Abstract
Oxsterols are oxygenated metabolites of cholesterol that are short-lived intermediates or end products in cholesterol excretion pathways. They are present in very low concentrations in mammalian systems, always accompanied by a high excess of cholesterol. According to current concepts, side-chain oxidized oxysterols may be mediators of many cholesterol-induced regulatory effects. When added to cultured cells in vitro, side-chain oxidized oxysterols limit intracellular cholesterol levels by at least three different mechanisms: 1) binding to Insig with subsequent block of the sterol regulatory element-binding proteins (SREBP)-mediated mechanism for regulation of sterol sensitive genes; 2) increasing degradation of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, eventually by a mechanism involving binding of Insig to the enzyme; 3) activation of LXR-mediated stimulation of cholesterol transporters and cholesterol metabolism. Addition of pure unesterified oxysterols to cell cultures is however highly unphysiological, and the in vivo relevance of such experiments is questionable. Transgenic mouse models with markedly reduced or increased concentration of some specific oxysterols do not present marked disturbances in cholesterol turnover and homeostasis. Oxysterol-binding proteins such as LXR have been conclusively shown to be of importance for cholesterol turnover in vivo, but their physiological ligands have not yet been defined with certainty. During the last few years, new experimental data has accumulated supporting the contention that side-chain oxysterols are involved in some LXR-mediated regulation in vivo, at least in some biological systems. The new findings will be critically reviewed here.
Keywords: 27-Hydroxycholesterol; 24S-hydroxycholesterol; 25-hydroxycholesterol; 24,25-epoxycholesterol
Oxysterols are oxygenated metabolites of cholesterol that are short-lived intermediates in cholesterol excretion pathways (as reviewed in Refs. 1–3). They are present in very low concentrations in mammalian systems, always accompanied by high excess of cholesterol. Oxysterols, in particular those with the extra hydroxyl group is present in the steroid side chain, have a high capacity to affect critical genes in cholesterol turnover under in vitro conditions. Because these metabolites are considerably more potent than cholesterol in this respect, they have been suggested to mediate a number of cholesterol-induced effects. Due to the high and unphysiological levels of free oxysterol used, with a ratio between the added free oxysterol and cholesterol often orders of magnitude higher than normal, it is not possible to evaluate the physiological importance of the effects from such experiments. The possibility has been discussed that high levels of free exogenous oxysterols may act upon plasma membranes by displacing cholesterol from phospholipid complexes (4). Such displacement may send some of the plasma membrane cholesterol to other intracellular compartments where it can result in multiple homeostatic effects. Such effects are less likely to occur under in vivo conditions.
Part of the explanation for the much higher effect of side-chain oxidized cholesterol than cholesterol itself may be their high mobility. Side-chain oxidized oxysterols are thus known to pass lipophilic biomembranes at rates up to 3 magnitudes faster than cholesterol (5, 6). This means that uptake of side-chain oxidized cholesterol species by the cell is likely to be less dependent on receptor-mediated mechanisms. This also means that oxysterols are suitable as transport forms of cholesterol and that conversion of cholesterol into a side-chain oxidized derivative is a strategy used in nature to eliminate excess cholesterol from the cells. The best examples of this is the elimination of cholesterol from cholesterol-loaded macrophages and endothelial cells by conversion into 27-hydroxycholesterol and cholestenoic acid and the elimination of cholesterol from the brain by conversion into 24S-hydroxycholesterol, which is able to pass the brain-blood barrier (2, 3).
In two previous reviews (2, 3) we pointed out that while it is evident that oxysterols are important intermediates in bile acid synthesis and significant transport forms of cholesterol, their physiological importance as regulators of cholesterol homeostasis in vivo is still uncertain and mainly based on indirect evidence. Transgenic mouse models with highly varying levels of side-chain oxidized oxysterols due to overexpression (7) or lack (8, 9) of the critical hydroxylase or the major oxysterol metabolizing enzyme (10), present modest changes only in over-all cholesterol homeostasis.
There are only a few examples of a high accumulation of side-chain oxidized free oxysterols in vivo. 27-Hydroxycholesterol, approximately 85% in esterified form, is known to accumulate in substantial amounts in human atheromas together with esterified cholesterol (11). As a consequence of the high level of cholesterol in the atheroma macrophages, conversion of cholesterol into 27-hydroxycholesterol is likely to increase. Also the ACAT activity will increase, resulting in increased esterification of cholesterol and oxysterols. Because there appears to be a preferential increase in oxysterol esterification, such a mechanism may explain the “trapping” of esterified 27-hydroxycholesterol in atheromas.
During the last few years, important new experimental data have accumulated supporting the contention that side-chain oxidized oxysterols may be of regulatory importance in vivo, at least in some biological systems. These findings will be critically reviewed here. The review will be restricted to effects of side-chain oxidized oxysterols on cholesterol homeostasis, and the recent work suggesting that 27-hydroxycholesterol may be involved in neurodegeneration and modulating estrogen receptor response will thus not be discussed here.
In principle, oxysterols may affect cholesterol-sensitive genes by three different mechanisms: 1) interaction with the sterol regulatory element-binding proteins (SREBP)-mechanism; 2) activation of the LXR mechanisms; 3) effect on the degradation of specific enzymes. With one exception, the present review will be restricted to oxysterols that are formed from cholesterol by oxidation of the steroid side-chain. The exception is 24,25-epoxycholesterol, which is derived from a precursor of cholesterol.
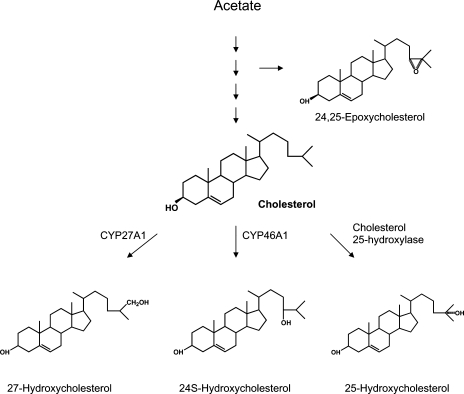
The formation and structures of the side-chain oxidized oxysterols discussed in this review is shown in Fig. 1.
Fig. 1.
Formation of the side-chain oxidized oxysterols discussed in this review.
INTERACTION WITH THE SREBP-MECHANISM
The role of SREBPs for cholesterol, homeostasis is well established by the elegant work from the laboratory of Goldstein, DeBose-Boyd, and Brown (12) utilizing in vivo and in vitro models. Cholesterol synthesis in mammals has thus been demonstrated to be controlled by a regulated transport of SREBPs from the endoplasmic reticulum to the Golgi, where the transcription factors are processed proteolytically to release active fragments. The SREBP-escort binding protein Scap and the anchor protein Insig are key proteins in this mechanism. Using purified recombinant versions of Insig and Scap cholesterol and oxysterols were shown to act by different mechanisms (13). Cholesterol acts by binding to Scap, thereby causing Scap to bind to the anchor protein Insig. In contrast, oxysterols bind preferentially to Insig with the consequence that Insig binds to Scap. It was further shown that two of the six transmembrane helices of Insig are important for its binding to oxysterols and to Scap.
It was suggested that the binding of oxysterols to Insig is important for the ability of oxysterols to inhibit cholesterol synthesis in animal cells.
The relevance of this mechanism for the situation in vivo is not obvious. Under in vivo conditions, the ratio between cholesterol and the oxysterol in the cell may be 103 or higher, and the situation may be similar in the critical membranes to which SREBP is bound. Whether Insig is able to selectively bind oxysterols in the presence of high excess of cholesterol has not been demonstrated.
It seems unlikely that the small variations expected to occur in the ratio between side-chain oxidized oxysterol and cholesterol under normal physiological conditions is of major importance for the SREBP-mediated mechanism.
ARE CHOLESTEROL-INDUCED EFFECTS ON SOME CRITICAL STEROL SENSITIVE GENES MEDIATED BY ACTIVATION OF LXR-SIGNALING BY 24-, 25-, OR 27-HYDROXYCHOLESTEROL IN EXTRACEREBRAL TISSUES?
It is well established that the nuclear receptors LXRα and LXRβ are of importance for cholesterol metabolism. A knock-out of the genes coding for these nuclear receptors do not cause any acute effects in young mice, and highly nonphysiological dietary challenges with 1% or 2% cholesterol in diet are necessary to demonstrate consequences of the gene defects (14). Aging mice, however, depleted for LXRα and LXRβ developed foam cells in spleen, lung, and arterial wall also on a normal diet (15). This is clearly consistent with a protective role of the LXR-systems in atherogenesis.
It is important to emphasize that the cholesterol-induced effects on cholesterol-sensitive genes mediated by the LXR systems are considerably lower than those possible to achieve with a strong synthetic agonist to LXR.
The LXRs became “adopted” orphan receptors when it was found that oxysterols present in biological systems are ligands and activators of LXR under in vitro conditions. Cholesterol accumulating in the circulation and in the tissues is thus believed to increase formation of oxysterols, which are ligands to the LXR-systems. Activation of the LXR-target genes (ABCA1, ABCG1, ABCG5, ABCG8, CYP7A1) can be regarded as a defense mechanism counteracting accumulation of cholesterol.
The physiological candidates for LXR-binding among the oxysterols are 27-hydroxycholesterol, 24S-hydroxycholesterol, 25-hydroxycholesterol, and 24,25-epoxycholesterol (as reviewed in Refs. 2 and 3). All these oxysterols bind and activate LXR, with the epoxide and 24S-hydroxycholesterol being most efficient.
The specific oxysterol(s) that may mediate the cholesterol-induced effects have however not been defined with certainty. The possibility has been discussed (S. Meaney, unpublished observations) that the LXR system is evolutionary designed to protect from exogenous steroids or other similar compounds that are side-chain oxidized and then eliminated as a consequence of the activation of LXR and the LXR-target genes ABCG5/ABCG8.
Under in vitro conditions, cholesterol has a very low affinity to the binding site of LXR, but because it is present in high excess, it may affect the binding of the oxysterol. When the ligand binding domain of LXR is exposed to mixtures of cholesterol and a side-chain oxidized oxysterol, a 500- to 1,000-fold excess of cholesterol will prevent the binding of the oxysterol (16). If this is the situation also in vivo, binding of an oxysterol to LXR would be possible only in biological systems with a ratio of the oxysterol to cholesterol that is higher than 1 per 1,000. This is the situation in the brain, in cholesterol-loaded macrophages, and possibly also in the lung. In these biological systems, variations in the oxysterol levels would be expected to lead to changes in the degree of activation of the LXR-systems. In view of the difficulties to measure oxysterol to cholesterol ratios in specific cell environments, it is difficult to draw firm conclusions from such measurements, however. At the present state it is not possible to exclude cholesterol as a physiological ligand or as an antagonist to the LXR systems (3).
As pointed out above, genetically engineered mice with marked changes in plasma levels of side-chain oxidized oxysterols have surprisingly small changes in cholesterol turnover and homeostasis (7–10). A potential problem is that disappearance of one oxysterol could be compensated for by another. In a recent elegant study by Chen et al. (17), attempts were made to interfere with activation of nuclear receptors by side-chain oxidized oxysterols under in vivo conditions.
The first approach was to overexpress an oxysterol catabolic enzyme, cholesterol sulfotransferase, in cultured mammalian cell lines. This overexpression resulted in inactivation of oxysterol-induced LXR signaling but did not alter the receptor response to a nonsterol agonist, T0901317. A likely explanation is an inactivation of the oxysterol by sulfonation. This approach was also used in a set of in vivo experiments, using mice infected with adenovirus expressing either β-galactosidase or sulfotransferase and fed diets consisting of normal chow or chow enriched with 1% cholesterol or the above nonsterol agonist. Addition of cholesterol to the diet led to the induction of the LXR target genes cholesterol 7α-hydroxylase (CYP7A1), SREBP-1, ABCG5, ABCG8 in mice transfected by the β-galactosidase virus but not in the mice infected by the sulfotransferase. Feeding the nonsterol agonist to the sulfotransferase-expressing mice resulted in a stimulation of all the above genes.
Because sulfotransferase is active not only toward oxysterols but also toward cholesterol, the possibility cannot be excluded that overexpression of the enzyme leads to depletion of cholesterol in critical membranes. In theory, such depletion may affect LXR-signaling by a mechanism that does not involve oxysterols.
A more selective approach was therefore used, using a transgenic mouse model in which three specific oxysterol biosynthetic genes were knocked out, the cholesterol 24-hydroxylase (Cyp46A1), the cholesterol 25-hydroxylase, and the cholesterol 27-hydroxylase (Cyp27). As expected, cholesterol feeding induced five established LXR target genes in wild-type mice [Cyp7A1, lipoprotein lipase, SREBP-1c, ABCG5, ABCG8]. Three of these genes, lipoprotein lipase, ABCG5, and ABCG8, were not induced in the triple-knockout mice. One target gene, SREBP-1c, responded partially to cholesterol feeding in the triple-knockout mice, and another, Cyp7A1, responded normally.
It was concluded that three of the LXR-target genes are likely to be activated by an oxysterol that is a product of Cyp27, cholesterol 25-hydroxylase, or Cyp46. The other LXR target genes may be activated by an oxysterol that is not a product of the depleted enzymes (e.g., 24,25-epoxycholesterol).
There are, however, some problems with the complicated triple-knockout model. The loss of the sterol 27-hydroxylase leads to a marked deficiency of bile acids with reduced absorption of cholesterol. These mice have therefore an upregulation of Cyp7A1, the rate-limiting enzyme in bile acids synthesis, and an upregulation of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol syntheis. This was compensated for in the experiment by supplementation with 0.025% cholic acid in diet given to all the animals, including the nontransgenic controls. The added cholic acid would be expected to suppress Cyp7A1 and normalize cholesterol absorption. In theory, differences in degree of absorption of cholesterol due to different exposure to bile acids in the intestine may have affected the results.
In view of the fact that the loss of the sterol 27-hydroxylase activity would be expected to give the most important effects, it would have been of interest to know if the results of the above experiments had been the same if it had been repeated in Cyp27 single knockout mice.
In spite of the above criticism, the above experiments are at present the best evidence available for the contention that side-chain oxidized steroids are important for at least some of the cholesterol-induced effects mediated by LXR in vivo. It should however be emphasized that feeding of 1% cholesterol to mice is a highly unphysiological challenge.
More indirect evidence for a role of an endogenous oxysterol in the activation of LXR was provided by the demonstration that silencing of an endogenous oxysterol-binding protein (ORP8) in a macrophage cell line induces transcription of LXR target genes, presumably because of a replacement of an oxysterol to LXR (18).
It may be mentioned that it has been reported that endogenously formed 27-hydroxycholesterol in fibroblasts is able to activate LXR in cholesterol-loaded skin fibroblasts resulting in increased activation of ABCA1 and ABCG1 (19). A difference was thus found between fibroblasts from control patients and a patient with a genetic deficiency of the sterol 27-hydroxylase (cerebrotendinous xanthomatosis, CTX). Evidence has also been presented that cholesterol overload in Niemann-Pick type C mutant cells at least in part is due to failure to activate the LXR-mechanism by formation of 25- and 27-hydroxycholesterol (20). In view of the apparent normal cholesterol homeostasis in mice with a disruption of the sterol 27-hydroxylase gene and treated with cholic acid and in CTX-patients treated with chenodeoxycholic acid, it is difficult to evaluate the importance of such a mechanism under in vivo conditions.
IS 24S-HYDROXYCHOLESTEROL A REGULATOR OF CHOLESTEROL HOMEOSTASIS IN THE BRAIN BY AN LXR-MEDIATED MECHANISM?
Because the ratio between the oxysterol 24S-hydroxycholesterol and cholesterol is high in the brain (cf. above), and that the level of LXRβ nuclear receptor also is high, there is a clear potential for LXR-mediated regulation by 24S-hydroxycholesterol in this organ. Normally the CYP46 enzyme responsible for generation of 24S-hydroxycholesterol from cholesterol is located exclusively in neurons. Neurons have a low rate of synthesis of cholesterol, and appear to be dependent upon a flux of cholesterol from astrocytes. Pfrieger (21) suggested the mechanism shown in Fig. 2. 24S-Hydroxycholesterol fluxes from the neurons to the astrocytes, resulting in activation of LXRβ with subsequent secretion of cholesterol from the astrocytes. The latter cholesterol may be taken up by the neuronal cells. Experimental evidence for a shuttle of cholesterol from astrocytes to neurons in an in vitro system has been presented, but there is yet no direct experimental evidence for a flux of 24S-hydroxycholesterol from the neurons to the astrocytes. ApoE appears to be required for the flux of cholesterol, and it was recently reported that 24S-hydroxycholesterol stimulates synthesis and excretion of apoE from cultured astrocytes (22).
Fig. 2.
Crosstalk between neuronal cells and astrocytes [modification of the mechanism suggested by Pfrieger (21)]. Neuronal cells produce 24S-hydroxycholesterol by CYP46A1, which fluxes from these cells to astrocytes where the oxysterol activates LXR and the LXR target genes ABCA1 and ABCG1. This causes a flux of cholesterol from the astrocytes with subsequent uptake by the neuronal cells. The cholesterol is secreted from the cells bound to apoE. ApoE is synthesized and excreted from the astrocytes by mechanisms stimulated by 24S-hydroxycholesterol (22). Neuronal cells have little capacity for cholesterol synthesis and seem to be dependent upon astrocytes for delivery of this steroid (21).
The above mechanism is attractive and consistent with in vitro results but needs to be confirmed in vivo.
If the mechanism shown in Fig. 2 is important in vivo, a correlation is likely to exist between flux of 24S-hydroxycholesterol and apoE secretion. Consistent with this, we recently reported a correlation between levels of apoE and 24S-hydroxycholesterol in spinal fluid from patients with Alzheimer's disease (23).
EFFECTS OF OXYSTEROLS ON PROTEOLYTIC DEGRADATION OF HMG-COA REDUCTASE
27-Hydroxycholesterol has been shown to be an efficient activator of hydroxymethylglutaryl-coenzyme A reductase (HMGR) ubiquitination and proteolytic inactivation under in vitro conditions. Formation of an HMG-Insig complex may proceed this inactivation, and the possibility has been discussed that an oxysterol-bound form of Insig can form a comlex with HMGR just as it can with Scap (13). If this is the case, the binding of the oxysterol to Insig is able to prevent cholesterol accumulation by two different mechanisms.
Experimental data were recently presented supporting the contention that 27-hydroxycholesterol may mediate rapid effects of excess cholesterol on HMGR degradation in cultured fibroblasts (4). In accordance with previous work, fibroblasts from patients with CTX who lack the sterol 27-hydroxylase were roughly normal in their total cellular sterol content, cholesterol esterification rate, and HMGR activity. The cells responded normally to a load of cholesterol. Cholesterol depletion resulted in a similar marked increase of HMG-CoA reductase activity in CTX and control fibroblasts. Surprisingly, however, the HMGR activity in the CTX cells was not suppressed by cholesterol enrichment. Addition of 27-hydroxycholesterol normalized the inactivating effect on HMGR activity in these cells. The authors concluded that the failure to inactivate HMGR upon cholesterol loading in the CTX cells is due to the absence of a production of 27-hydroxycholesterol.
It should be mentioned that one previous study failed to demonstrate a role of an oxysterol for the acute down-regulation of HMGR in CHO-215 cells (24).
While the results of the above study give experimental support for the contention that 27-hydroxycholesterol is able to mediate rapid downregulation of HMGR in cultured fibroblasts under some specific conditions, the relevance of this mechanism for the in vivo situation is difficult to evaluate. Under physiological conditions the situation may be more similar to that in the previous study (24) in which no role of an oxysterol could be demonstrated.
ROLE OF 24,25-EPOXYCHOLESTEROL
24(S),25-epoxycholesterol is formed as a side product in the mevalonate pathway (Fig. 1). After administration of mevalonate to rats, the level of 24(S),25-epoxycholesterol was reported to increase with a factor of 2, whereas the levels of the other side-chain oxidized oxysterols were unchanged (25). It was suggested that the epoxide, in contrast to the other oxysterols, could be a potential mediator of the suppressive effects of mevalonate on HMGR, possibly by increasing the degradation.
It should be mentioned that quantitation of endogenous levels of 24(S),25-epoxycholesterol is difficult and it has been reported that this compound may not survive the temperature required for GC-MS analysis. The varying levels of the epoxide reported in different studies may be related to methodological problems.
Increased cholesterol synthesis would be expected to increase the levels of the epoxide, at least under conditions in which there is a partial inhibition of the 2,3-oxidosqualene-lanosterol cyclase. This would be expected to lead to activation of target genes of LXR (ABCA1, ABCG1, ABCG5, ABCG8, Cyp7A1). Such a response would tend to counteract the accumulation of newly synthesized cholesterol.
Studies by Rowe et al. (26), and Wong, Quinn, and Brown (27) have given strong support for the contention that 24(S),25-epoxycholesterol is important as a modulator of cholesterol homeostasis under some specific conditions. Thus it was reported that treatment with a statin reduced cholesterol efflux from human macrophages as a consequence of reduced expression of ABCA1, ABCG1, and LXRa. The beneficial effects of statins thus appear to be counteracted by a proatherogenic mechanism that is obviously weaker than the sum of all the antiatherogenic effects of the drug. Evidence was given that the statin effect on cholesterol efflux was mediated by 24(S),25-epoxycholesterol and the levels of this oxysterol changed in parallel with the changes observed in the activation of the LXR target genes. In the studies by Rowe et al. (26), and Wong, Quinn, and Brown (27), formation of the epoxide increased when the cells were treated with an inhibitor of 2,3-oxidosqualene-lanosterol cyclase. In accordance with this, it was shown that a cyclase inhibitor within a certain concentration range had a clear effect on foam cell formation, and the possibility was discussed that partial inhibition of this enzyme could represent a new strategy for prevention of atherosclerosis.
Increased cholesterol synthesis would be expected to increase formation of 24(S),25-epoxycholesterol, a mechanism that would tend to counteract the accumulation of newly formed cholesterol in the cell. The importance of this mechanism under conditions in vivo remains to be established, however.
GENERAL CONCLUSIONS
Side-chain oxysterols have a unique capacity to limit cholesterol levels in cultured cells by all the three major mechanisms known for regulation of cholesterol homeostasis. Evidence has now been presented that interaction between LXR and side-chain oxidized steroids is of importance also in vivo, at least in connection with treatment of mice with very high doses of cholesterol. The relevance of this mechanism in humans is however still uncertain.
Published, JLR Papers in Press, October 23, 2008.
References
- 1.Schroepfer G. J. 2000. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 80 361–554. [DOI] [PubMed] [Google Scholar]
- 2.Björkhem I., and U. Diczfalusy. 2002. Oxysterols: friends, foes or just fellow passengers. Arterioscler. Thromb. Vasc. Biol. 22 734–742. [DOI] [PubMed] [Google Scholar]
- 3.Björkhem I. 2002. Do oxysterols control cholesterol homeostasis? J. Clin. Invest. 110 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lange Y., D. S. Ory, Y. Jin, L. H. Lanier, F. Hsu, and T. L. Steck. 2008. Effectors of rapid homeostatic responses of endoplasmic reticulum cholesterol and HMG CoA reductase. J. Biol. Chem. 283 1445–1455. [DOI] [PubMed] [Google Scholar]
- 5.Lange Y., J. Ye, and F. Strebel. 1995. Movement of 25-hydroxycholesterol from the plasma membrane to the rough endoplasmic reticulum in cultured hepatoma cells. J. Lipid Res. 36 1092–1097. [PubMed] [Google Scholar]
- 6.Meaney S., K. Bodin, U. Diczfalusy, and I. Björkhem. 2002. On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: importance of the position of the oxygen function. J. Lipid Res. 43 2130–2135. [DOI] [PubMed] [Google Scholar]
- 7.Meir K., D. Kitsberg, I. Alkalay, F. Shkedy, H. Rosen, S. Shptzen, L. Ben-Avi, B. Staels, C. Fievet, V. Meiner, et al. 2002. Human sterol 27-hydroxylase (CYP27) overexpression transgenic mouse model. Evidence against 27-hydroxycholesterol as a critical regulator of cholesterol homeostasis. J. Biol. Chem. 277 34036–34041. [DOI] [PubMed] [Google Scholar]
- 8.Rosen H., A. Reshef, N. Meada, A. Lippoldt, L. Triger, S. Shpizen, L. Triger, G. Eggertsen, I. Björkhem, and E. Leitersdorf. 1998. Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with a disrupted sterol 27-hydroxylase gene. J. Biol. Chem. 273 14805–14812. [DOI] [PubMed] [Google Scholar]
- 9.Lund E. G., C. Xie, T. Kotti, S. D. Turley, J. M. Dietschy, and D. W. Russel. 2003. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 278 22980–22988. [DOI] [PubMed] [Google Scholar]
- 10.Li-Hawkins J., E. G. Lund, S. D. Turley, and D. W. Russell. 2000. J. Biol. Chem. 275 16536–16542. [DOI] [PubMed] [Google Scholar]
- 11.Brown A. J., and W. Jessup. 1999. Oxysterols and atherosclerosis. Atherosclerosis. 142 1–28. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein J. L., R. A. DeBose-Boyd, and M. S. Brown. 2006. Cell. 124 35–46. [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan A., I. Yukio, H. J. Kwon, M. S. Brown, and J. L. Goldstein. 2007. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. USA. 104 6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberti S., G. Schuster, P. Parini, D. Feltkamp, U. Diczfalusy, M. Rudling, B. Angelin, I. Björkhem, S. Pettersson, and J. A. Gustafsson. 2001. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXR-deficient mice. J. Clin. Invest. 107 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster G., P. Parini, L. Wang, S. Alberti, K. Steffensen, G. Hansson, B. Angelin, and J. A.Gustafsson. 2002. Accumulation of foam cells in liver X receptor-deficient mice. Circulation. 106 1147–1153. [DOI] [PubMed] [Google Scholar]
- 16.Meaney, S. 2003. Studies on oxysterols. Origins, properties and roles. Academic Thesis Karolinska Institutet.
- 17.Chen W., G. Chen, D. L. Head, D. J. Mangelsdorf, and D. W. Russell. 2007. Enzymatic reduction of oxysterols impairs LXR signalling in cultured cells and the livers of mice. Cell Metab. 5 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan D., M. I. Mayranpaa, J. Wong, J. Perttila, M. Lehto, et al. 2008. J. Biol. Chem. 283 332–340. [DOI] [PubMed] [Google Scholar]
- 19.Fu X., J. G. Menke, Y. Chen, G. Zhou, K. L. MacNaul, S. D. Wright, C. P. Sparrow, and E. G. Lund. 2001. 27-Hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J. Biol. Chem. 276 38378–38387. [DOI] [PubMed] [Google Scholar]
- 20.Frolov A., S. E. Zielinski, J. R. Crowley, N. Dudley-Ruckert, J. E. Schaffer, and D. S. Ory. 2003. NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol derived oxysterols. J. Biol. Chem. 278 25517–25525. [DOI] [PubMed] [Google Scholar]
- 21.Pfrieger F. W. 2003. Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes? Bioessays. 25 72–78. [DOI] [PubMed] [Google Scholar]
- 22.Abildayeva K., P. J. Jansen, V. Hirsch-Reinshage, V. W. Bloks, A. H. F. Bakker, F. C. Ramaekers, J. De Vente, A. K. Groen, C. L. Wellington, F. Kuipers, et al. 2006. 24S-Hydroxycholesterol participates in a liver X-receptor controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 281 12799–12808. [DOI] [PubMed] [Google Scholar]
- 23.Shafaati M., A. Solomon, M. Kivipelto, I. Björkhem, and V. Leoni. 2007. Neurosci. Lett. 425 78–82. [DOI] [PubMed] [Google Scholar]
- 24.Plemenitas A., and J. A. Watson. 1999. Down-regulation of mammalian HMGR with highly purified liposomal cholesterol. Eur. J. Biochem. 266 317–326. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z., D. Li, D. E. Blanchard, S. R. Lear, S. K. Erickson, and T. A. Spencer. 2001. Key regulatory oxysterols in the liver: analysis as delta-4–3-ketone derivatives by HPLC and response to physiological perturbations. J. Lipid Res. 42 649–658. [PubMed] [Google Scholar]
- 26.Rowe A., H., C. A. Argmann, J. Y. Edwards, C. G. Sawyez, O. H. Morand, et al. 2003. Enhanced synthesis of the oxysterol 24(S),25-epoxycholesterol in macrophages by inhibition of 2,3-oxidosqualene-lanosterol cyclase. Circ. Res. 93 717–725. [DOI] [PubMed] [Google Scholar]
- 27.Wong J., C. M. Quinn, and A. J. Brown. 2004. Statins inhibit synthesis of an oxysterol ligand for the liver X receptor in human macrophages with consequences for cholesterol flux. Arterioscler. Thromb. Vasc. Biol. 24 2365–2371. [DOI] [PubMed] [Google Scholar]