxLDL, oxidized LDL
OxPL, oxidized phopholipid
PAMP, pathogen-associated molecular pattern
PC, phosphocholine
POVPC, 1-palmitoyl-2-(5′-oxovaleroyl)-sn-glycero-3-phosphocholine
PRR, pattern recognition receptor
SR, scavenger receptor
Published, JLR Papers in Press, December 22, 2008.
Footnotes
Guest editor for this article was Linda Curtiss, the Scripps Research Institute.
References
- 1.Binder C. J., M. K. Chang, P. X. Shaw, Y. I. Miller, K. Hartvigsen, A. Dewan, and J. L. Witztum. 2002. Innate and acquired immunity in atherogenesis. Nat. Med. 8 1218–1226. [DOI] [PubMed] [Google Scholar]
- 2.Hansson G. K., and P. Libby. 2006. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 6 508–519. [DOI] [PubMed] [Google Scholar]
- 3.Getz G. S. 2005. Thematic review series: the immune system and atherogenesis. Immune function in atherogenesis. J. Lipid Res. 46 1–10. [DOI] [PubMed] [Google Scholar]
- 4.Binder C. J., P. X. Shaw, M. K. Chang, A. Boullier, K. Hartvigsen, S. Hörkkö, Y. I. Miller, D. A. Woelkers, M. Corr, and J. L. Witztum. 2005. The role of natural antibodies in atherogenesis. J. Lipid Res. 46 1353–1363. [DOI] [PubMed] [Google Scholar]
- 5.Wright S. D., C. Burton, M. Hernandez, H. Hassing, J. Montenegro, S. Mundt, S. Patel, D. J. Card, A. Hermanowski-Vosatka, J. D. Bergstrom, et al. 2000. Infectious agents are not necessary for murine atherogenesis. J. Exp. Med. 191 1437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palinski W., S. Ylä-Herttuala, M. E. Rosenfeld, S. W. Butler, S. A. Socher, S. Parthasarathy, L. K. Curtiss, and J. L. Witztum. 1990. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 10 325–335. [DOI] [PubMed] [Google Scholar]
- 7.Palinski W., S. Hörkkö, E. Miller, U. P. Steinbrecher, H. C. Powell, L. K. Curtiss, and J. L. Witztum. 1996. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Invest. 98 800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld M. E., W. Palinski, S. Ylä-Herttuala, S. Butler, and J. L. Witztum. 1990. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 10 336–349. [DOI] [PubMed] [Google Scholar]
- 9.Horie K., T. Miyata, K. Maeda, S. Miyata, S. Sugiyama, H. Sakai, C. Y. Strihou, V. M. Monnier, J. L. Witztum, and K. Kurokawa. 1997. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J. Clin. Invest. 100 2995–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neale T. J., P. P. Ojha, M. Exner, H. Poczewski, B. Ruger, J. L. Witztum, P. Davis, and D. Kerjaschki. 1994. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J. Clin. Invest. 94 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dei R., A. Takeda, H. Niwa, M. Li, Y. Nakagomi, M. Watanabe, T. Inagaki, Y. Washimi, Y. Yasuda, K. Horie, et al. 2002. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer's disease. Acta Neuropathol. 104 113–122. [DOI] [PubMed] [Google Scholar]
- 12.Chang M. K., C. Bergmark, A. Laurila, S. Hörkkö, K. H. Han, P. Friedman, E. A. Dennis, and J. L. Witztum. 1999. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: Evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA. 96 6353–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsimikas S., E. S. Brilakis, R. J. Lennon, E. R. Miller, J. L. Witztum, J. P. McConnell, K. S. Kornman, and P. B. Berger. 2007. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 48 425–433. [DOI] [PubMed] [Google Scholar]
- 14.Binder C. J., K. Hartvigsen, and J. L. Witztum. 2007. Promise of immune modulation to inhibit atherogenesis. J. Am. Coll. Cardiol. 50 547–550. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson J., G. K. Hansson, and P. K. Shah. 2005. Immunomodulation of atherosclerosis: implications for vaccine development. Arterioscler. Thromb. Vasc. Biol. 25 18–28. [DOI] [PubMed] [Google Scholar]
- 16.Caligiuri G., J. Khallou-Laschet, M. Vandaele, A. T. Gaston, S. Delignat, C. Mandet, H. V. Kohler, S. V. Kaveri, and A. Nicoletti. 2007. Phosphorylcholine-targeting immunization reduces atherosclerosis. J. Am. Coll. Cardiol. 50 540–546. [DOI] [PubMed] [Google Scholar]
- 17.Hörkkö S., D. A. Bird, E. Miller, H. Itabe, N. Leitinger, G. Subbanagounder, J. A. Berliner, P. Friedman, E. A. Dennis, L. K. Curtiss, et al. 1999. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 103 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw P. X., S. Horkko, M. K. Chang, L. K. Curtiss, W. Palinski, G. J. Silverman, and J. L. Witztum. 2000. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman P., S. Hörkkö, D. Steinberg, J. L. Witztum, and E. A. Dennis. 2002. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol concentration. J. Biol. Chem. 277 7010–7020. [DOI] [PubMed] [Google Scholar]
- 20.Boullier A., K. L. Gillotte, S. Hörkkö, S. R. Green, P. Friedman, E. A. Dennis, J. L. Witztum, D. Steinberg, and O. Quehenberger. 2000. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J. Biol. Chem. 275 9163–9169. [DOI] [PubMed] [Google Scholar]
- 21.Gillotte-Taylor K., A. Boullier, J. L. Witztum, D. Steinberg, and O. Quehenberger. 2001. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 42 1474–1482. [PubMed] [Google Scholar]
- 22.Boullier A., P. Friedman, R. Harkewicz, K. Hartvigsen, S. R. Green, F. Almazan, E. A. Dennis, D. Steinberg, J. L. Witztum, and O. Quehenberger. 2005. Phosphocholine as a pattern recognition ligand for CD36. J. Lipid Res. 46 969–976. [DOI] [PubMed] [Google Scholar]
- 23.Hardy R. R., C. J. Wei, and K. Hayakawa. 2004. Selection during development of VH11+ B cells: a model for natural autoantibody-producing CD5+ B cells. Immunol. Rev. 197 60–74. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y., J. W. Tung, E. E. B. Ghosn, L. A. Herzenberg, and L. A. Herzenberg. 2007. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc. Natl. Acad. Sci. USA. 104 4542–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binder C. J., M. Y. Chou, L. Fogelstrand, K. Hartvigsen, P. X. Shaw, A. Boullier, and J. L. Witztum. 2008. Natural antibodies in murine atherosclerosis. Curr. Drug Targets. 9 190–195. [DOI] [PubMed] [Google Scholar]
- 26.Baumgarth N., J. W. Tung, and L. A. Herzenberg. 2005. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 26 347–362. [DOI] [PubMed] [Google Scholar]
- 27.Mi Q. S., L. Zhou, D. H. Schulze, R. T. Fischer, A. Lustig, L. J. Rezanka, D. M. Donovan, D. L. Longo, and J. J. Kenny. 2000. Highly reduced protection against Streptococcus pneumoniae after deletion of a single heavy chain gene in mouse. Proc. Natl. Acad. Sci. USA. 97 6031–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang M. K., C. J. Binder, Y. I. Miller, G. Subbanagounder, G. J. Silverman, J. A. Berliner, and J. L. Witztum. 2004. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 200 1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binder C. J., S. Hörkkö, A. Dewan, M. K. Chang, E. P. Kieu, C. S. Goodyear, P. X. Shaw, W. Palinski, J. L. Witztum, and G. J. Silverman. 2003. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 9 736–743. [DOI] [PubMed] [Google Scholar]
- 30.Faria-Neto J. R., K. Y. Chyu, X. Li, P. C. Dimayuga, C. Ferreira, J. Yano, B. Cercek, and P. K. Shah. 2005. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 189 83–90. [DOI] [PubMed] [Google Scholar]
- 31.Huber J., A. Vales, G. Mitulovic, M. Blumer, R. Schmid, J. L. Witztum, B. R. Binder, and N. Leitinger. 2002. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler. Thromb. Vasc. Biol. 22 101–107. [DOI] [PubMed] [Google Scholar]
- 32.Imai Y., K. Kuba, G. G. Neely, R. Yaghubian-Malhami, T. Perkmann, G. van Loo, M. Ermolaeva, R. Veldhuizen, Y. H. C. Leung, H. Wang, et al. 2008. Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 133 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuominen A., Y. I. Miller, L. F. Hansen, Y. A. Kesaniemi, J. L. Witztum, and S. Hörkkö. 2006. A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 26 2096–2102. [DOI] [PubMed] [Google Scholar]
- 34.Kagan V. E., G. G. Borisenko, Y. Y. Tyurina, V. A. Tyurin, J. Jiang, A. I. Potapovich, V. Kini, A. A. Amoscato, and Y. Fujii. 2004. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic. Biol. Med. 37 1963–1985. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein J. L., Y. K. Ho, S. K. Basu, and M. S. Brown. 1979. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA. 76 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pluddemann A., C. Neyen, and S. Gordon. 2007. Macrophage scavenger receptors and host-derived ligands. Methods. 43 207–217. [DOI] [PubMed] [Google Scholar]
- 37.Krieger M. 1992. Molecular flypaper and atherosclerosis: structure of the macrophage scavenger receptor. Trends Biochem. Sci. 17 141–146. [DOI] [PubMed] [Google Scholar]
- 38.Pluddemann A., S. Mukhopadhyay, and S. Gordon. 2006. The interaction of macrophage receptors with bacterial ligands. Expert Rev. Mol. Med. 8 1–25. [DOI] [PubMed] [Google Scholar]
- 39.Miller Y. I., S. Viriyakosol, D. S. Worrall, A. Boullier, S. Butler, and J. L. Witztum. 2005. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler. Thromb. Vasc. Biol. 25 1213–1219. [DOI] [PubMed] [Google Scholar]
- 40.Erridge C., S. Kennedy, C. M. Spickett, and D. J. Webb. 2008. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 283 24748–24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walton K. A., X. Hsieh, N. Gharavi, S. Wang, G. Wang, M. Yeh, A. L. Cole, and J. A. Berliner. 2003. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 278 29661–29666. [DOI] [PubMed] [Google Scholar]
- 42.Hazen S. L. 2008. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 283 15527–15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenberg M. E., M. Sun, R. Zhang, M. Febbraio, R. Silverstein, and S. L. Hazen. 2006. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 203 2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman M., Y. Ekkel, L. Rohrer, M. Penman, N. J. Freedman, G. M. Chisolm, and M. Krieger. 1991. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA. 88 4931–4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang M. K., C. J. Binder, M. Torzewski, and J. L. Witztum. 2002. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. USA. 99 13043–13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griselli M., J. Herbert, W. L. Hutchinson, K. M. Taylor, M. Sohail, T. Krausz, and M. B. Pepys. 1999. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J. Exp. Med. 190 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savill J., I. Dransfield, C. Gregory, and C. Haslett. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2 965–975. [DOI] [PubMed] [Google Scholar]
- 48.Ogden C. A., R. Kowalewski, Y. Peng, V. Montenegro, and K. B. Elkon. 2005. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 38 259–264. [DOI] [PubMed] [Google Scholar]
- 49.Tsimikas S., Y. T. Lau, K. H. Han, B. P. Shortal, E. Miller, A. Segev, L. K. Curtiss, J. L. Witztum, and B. H. Strauss. 2004. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and Lp(a): acute and long term immunological responses to oxidized LDL. Circulation. 109 3164–3170. [DOI] [PubMed] [Google Scholar]
- 50.Briley-Saebo K. C., P. X. Shaw, W. J. Mulder, S. H. Choi, E. Vucic, J. G. Aguinaldo, J. L. Witztum, V. Fuster, S. Tsimikas, and Z. A. Fayad. 2008. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation. 117 3206–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou M. Y., K. Hartvigsen, L. F. Hansen, L. Fogelstrand, P. X. Shaw, A. Boullier, C. J. Binder, and J. L. Witztum. 2008. Oxidation-specific epitopes are important targets of innate immunity. J. Intern. Med. 263 479–488. [DOI] [PubMed] [Google Scholar]