Abstract
Adipose tissue metabolism exerts a profound impact on whole-body metabolism. We review how fuel partitioning between adipocytes and other tissues affects insulin signaling pathways. We discuss the role of adipose tissue inflammation in adipocyte metabolism and whole-body insulin sensitivity. Finally, we mention the role of adipokines in autocrine and paracrine signaling.
Keywords: adiponectin, leptin, inflammation
Obesity is associated with changes in adipocyte gene expression spanning many pathways. These changes alter fuel partitioning between adipose tissue and other tissues. In addition, they alter the hormonal milieu by changing the relative expression of adipose hormones, adipokines. Finally, many metabolites act as signaling molecules; thus, their redistribution alters signaling pathways in adipose and other tissues.
The role of inflammation in obesity has gained attention, beginning with the discovery that the macrophage content of adipose tissue in obese humans and rodents increases dramatically [reviewed in (1)]. Thus, in evaluating gene expression from adipose tissue, one has to consider the change in tissue composition and the role of macrophage-derived molecules in the alteration of adipocyte gene expression.
In the context of overnutrition, most of the attention has been directed to dietary fat and carbohydrates. However, high-calorie diets are often associated with increased protein intake. Of particular interest in relation to extrahepatic tissues are the branched chain amino acids (BCAAs). BCAA levels are increased in the bloodstream of obese humans and in animal models of obesity [reviewed in (2)]. Owing to the absence of the mitochondrial branched chain amino transferase in the liver, BCAAs bypass the liver and are selectively metabolized in extraphepatic tissues. BCAAs modulate food intake through hypothalamic signaling and regulate leptin production in adipose tissue. The increase in BCAAs in obesity appears to be due in part to reduced expression of mitochondrial branched chain amino transferase in adipose tissue (2).
Amino acid excess affects insulin signaling and glucose metabolism. This results in a direct stimulation of gluconeogenesis (3) and decreased glucose transport and glycogen synthesis in muscle (4). Through their activation of the mTor/S6 kinase pathway, amino acids stimulate serine phosphorylation of IRS1 and blunt insulin signaling (5).
FUEL PARTITIONING
Although animals go through feeding/fasting cycles, the liver maintains a relatively constant fatty acid flux into triglyceride biosynthesis [reviewed in (6)]. During fasting, the free fatty acid is derived from lipolysis of adipose tissue triglyceride, is transported to the liver, and is re-esterified to form hepatic triglyceride. With a lipogenic diet, the fatty acids are synthesized de novo in liver and adipose tissue. In a healthy animal, hepatocytes are able to maintain a rate of VLDL secretion sufficient to prevent the accumulation of excess triglyceride, a condition termed hepatic steatosis. Hepatic steatosis occurs in >20% of the US population, with some ethnic groups as high as 45% (7). It is especially common in obese individuals. The heritability of hepatic steatosis is quite high, and a candidate susceptibility gene, PNPLA3, a gene expressed in liver and adipose tissue, has recently been identified in a genome-wide association study (8).
Early microarray experiments indicated that genes involved in lipogenesis are downregulated in adipose tissue of obese leptin-deficient mice (9, 10). This also includes the master regulator of these genes, Srebp1c. Conversely, these same genes are upregulated in the liver. Interestingly, a comparison hepatic gene expression in diabetes-resistant (C57BL/6) versus diabetes-susceptible (BTBR) leptin-deficient mice showed that only the nondiabetic strain exhibited the induction of lipogenic genes in the liver (9). In the livers of obese mice from a diabetes-susceptible strain, Srebp1c and its target genes were not upregulated. The same trend is seen for Pparg, a gene normally not expressed at a high level in the liver, but one that is induced in the liver of obese and lipodystrophic animals (9, 11). The hepatic steatosis phenotype is rescued by ablation of the hepatic Pparg gene (11). This creates an interesting dichotomy between hepatic steatosis and diabetes susceptibility; the diabetes-resistant mouse strain is more susceptible to hepatic steatosis. Similar results have been observed in mice with lipodystrophy when the lipodystrophy mutation is studied in C57BL/6 versus a diabetes-susceptible strain, FVB (11). In short, the relative expression level of at least two key transcription factors, Srepb1c and Pparg, in adipose tissue and liver, plays a role in fuel partitioning between the two tissues. There is reason to believe that several factors in adipose tissue locally have an impact on peroxisome proliferator-activated receptor γ (PPARγ) activity. Such factors include the cytoplasmic lipid binding protein aP2, whose absence leads to increased PPARγ activity (12), as well as the adipokine adiponectin, whose overexpression in adipose tissue causes a net increase in the transcription of PPARγ targets (13).
The partitioning of lipids between adipose tissue and other tissues plays an important role in insulin signaling and cellular viability. Adipose tissue is specialized for triglyceride storage and has a very high capacity to accumulate triglycerides. Thus, the enhanced lipolysis and consequent free fatty acid flux from adipose tissue in obesity exposes other tissues to a substantial fatty acid burden (14). These other tissues can accumulate triglycerides, and this is associated with cell pathology and insulin resistance. However, it is important to point out that current evidence argues against triglyceride itself being the culprit in these fatty-acid-mediated actions. Rather, strong evidence supports a role for diacylglycerol in the blunting of insulin signaling. Increased expression of diacylglycerol acyl transferase improves insulin sensitivity (15), whereas inhibition of diacylglycerol kinase suppresses insulin signaling (16). A likely target for diacylglycerol action is protein kinase C-Θ (17), which upon stimulation by diacylglycerol, phosphorylates serine residues on insulin receptor substrates, blunting the insulin signal (18).
In addition to diacylglycerol, there is also strong evidence implicating ceramide in the modulation of insulin sensitivity. Ceramide is structurally analogous to diacylglycerol by having two fatty acid moieties attached to a backbone. Instead of glycerol, the backbone is serine, and instead of giving rise to glycerolipids, ceramide is an intermediate in sphingolipid synthesis. Like diacylglycerols, ceramides are signaling molecules. They activate protein phosphatase 2A, which dephosphorylates and thus inactivates Akt/PKB, a key arm of the insulin signaling pathway (19). In addition, ceramides inhibit the translocation of Akt/PKB to the plasma membrane (20).
LEPTIN AND ADIPONECTIN
Leptin also plays a profound role in fuel partitioning. Diet-induced obesity simultaneously leads to increased leptin secretion and a blunting of the autocrine leptin signal in adipocytes. The leptin signal tends to be anti-adipogenic, a conclusion that emerged from the protection from obesity afforded to transgenic mice overexpressing the leptin b receptor in adipocytes (21). This illustrates the point that adipose tissue expansion during a positive energy balance (i.e., overeating) is an integral component of the maintenance of energy homeostasis. These mice are deficient in both adipocyte hypertrophy and hyperplasia, resulting in an inability to expand adipose tissue mass under these conditions. Functionally, the net result is comparable to states of partial or complete lipodystrophy; for example, excess triglycerides accumulate ectopically in tissues such as liver, muscle, and β-cells, where they contribute significantly toward the lipotoxic effects of lipids, as discussed above.
On the other end of the spectrum, we find a recently described mouse obesity model that overexpresses the adipocyte-derived circulating factor adiponectin (13). In the context of a challenge with the Leptinob/ob mutation, these mice retain adiponectin levels at concentrations found in a lean mouse. Under these conditions, the mice further expand their adipose tissue mass quite dramatically, far beyond the excess adiposity conventionally seen in the Leptinob/ob model. Surprisingly, despite the Leptinob/ob mutation and the huge excess of adipose tissue, these mice have a fairly good metabolic profile and retain near normal insulin sensitivity, a normalized lipid profile, and hallmarks of a significant improvement in adipose tissue histology. An increased number of smaller fat cells is apparent, with a reduced infiltration of macrophages. Hepatic steatosis, which is conventionally seen at high levels in the Leptinob/ob mouse, is reduced. This is an example in which the ectopic accumulation of lipids is reduced, presumably because excess lipids are neutralized by storage in subcutaneous fat pads. As a result, insulin sensitivity is preserved.
These results suggest that adipose tissue can play a protective and a detrimental role in maintaining a favorable metabolic profile. This was quite elegantly demonstrated in a fat transplantation study that followed a 2 × 2 experimental design in which subcutaneous and visceral fat was transplanted into either a subcutaneous or visceral adipose site (22). Transplantation of subcutaneous fat into a visceral site produced significant improvement in insulin sensitivity and a drop in plasma glucose and insulin levels. Surprisingly, the animals also experienced a drop in leptin and adiponectin, suggesting that other fat-derived factors play a role in the improved metabolic profile. Since transplantation into the subcutaneous site did not alter the metabolic profile, there appear to be both donor and recipient site-specific factors required for these metabolic changes.
This raises the important question as to what the critical initial events are that prevent adipose tissue from assuming its appropriate physiological role as a triglyceride storage compartment, leading to the forced accumulation of ectopic lipids in alternative tissues. From an evolutionary point of view, the “thrifty gene hypothesis” suggests that we are all geared toward maximizing our ability to store fat during times of plenty so these reserves can be tapped during times of food shortage. On the other hand, “too much of a good thing” (i.e., excess adipose tissue accumulation) may significantly impair the ability to effectively escape a predator. Leptin is clearly one leg of a feedback loop designed to reduce food intake and increase energy expenditure during times of excessive adipose tissue growth. However, the system is clearly not very effective, as clear signs of “leptin resistance” occur during early stages of obesity. On the other hand, the well-established obesity-associated downregulation of adiponectin may be a second leg on which this feedback loop critically depends. If adiponectin is indeed an anabolic hormone that potently drives free fatty acids into adipocytes for esterification, its downregulation during adipose tissue growth may prevent excessive expansion of fat mass. However, this comes at the price of ectopic accumulation of a fraction of these excess lipids in other tissues.
ADIPOSE TISSUE INFLAMMATION
The obesity-associated increased infiltration of immune cells, especially macrophages, is well established at this stage (23). The infiltration of macrophages per se can trigger increased local and systemic inflammation, which is associated with decreased insulin sensitivity (Fig. 1). For instance, transgenic overexpression of monocyte chemotactic protein-1 in adipose tissue (24) is necessary and sufficient to trigger increased infiltration of macrophages. However, it is not clear whether under normal physiological conditions increased macrophage infiltration and subsequent inflammation is the result of a sophisticated chemokine-based signaling mechanism or simply the result of an increased incidence of necrotic adipocytes, frequently seen during rapid tissue expansion. Independent of its origin, a reduction of local inflammation in adipose tissue, either through pharmacological intervention or through genetic manipulation of pro-inflammatory pathways, is invariably associated with improvements in local and systemic insulin sensitivity. While inflammatory pathways are the ultimate mediators of insulin resistance, other events in adipose tissue may precede the initiation of inflammation-induced adipose tissue dysfunction. In addition, since the macrophage-induced inflammation is preceded by the activation of the classical inflammatory transcription program in the macrophages [the “M1” activation pathway (25)], there may be critical factors in adipose tissue that elicit this program in adipose tissue macrophages.
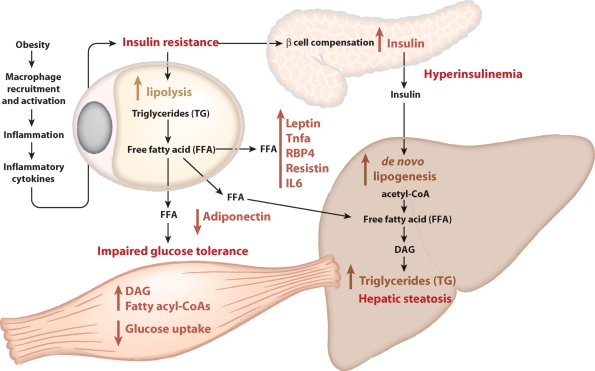
Fig. 1.
Three links between adipocyte biology and metabolic syndrome. Obesity leads to the recruitment by adipocytes of macrophages. These macrophages are activated to produce inflammatory cytokines, which blunt insulin signaling. In adipocytes, insulin resistance leads to an impaired ability of insulin to suppress lipolysis, leading to an increased flux of free fatty acids from adipocytes to other tissues. In muscle, increased fatty acid flux leads to impaired glucose uptake, leading to whole-body impaired glucose tolerance. In the liver, the increased flux of free fatty acid contributes to increased triglyceride synthesis and hepatic steatosis. Insulin resistance causes pancreatic β-cells to compensate with increased insulin production, leading to hyperinsulinemia. This in turn stimulates de novo lipogenesis in the liver, contributing to the pool of free fatty acids available for triglyceride production. Obesity also alters the balance of adipokines produced by adipocytes, with an increase in leptin, TNFα, RBP4, resistin, and IL6, and a decrease in adiponectin. This altered balance contributes to impaired glucose tolerance and insulin resistance.
An attractive model that is supported by limited data is that adipocyte growth (both hypertrophy and hyperplasia) places demands for increased vascularization and tissue remodeling. If these two processes lag behind the expansion of adipose mass, hypoxic conditions emerge, which then stimulate a specific program of gene expression and may also lead to the recruitment of macrophages to the adipose tissue. Gene expression changes consistent with this model have been observed in a comparison of responsive versus nonresponsive (in terms of weight gain) mice of a single strain fed a high-fat diet (26).
Hypoxia has been directly detected by immunohistochemistry and by measuring the perfusion of adipose tissue with radiolabeled microspheres in genetically obese [KKAy (27) or leptin-deficient (28)] mice. These changes are associated with reduced adiponectin expression and an increase in the expression of genes associated with hypoxia, Hif-1α, Glut1, and Pdk1, and inflammation, TNFα, IL-1, IL-6, and TGF-β (28). The latter genes were induced in both adipocytes and macrophages.
In many other tissues, hypoxia is associated with an increased level of fibrosis through an upregulation of many extracellular matrix proteins [reviewed in (29)]. As such, it is likely that the prevailing hypoxia in expanding adipose tissue may also be associated with an increased degree of extracellular matrix deposition in adipose tissue. It is not clear whether this increased density of extracellular support structures in adipose tissue contributes directly toward an increased rate of cell death observed in expanding adipose tissue. Genetic and pharmacological studies will be required to see if the upregulation of these extracellular matrix components in adipose tissue is a simple epiphenomenon associated with expanding adipose tissue or if it presents a worthwhile area to interfere with in the context of an increased fibrotic content in adipose tissue.
THE ADIPOCYTE: DO ENDOPLASMIV RETICULUM STRESS AND THE UNFOLDED PROTEIN RESPONSE PLAY A ROLE?
Endoplasmic reticulum stress and the associated unfolded protein response (UPR) have been associated with cellular dysfunction and cell death in a number of different cell types relevant for metabolic homeostasis. This is particularly relevant for cell types with a very active secretory pathway. The pancreatic β-cell, with its high-level production of insulin, is highly prone to stress in the endoplasmic reticulum. The rate of protein secretion from adipocytes is frequently underestimated. Whereas the role of the adipocyte as an endocrine cell is widely appreciated, many of the adipokines that the fat cell produces have relatively short half-lives but circulate at rather high levels (e.g., adiponectin, several complement factors, and acute phase reactants). This imposes major challenges for the proper folding and assembly of some of these factors, which in some instances need to form highly complex quaternary structures. Thus, the UPR may play an important role in the cellular homeostasis of the adipocyte in the lean as well as in the obese state.
All three “classical” pathways of the UPR (such as the PERK, IRE-1, and the ATF-6 pathways) are present and can be activated in the adipocyte [reviewed in (30)]. Even in human adipose tissue, these pathways are highly relevant, and the degree of activation correlates positively with overall adiposity within an individual (31). However, in this study, the activation state of the pathway did not correlate with systemic insulin resistance. This is surprising in light of the fact that endoplasmic reticulum stress can trigger activation of the jun kinase pathway and NF-κB, while at the same time, local inflammation in adipose tissue can trigger the UPR. The connection between the UPR and inflammation is a reflection of crosstalk at multiple levels, including the increased production of reactive oxygen species that are generated as a result of the activation of the UPR. These questions will need to be further studied, particularly because a recent article implicated Xbp1, an important downstream mediator of the UPR, as a master regulator of lipogenesis in the liver; deletion of Xbp1 in the liver caused hypocholesterolemia and reduced triglyceride accumulation as a result of decreased lipogenesis (32). Whether Xbp1 exerts similar functions on lipogenesis and/or lipid storage in adipocytes may indicate that the differential activation of the UPR in liver and adipose tissue plays a role in the fuel partitioning of lipids between these two tissues.
MITOCHONDRIAL DYSFUNCTION: ALSO IMPORTANT FOR THE WHITE ADIPOCYTE?
Mitochondrial function is key for proper maintenance of energy homeostasis. This also holds true for white adipocytes, where proper mitochondrial function is likely to be key for systemic insulin sensitivity. Insulin-sensitizing drugs, such as the PPARγ agonists, induce a host of mitochondrial proteins and improve mitochondrial function in adipocytes (33). Impaired mitochondrial respiratory function triggers a reduction in translocation of Glut4 to the plasma membrane, but surprisingly, enhances Akt signaling (34). Even modest changes at the level of mitochondrial function have a dramatic effect on production and release of adiponectin (34). Particularly in the hyperglycemic state, excess intracellular glucose availability causes a dramatic increase in mitochondrial ROS production and hence increased local inflammation (35). It is therefore very likely that proper mitochondrial function in white adipocytes is key for appropriate energy balance between different tissues, particularly during times of excess energy intake.
CONCLUSIONS AND OUTLOOK
Adipose tissue and the liver constitute an interesting organ pair that is in constant communication with each other via adipokines, lipid factors, and lipoprotein particles. The adipohepatic axis affects lipid and carbohydrate usage and flux. Dysregulation in either of the two tissues is detrimental to the other and ultimately for the entire system. One of the first organs to be affected when adipose tissue becomes dysfunctional and inflamed is the liver. Secondary to that, changes in free fatty acid concentration and flux affect other cell types, such as muscle cells and pancreatic β-cells, which are susceptible to fatty-acid-induced lipotoxicity. The associated insulin resistance imposes increased demands on the secretory capacity of β-cells, which under these conditions, is vulnerable to UPR-induced cell death.
Many new avenues of research are currently opening up that will allow for further study of the complex relationship between proper adipocyte hypertrophy and hyperplasia and downstream events such as inflammation, mitochondrial dysfunction, and lipid accumulation in secondary organs such as the liver, muscle, and β-cells.
Abbreviations
BCAA, branched chain amino acid
PPARγ, peroxisome proliferator-activated receptor γ
UPR, unfolded protein response
The authors' research is supported by the National Institutes of Health Grants R01-DK 66539 and R01-DK 58037 (A.D.A.), and R01-DK55758 and R01-CA112023 (P.E.S.). A.D.A. and P.E.S. are also supported by Juvenile Diabetes Research Foundation Grant 17-2007-1026.
Published, JLR Papers in Press, November 17, 2008.
References
- 1.de Luca C., and J. M. Olefsky. 2008. Inflammation and insulin resistance. FEBS Lett. 582 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.She P., C. Van Horn, T. Reid, S. M. Hutson, R. N. Cooney, and C. J. Lynch. 2007. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 293 E1552–E1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs M., A. Brehm, M. Krssak, C. Anderwald, E. Bernroider, P. Nowotny, E. Roth, V. Chandramouli, B. R. Landau, W. Waldhausl, et al. 2003. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia. 46 917–925. [DOI] [PubMed] [Google Scholar]
- 4.Krebs M., M. Krssak, E. Bernroider, C. Anderwald, A. Brehm, M. Meyerspeer, P. Nowotny, E. Roth, W. Waldhausl, and M. Roden. 2002. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 51 599–605. [DOI] [PubMed] [Google Scholar]
- 5.Tremblay F., M. Krebs, L. Dombrowski, A. Brehm, E. Bernroider, E. Roth, P. Nowotny, W. Waldhausl, A. Marette, and M. Roden. 2005. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 54 2674–2684. [DOI] [PubMed] [Google Scholar]
- 6.Blasiole D. A., R. A. Davis, and A. D. Attie. 2007. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol. Biosyst. 3 608–619. [DOI] [PubMed] [Google Scholar]
- 7.Browning J. D., L. S. Szczepaniak, R. Dobbins, P. Nuremberg, J. D. Horton, J. C. Cohen, S. M. Grundy, and H. H. Hobbs. 2004. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 40 1387–1395. [DOI] [PubMed] [Google Scholar]
- 8.Romeo S., J. Kozlitina, C. Xing, A. Pertsemlidis, D. Cox, L. A. Pennacchio, E. Boerwinkle, J. C. Cohen, and H. H. Hobbs. 2008. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 40 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadler S. T., J. P. Stoehr, K. L. Schueler, G. Tanimoto, B. S. Yandell, and A. D. Attie. 2000. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc. Natl. Acad. Sci. USA. 97 11371–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soukas A., P. Cohen, N. D. Socci, and J. M. Friedman. 2000. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev. 14 963–980. [PMC free article] [PubMed] [Google Scholar]
- 11.Reitman M. L. 2002. Metabolic lessons from genetically lean mice. Annu. Rev. Nutr. 22 459–482. [DOI] [PubMed] [Google Scholar]
- 12.Makowski L., K. C. Brittingham, J. M. Reynolds, J. Suttles, and G. S. Hotamisligil. 2005. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J. Biol. Chem. 280 12888–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J. Y., E. van de Wall, M. Laplante, A. Azzara, M. E. Trujillo, S. M. Hofmann, T. Schraw, J. L. Durand, H. Li, G. Li, et al. 2007. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117 2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz J. F., and S. Klein. 2000. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am. J. Physiol. Endocrinol. Metab. 278 E1144–E1152. [DOI] [PubMed] [Google Scholar]
- 15.Monetti M., M. C. Levin, M. J. Watt, M. P. Sajan, S. Marmor, B. K. Hubbard, R. D. Stevens, J. R. Bain, C. B. Newgard, R. V. Farese, Sr., et al. 2007. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6 69–78. [DOI] [PubMed] [Google Scholar]
- 16.Chibalin A. V., Y. Leng, E. Vieira, A. Krook, M. Bjornholm, Y. C. Long, O. Kotova, Z. Zhong, F. Sakane, T. Steiler, et al. 2008. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 132 375–386. [DOI] [PubMed] [Google Scholar]
- 17.Kim J. K., J. J. Fillmore, M. J. Sunshine, B. Albrecht, T. Higashimori, D. W. Kim, Z. X. Liu, T. J. Soos, G. W. Cline, W. R. O'Brien, et al. 2004. PKC-theta knockout mice are protected from fat-induced insulin resistance. J. Clin. Invest. 114 823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotamisligil G. S., P. Peraldi, A. Budavari, R. Ellis, M. F. White, and B. M. Spiegelman. 1996. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 271 665–668. [DOI] [PubMed] [Google Scholar]
- 19.Summers S. A., L. A. Garza, H. Zhou, and M. J. Birnbaum. 1998. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18 5457–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratford S., K. L. Hoehn, F. Liu, and S. A. Summers. 2004. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279 36608–36615. [DOI] [PubMed] [Google Scholar]
- 21.Wang M. Y., L. Orci, M. Ravazzola, and R. H. Unger. 2005. Fat storage in adipocytes requires inactivation of leptin's paracrine activity: implications for treatment of human obesity. Proc. Natl. Acad. Sci. USA. 102 18011–18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran T. T., Y. Yamamoto, S. Gesta, and C. R. Kahn. 2008. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 7 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisberg S. P., D. McCann, M. Desai, M. Rosenbaum, R. L. Leibel, and A. W. Ferrante, Jr. 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda H., S. Tateya, Y. Tamori, K. Kotani, K. Hiasa, R. Kitazawa, S. Kitazawa, H. Miyachi, S. Maeda, K. Egashira, et al. 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng C. N., J. L. Bodzin, and A. R. Saltiel. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koza R. A., L. Nikonova, J. Hogan, J. S. Rim, T. Mendoza, C. Faulk, J. Skaf, and L. P. Kozak. 2006. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2 e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosogai N., A. Fukuhara, K. Oshima, Y. Miyata, S. Tanaka, K. Segawa, S. Furukawa, Y. Tochino, R. Komuro, M. Matsuda, et al. 2007. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 56 901–911. [DOI] [PubMed] [Google Scholar]
- 28.Ye J., Z. Gao, J. Yin, and Q. He. 2007. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 293 E1118–E1128. [DOI] [PubMed] [Google Scholar]
- 29.Higgins D. F., K. Kimura, M. Iwano, and V. H. Haase. 2008. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 7 1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregor M. F., and G. S. Hotamisligil. 2007. Thematic review series: adipocyte biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J. Lipid Res. 48 1905–1914. [DOI] [PubMed] [Google Scholar]
- 31.Sharma J. K., K. D. Swapan, A. K. Mondal, O. G. Hackney, W. S. Chu, P. L. Kern, N. Rasouli, H. J. Spencer, A. Yao-Borengasser, and S. C. Elbein. 2008. Endoplasmic reticulum stress markers are associated with obesity in non-diabetic subjects. J. Clin. Endocrinol. Metab. 93 4532–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaser A., A. H. Lee, A. Franke, J. N. Glickman, S. Zeissig, H. Tilg, E. E. Nieuwenhuis, D. E. Higgins, S. Schreiber, L. H. Glimcher, et al. 2008. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 134 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson-Fritch L., S. Nicoloro, M. Chouinard, M. A. Lazar, P. C. Chui, J. Leszyk, J. Straubhaar, M. P. Czech, and S. Corvera. 2004. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Invest. 114 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi X., A. Burkart, S. M. Nicoloro, M. P. Czech, J. Straubhaar, and S. Corvera. 2008. Paradoxical effect of mitochondrial respiratory chain impairment on insulin signaling and glucose transport in adipose cells. J. Biol. Chem. 283 30658–30667. [DOI] [PMC free article] [PubMed]
- 35.Lin Y., A. H. Berg, P. Iyengar, T. K. Lam, A. Giacca, T. P. Combs, M. W. Rajala, X. Du, B. Rollman, W. Li, et al. 2005. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J. Biol. Chem. 280 4617–4626. [DOI] [PubMed] [Google Scholar]