Abstract
The physiological effects of many extracellular stimuli are initiated through receptor-promoted activation of phospholipase C and inositol lipid signaling pathways. The historical view that phospholipase C-promoted signaling primarily occurs through activation of heterotrimeric G proteins or tyrosine kinases has expanded in recent years with the realization that at least three different mammalian phospholipase C isozymes are directly activated by members of the Ras superfamily of GTPases. Thus, Ras, Rap, Rac, and Rho GTPases all specifically regulate certain phospholipase C isozymes, and insight into the physiological significance of these signaling responses is beginning to accrue. High resolution three-dimensional structures of phospholipase C isozymes also are beginning to shed light on their mechanism of activation.
Keywords: inositol lipid signaling, Rac, Rho, Rap
Phospholipase C (PLC) isozymes hydrolyze PtdIns(4,5)P2 to Ins(1,4,5)P3 and diacylglycerol. Discovery over two decades ago of Ins(1,4,5)P3 as the Ca2+-mobilizing second messenger incontrovertibly connected agonist-promoted activation of PLC at the plasma membrane to the cellular and physiological actions of a broad range of hormones, neurotransmitters, growth factors, and other extracellular stimuli (1). The significance of PLC-catalyzed alterations in membrane PtdIns(4,5)P2 levels subsequently broadened with the realization that activities of many cellular proteins are regulated by binding to this inositol lipid (2).
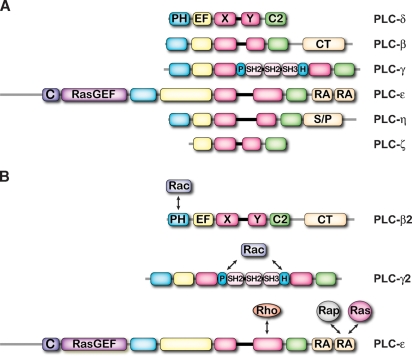
PLC isozymes were first purified in the 1980s and their genes cloned soon thereafter (3). This class of signaling proteins initially included PLC-β, PLC-γ, and PLC-δ, but the mammalian family has expanded in the last decade to include PLC-ɛ, PLC-ζ, and two PLC-η isozymes (4). Although PLC isozymes exhibit relatively low overall homology, each contains a conserved catalytic core (Fig. 1A). The historically designated X and Y domains comprise the two halves of a highly conserved catalytic triose phosphate isomerase (TIM) barrel flanked on the N-terminal side by a series of elongation factor (EF)-hand domains and by a C2 domain at the C terminus. With the exception of PLC-ζ, all PLC isozymes contain an N-terminal pleckstrin homology (PH) domain, which potentially binds membrane phosphoinositides or regulatory proteins. The catalytic core of most of the 13 mammalian PLC isozymes is elaborated with unique domains that underlie the evolution of unique modes of regulation in these signaling proteins.
Fig. 1.
Domain architecture of PLCs and their control by Ras superfamily GTPase. Domain architecture is drawn to scale for representative members of each class of PLC isozyme (A), and major sites of interaction between GTPases and PLCs are indicated (B). Individual domains are discussed in the main text except for a cysteine-rich region (C) at the N terminus of PLC-ɛ and a serine- and proline-rich region (S/P) at the C terminus of PLC-η.
Essen et al. (5) provided the first high-resolution structures and unambiguous mechanistic insight into the active site of a PLC isozyme. The catalytic TIM barrel of PLC-δ1 is sequestered between the series of EF-hands from the N-terminal region and the C2 domain at the C terminus. Portions of the first two EF-hands and the X/Y-linker that splits the catalytic TIM barrel are not visible in several crystal structures, indicating their intrinsic mobility in PLC-δ1. PLC isozymes are Ca2+ dependent, and the active site binds a Ca2+ ion required for hydrolysis of phosphoinositides (5). The C2 domain of PLC-δ also binds several Ca2+ ions possibly to facilitate interaction with negatively charged membranes (6).
Structural and kinetic analyses indicate that substrate hydrolysis catalyzed by mammalian PLCs proceeds through two steps (5, 7). Diacylglycerol and a cyclic inositol intermediate are produced in a phosphotransfer step that involves a general acid/base catalysis. An acyclic inositol is then produced in a phosphohydrolysis step that involves Ca2+ cofactor-mediated lowering of the PKa of the 2-hydroxyl group of the inositol with consequential deprotonation and nucleophilic attack on the 1-phosphate. Invariantly conserved histidines in the active site coordinate the 1-phosphoryl group and function as general bases in catalysis: four acidic groups ligate the Ca2+ cofactor; a similarly conserved trio of lysine, serine, and arginine coordinate the 4-phosphoryl group; and a conserved lysine binds the 5-phosphoryl group. These active-site residues initially revealed in the structure of PLC-δ1 are present in all mammalian PLC isozymes. Moreover, the recently solved structure of PLC-β2 revealed an active-site architecture identical to that of PLC-δ1 (8).
Receptor-promoted regulation of PLC historically has been thought to occur by one of two major signaling mechanisms (4, 9, 10). In the first case, hormones and neurotransmitters stimulate G-protein-coupled receptors (GPCRs) that in turn activate ubiquitously expressed members of the Gq subfamily of heterotrimeric G proteins. All four PLC-β isozymes are directly activated by Gα-subunits of the Gq subfamily. Activation of GPCRs that couple to other G proteins (e.g., Gi) also can promote inositol lipid signaling since certain of the PLC-β isozymes (PLC-β2 and PLC-β3) are directly activated by Gβγ dimers released from heterotrimeric G proteins. The molecular basis for activation of PLC-β isozymes by Gα- and Gβγ-subunits remains undefined. However, these isozymes contain an extended C-terminal domain that is necessary for membrane association, Gαq-dependent activation, and promotion of the GTPase activity of Gαq and related α-subunits. Gβγ has been proposed to interact with both the PH domain and catalytic core of PLC-β2 and PLC-β3.
Polypeptide growth factors promote inositol lipid signaling through a second major mechanism involving receptor autophosphorylation and generation of phosphotyrosine binding sites for PLC-γ isozymes on the activated receptor (10). Tyrosine kinase receptor-mediated phosphorylation of PLC-γ then occurs on several tyrosines, at least one of which is critical for PLC-γ activation. Receptors for antigens and IgE are not tyrosine kinases, but their activation results in activation of nonreceptor tyrosine kinases, such as Src, that also phosphorylate PLC-γ isozymes on these tyrosines. Two SH2 domains, a single SH3 domain, and a split PH domain are inserted between the X and Y domains of the catalytic TIM barrel of PLC-γ1 and PLC-γ2. Phosphorylation of Tyr-783 (and possibly Tyr-771) plays an obligatory role in activation of PLC-γ1 (11), although delineation of the molecular details of this key step remains elusive.
Although the roles of heterotrimeric G proteins and tyrosine kinases in regulation of inositol lipid signaling are broad and well established, the existence of 13 mammalian PLC isozymes divided into six structural subclasses suggests greater complexity in receptor-regulated PLC-dependent signaling than initially envisioned. Below, we focus on recent studies that have revealed a remarkably diverse regulation of inositol lipid signaling through small GTPases of the Ras superfamily.
REGULATION OF PHOSPHOLIPASE C BY Ras GTPases
The Ras superfamily of small GTPases comprises >150 members (12). These molecular switches fulfill critical roles in human physiology and disease, including regulation of cell proliferation, differentiation, survival, polarity, shape, and movement, as well as regulation of gene expression, vesicle transport, and many other activities. Legions of upstream activators and regulators of Ras GTPases exist, and many different proteins are downstream effectors of activated GTP-bound forms of these GTPases. Although signaling through Ras superfamily GTPases often necessarily focuses on a single GTPase in a single pathway, the existence of multiple upstream inputs and downstream outputs as well as extensive crosstalk among pathways involving these GTPases results in remarkably complex signaling networks. Many GPCR-initiated events also activate Ras superfamily GTPases further increasing crosstalk. For example, GTP-bound forms of Gα12/13 and Gαq directly activate specific GEFs, e.g., p115-RhoGEF and p63 RhoGEF, that subsequently activate Rho GTPases (13). Similarly, active heterotrimeric G proteins lead to the activation of Ca2+/diacylglycerol- or cyclic AMP-activated Ras/Rap GEFs, e.g., Epac (14).
The elegant work of Illenberger and Gierschik and their colleagues (15, 16) first convincingly implicated a role for small GTPases in inositol lipid signaling, and we now realize that at least three different PLC isozymes (PLC-β2, -γ2, and -ɛ) are robustly activated by Ras superfamily GTPases (4). This regulation occurs through specific recognition of Ras, Rap, or Rho GTPases by at least four different binding interfaces (Fig. 1B). Thus, scores of inputs into inositol lipid signaling potentially exist in addition to those historically understood to be mediated by heterotrimeric G proteins and tyrosine kinases. Since Ras superfamily GTPases exhibit remarkable differences in subcellular localization, selective activation of PLC isozymes occurs in various subcellular compartments. Certain PLC isozymes function as signaling nodes receiving activating input from both heterotrimeric and Ras superfamily GTPases, and at least in the case of PLC-ɛ, this upstream regulation results in the subsequent, direct activation of additional Ras GTPases. Knowledge of the signaling consequences and physiological significance of small GTPase activated PLCs continues to expand and evolve.
Rac-MEDIATED ACTIVATION OF PLC-β2
Direct activation of a PLC isozyme by a small GTPase was first described by Illenberger, Gierschik, and colleagues (15, 16), who showed that PLC-β2 could be purified from cytosol of neutrophils in a GTPγS-dependent complex with a Rho-family GTPase. Guanine nucleotide-dependent activation of purified PLC-β2 was observed with recombinant Rac1, Rac2, and Cdc42, although Cdc42 was the least potent and efficacious of these GTPases. By contrast, RhoA was inactive. This regulatory effect apparently is limited to PLC-β2 because only weak Rac-promoted activation was observed with PLC-β3, and no effect was observed with PLC-β1.
Snyder et al. (17) reached similar conclusions in studies of protein-protein interactions quantified using surface plasmon resonance. Thus, Rac1, Rac2, and Rac3, but not Cdc42, Ras, Rho, or Rap, bound PLC-β2 with high affinity and in a GTP-dependent fashion. PLC-β3 also bound Rac but with lower affinity relative to PLC-β2, and no binding occurred with PLC-β1. This specificity of GTP-dependent binding of GTPases was entirely recapitulated with the PH domain of PLC- β2 but not the PH domain of G protein receptor kinase2. Evidence of binding of Rac to the PH domain of PLC-β2 also was obtained by Illenberger et al. (18, 19), who illustrated in studies of a series of chimeras of PLC-β2 and PLC-β1 that Rac-dependent increases in membrane association and phospholipase activity required the presence of the PH domain from PLC-β2. As discussed below, a high-resolution crystallographic structure of a GTP-dependent complex of Rac1 and PLC-β2 unambiguously confirmed that the GTPase/PLC-β2 interface is entirely accounted for by interaction of Rac with the PH domain (8).
Ras-MEDIATED ACTIVATION OF PLC-ɛ
Kataoka and his colleagues discovered PLC-ɛ as a Ras binding protein in Caenorhabditis elegans in 1998 (20), and molecular cloning of the mammalian homolog of this isozyme revealed the first PLC with conserved Ras binding domains (21–23). Although the first of two Ras-associating (RA) domains in the C terminus was reported to be the Ras-targeted domain of the C. elegans enzyme (20), multiple lines of evidence, including structural analyses (24), establish that the second C-terminal RA domain, RA2, is primarily responsible for Ras binding to mammalian PLC-ɛ (Fig. 1). Mutation of two adjoining lysines (K2150 and K2152) in RA2 of mouse PLC-ɛ resulted in loss of GTP-dependent binding of H-Ras, and this mutant PLC-ɛ was no longer activated after coexpression with H-Ras in COS-7 cells (21). The specificity of interaction of Ras subfamily GTPases with PLC-ɛ has not been fully studied, but H-Ras, K-Ras, M-Ras, Rap1A, Rap1B, and Rap2B all appear to be activators (21, 23, 25, 26).
Although research to date largely has focused on PLC-ɛ as a downstream effector of Ras superfamily GTPases, this novel PLC isozyme contains an N-terminal CDC25 GEF domain that confers function to this protein as an upstream activator of small GTPases (22, 23, 25). Neither the GTPase selectivity nor the physiological significance of this conserved domain has been fully established. However, several studies suggested that i) PLC-ɛ activates both Ras and Rap GTPases; ii) activity of the GEF domain may be important for subcellular localization of the isozyme; iii) feed-forward activation of GTPases that interact with the C-terminal RA2 domain may impart unique kinetics of activation to this PLC isoform; and iv) physiological effects of this isozyme may concomitantly involve its lipase and GEF activities (4, 27).
Growth-factor-mediated activation of a variety of transmembrane receptor tyrosine kinases results in activation of the PLC-ɛ by a mechanism that requires the RA2 domain (26, 28). GPCR-dependent activation of PLC-ɛ also occurs through cyclic AMP-dependent activation of the GEF, Epac, which activates Rap2B (29).
Targeted disruption of PLC-ɛ has begun to reveal biological functions that involve regulation of PLC-ɛ by small GTPases. For example, studies of PLC-ɛ-null mice revealed an importance of this isozyme in cardiac development and function (30, 31). Oestreich et al. (32) recently illustrated that the physiological regulation of Ca2+-induced Ca2+ release in cardiac myocytes by catecholamine-activated β-adrenergic receptors involves cyclic AMP-dependent activation of Epac resulting in activation of Rap. Rap-activated PLC-ɛ then promotes diacyglycerol/Ca2+-dependent activation of a protein kinase C and release of Ca2+ from the sarcoplasmic reticulum.
PLC-ɛ-deficient mice are resistant to skin tumor formation in a chemical carcinogen-induced model (33), and several lines of evidence suggest that PLC-ɛ increases tumor formation by augmenting inflammatory responses in dermal fibroblasts (34). Although the involved signaling pathways have not been unambiguously elucidated, PLC-ɛ apparently functions downstream of activation of a calmodulin/diacylglycerol-regulated RasGEF and Rap1.
Rho-MEDIATED ACTIVATION OF PLC-ɛ
Wing et al. (35) demonstrated that coexpression of PLC-ɛ with GTPase-deficient RhoA, RhoB, or RhoC, but not Rac or Cdc42, resulted in marked elevation of intracellular inositol phosphate accumulation. In contrast with the effect of Ras, truncated forms of PLC-ɛ lacking the C-terminal RA domains retained capacity to be activated by Rho. Studies with purified proteins have confirmed that PLC-ɛ is a direct effector of GTP-bound Rho through a mechanism entirely independent of the RA domains (36). Indeed, dual and partially synergistic activation of PLC-ɛ occurs in the combined presence of activated Rho and Ras (37).
Early studies of mammalian PLC-ɛ revealed that expression of Gα12 and Gα13, but not other heterotrimeric G protein α-subunits, resulted in PLC-ɛ-dependent increases in inositol phosphate accumulation (22, 38). Certain RhoGEFs, e.g., p115-RhoGEF and LARG, are effectors of Gα12 and Gα13 (13), and subsequent work has shown that activation of PLC-ɛ by Gα12 and Gα13 occurs through RhoGEF-mediated activation of Rho. Similarly, activators, e.g., thrombin or lysophosphatidic, of GPCRs that couple to Gα12 and Gα13 activate PLC-ɛ in a Rho-dependent fashion (28). Activation of natively expressed PLC-ɛ downstream of thrombin or lysophosphatidic acid receptors in rat-1 fibroblasts is much more sustained than is the concomitant activation of PLC-β3 (39).
Studies of astrocytes isolated from wild-type and PLC-ɛ-deficient mice illustrated that inositol lipid signaling responses to thrombin, sphingosine 1-phosphate, and lysophosphatidic acid occur through mechanisms involving activation of PLC-ɛ (40). The lysophosphatidic acid and sphingosine-1P-promoted effects depend on activation of Gi-coupled GPCRs and presumably release of Gβγ. By contrast, the effects of thrombin on PLC-ɛ occur through activation of Rho. PLC-ɛ also is required for the stimulatory effects of thrombin on ERK phosphorylation and DNA synthesis, and PLC-ɛ is both a downstream effector and upstream activator of small GTPases in this cell proliferative response. That is, thrombin-promoted proliferative effects depend on Rho-promoted coupling to PLC-ɛ, which promotes activation of Rap via the CDC25 GEF domain of PLC-ɛ.
Rac-MEDIATED ACTIVATION OF PLC-γ2
Gierschik and his colleagues also discovered that coexpression of PLC-γ2 with GTPase-deficient mutants of Rac1, Rac2, or Rac3 (but not Cdc42 or RhoA) results in marked increases in inositol phosphate accumulation (41). No evidence for activation of PLC-γ1 was observed with any of these GTPases. A similar selectivity for GTP-dependent activation of PLC-γ2 by Rac was observed in studies with homogenates from cells overexpressing these signaling proteins. Since mutation of tyrosines known to be important for phosphorylation-dependent activation of PLC-γ2 had no effect on Rac-promoted activation, this GTPase-regulated signaling response apparently occurs independently of tyrosine kinase-mediated signaling.
Like all PLC isozymes, PLC-γ1 and PLC-γ2 contain an N-terminal PH domain. However, they also contain a second PH domain within the X/Y-linker that separates the halves of the catalytic TIM barrel (Fig. 1). This PH domain is “split' in primary sequence by two SH2 domains and an SH3 domain forming a unique X/Y-linker. Walliser et al. (42) recently investigated the basis for selective interaction of Rac GTPases with PLC-γ2 versus PLC-γ1 and discovered that the split PH domain of PLC-γ2 accounts for GTP-dependent binding of these GTPases. Mutational studies and NMR spectroscopy revealed that the Rac binding interface resides in β-strand 5 and the α-helix region of the split PH domain. This interface notably differs from the Rac1 binding surface of the PH domain of PLC-β2, which was shown by Jezyk et al. (8) to involve primarily β-strand 1 and loop regions in structural proximity to this strand. The mechanism of Rac-promoted activation of PLC-γ2 remains unclear, although relief of an autoinhibited state has been suggested. The physiological significance of Rac-promoted PLC-γ2-dependent signaling also remains undefined, although both PLC-γ2 and Rac2 are involved in B lymphocyte development and signaling (41).
MECHANISM OF GTPase-PROMOTED ACTIVATION OF PLC ISOZYMES
Significant insight into the mechanism of activation of PLC isozymes by Ras-family GTPases recently accrued from high-resolution, three-dimensional structures of a GTP-dependent complex of Rac1 and PLC-β2 and of PLC-β2 in the absence of G protein activator (8, 43). No conformational changes are induced in PLC-β2 by activated Rac1, and the structures suggested autoinhibition of the isozyme occurs due to occlusion of the active site by a portion of the X/Y-linker. This linker connects the highly conserved halves of the catalytic TIM barrel but is highly variable in length and sequence across all 13 PLC isozymes. This region also is highly disordered, as it was not observed in the high-resolution structure of PLC-δ1, and only 20 of the ∼70 residues of the X/Y-linker are observed in the two PLC-β2 structures.
Occlusion of the active site of PLC-β2 by an ordered portion of the X/Y-linker led to the hypothesis that this isozyme exists in an autoinhibited state (Fig. 2). Consistent with this idea, removal of the ordered portion of the X/Y-linker resulted in marked increases in lipase activity (43). Indeed, mutation of a single glycine of the X/Y-linker that makes backbone and side chain contacts with catalytic residues in the active site resulted in a markedly activated enzyme. Several results suggest a still to be defined interplay between this ordered region and the remainder of the X/Y-linker in regulation of enzyme function. For example, removal of the disordered region of PLC-β2 resulted in larger activation than did removal of the ordered region, and removal of the entire X/Y-linker of PLC-β2 resulted in even greater activation than observed with removal of either the ordered or disordered regions alone. Activation of PLC-β2 by Rac GTPases requires an intact PH domain (17, 18), activation by Gαq requires the C terminus, and activation by Gβγ requires the PH domain and/or sequence in the Y-box of the catalytic TIM barrel (4, 10). The very active X/Y-linker-deleted mutants of PLC-β2 nonetheless retain capacity to be activated by all three G protein activators (43). These observations and the absence of Rac-induced conformational changes in the active site of PLC-β2 indicate that G-protein-dependent activation involves recruitment and orientation of the isozyme at the plasma membrane. Long stretches of acidic amino acids exist in the X/Y-linker, and these areas of dense negative charge likely are repelled from negatively charged membranes upon binding of PLC-β2 to membrane-resident G protein activators. This repulsion presumably removes the X/Y-linker from near the active site of PLC-β2 to relieve autoinhibition (Fig. 2).
Fig. 2.
Model for the autoinhibition and general activation of PLC isozymes. The active site (yellow) of a PLC isozyme is blocked by a highly mobile and negatively charged X/Y-linker that also prevents high-affinity binding of the PLC to membranes. An upstream input, e.g., GTP-bound Rac, favors the binding of the PLC to membranes to promote the movement of the X/Y-linker away from the active site while simultaneously orienting the active site for optimal access of phosphoinositide substrates.
Whereas mammalian PLC isozymes differ in length and sequence of their X/Y-linkers, most of these proteins possess areas of dense negative charge in this region. Moreover, removal of the linker region of PLC-δ1 or PLC-ɛ also results in remarkable activation of these signaling proteins (43), strongly suggesting that a common mechanism of autoinhibition and activation controlled by the X/Y-linker occurs in most if not all PLC isozymes.
CONCLUSION
PLC isozymes receive and coordinate multiple upstream inputs, many of which involve Ras superfamily GTPases. It will be important to more fully place these PLC-dependent signaling nodes in the wider context of the remarkably large set of biological responses associated with activation of Ras family GTPases. Recent structural analyses also suggest that the mechanism of activation of PLC isozymes by Ras GTPases and by heterotrimeric G proteins or by tyrosine phosphorylation will soon be revealed.
Abbreviations
EF, elongation factor
GPCR, G-protein-coupled receptor
PH, pleckstrin homology
PLC, phospholipase C
RA, Ras-associating
TIM, triose phosphate isomerase
This work was supported by National Institutes of Health Grant GM-57391 (T.K.H. and J.S.) and a Ruth L. Kirschstein National Research Service Award fellowship F32GM074411 (S.N.H.).
Published, JLR Papers in Press, November 24, 2008.
References
- 1.Berridge M. J. 1987. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu. Rev. Biochem. 56 159–193. [DOI] [PubMed] [Google Scholar]
- 2.Lemmon M. A. 2003. Phosphoinositide recognition domains. Traffic. 4 201–213. [DOI] [PubMed] [Google Scholar]
- 3.Rhee S. G., and K. D. Choi. 1992. Regulation of inositol phospholipid-specific phospholipase C isozymes. J. Biol. Chem. 267 12393–12396. [PubMed] [Google Scholar]
- 4.Harden T. K., and J. Sondek. 2006. Regulation of phospholipase C isozymes by Ras superfamily GTPases. Annu. Rev. Pharmacol. Toxicol. 46 355–379. [DOI] [PubMed] [Google Scholar]
- 5.Essen L. O., O. Perisic, R. Cheung, M. Katan, and R. L. Williams. 1996. Crystal structure of a mammalian phosphoinositide-specific phospholipase C δ. Nature. 380 595–602. [DOI] [PubMed] [Google Scholar]
- 6.Essen L. O., O. Perisic, D. E. Lynch, M. Katan, and R. L. Williams. 1997. A ternary metal binding site in the C2 domain of phosphoinositide-specific phospholipase C-δ1. Biochemistry. 36 2753–2762. [DOI] [PubMed] [Google Scholar]
- 7.Ellis M. V., S. R. James, O. Perisic, C. P. Downes, R. L. Williams, and M. Katan. 1998. Catalytic domain of phosphoinositide-specific phospholipase C (PLC). Mutational analysis of residues within the active site and hydrophobic ridge of Plcδ1. J. Biol. Chem. 273 11650–11659. [DOI] [PubMed] [Google Scholar]
- 8.Jezyk M. R., J. T. Snyder, S. Gershberg, D. K. Worthylake, T. K. Harden, and J. Sondek. 2006. Crystal structure of Rac1 bound to its effector phospholipase C-β2. Nat. Struct. Mol. Biol. 13 1135–1140. [DOI] [PubMed] [Google Scholar]
- 9.Exton J. H. 1996. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu. Rev. Pharmacol. Toxicol. 36 481–509. [DOI] [PubMed] [Google Scholar]
- 10.Rhee S. G. 2001. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70 281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulin B., F. Sekiya, and S. G. Rhee. 2005. Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-γ1. Proc. Natl. Acad. Sci. USA. 102 4276–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wennerberg K., K. L. Rossman, and C. J. Der. 2005. The Ras superfamily at a glance. J. Cell Sci. 118 843–846. [DOI] [PubMed] [Google Scholar]
- 13.Sternweis P. C., A. M. Carter, Z. Chen, S. M. Danesh, Y. F. Hsiung, and W. D. Singer. 2007. Regulation of Rho guanine nucleotide exchange factors by G proteins. Adv. Protein Chem. 74 189–228. [DOI] [PubMed] [Google Scholar]
- 14.Bos J. L. 2006. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 31 680–686. [DOI] [PubMed] [Google Scholar]
- 15.Illenberger D., F. Schwald, and P. Gierschik. 1997. Characterization and purification from bovine neutrophils of a soluble guanine-nucleotide-binding protein that mediates isozyme-specific stimulation of phospholipase C β2. Eur. J. Biochem. 246 71–77. [DOI] [PubMed] [Google Scholar]
- 16.Illenberger D., F. Schwald, D. Pimmer, W. Binder, G. Maier, A. Dietrich, and P. Gierschik. 1998. Stimulation of phospholipase C-β2 by the Rho GTPases Cdc42Hs and Rac1. EMBO J. 17 6241–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder J. T., A. U. Singer, M. R. Wing, T. K. Harden, and J. Sondek. 2003. The pleckstrin homology domain of phospholipase C-β2 as an effector site for Rac. J. Biol. Chem. 278 21099–21104. [DOI] [PubMed] [Google Scholar]
- 18.Illenberger D., C. Walliser, B. Nurnberg, M. Diaz Lorente, and P. Gierschik. 2003. Specificity and structural requirements of phospholipase C-β stimulation by Rho GTPases versus G protein βγ dimers. J. Biol. Chem. 278 3006–3014. [DOI] [PubMed] [Google Scholar]
- 19.Illenberger D., C. Walliser, J. Strobel, O. Gutman, H. Niv, V. Gaidzik, Y. Kloog, P. Gierschik, and Y. I. Henis. 2003. Rac2 regulation of phospholipase C-β2 activity and mode of membrane interactions in intact cells. J. Biol. Chem. 278 8645–8652. [DOI] [PubMed] [Google Scholar]
- 20.Shibatohge M., K. Kariya, Y. Liao, C. D. Hu, Y. Watari, M. Goshima, F. Shima, and T. Kataoka. 1998. Identification of PLC210, a Caenorhabditis elegans phospholipase C, as a putative effector of Ras. J. Biol. Chem. 273 6218–6222. [DOI] [PubMed] [Google Scholar]
- 21.Kelley G. G., S. E. Reks, J. M. Ondrako, and A. V. Smrcka. 2001. Phospholipase C-ɛ: a novel Ras effector. EMBO J. 20 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez I., E. C. Mak, J. Ding, H. E. Hamm, and J. W. Lomasney. 2001. A novel bifunctional phospholipase C that is regulated by Gα12 and stimulates the Ras/mitogen-activated protein kinase pathway. J. Biol. Chem. 276 2758–2765. [DOI] [PubMed] [Google Scholar]
- 23.Song C., C. D. Hu, M. Masago, K. Kariyai, Y. Yamawaki-Kataoka, M. Shibatohge, D. Wu, T. Satoh, and T. Kataoka. 2001. Regulation of a novel human phospholipase C, PLCɛ, through membrane targeting by Ras. J. Biol. Chem. 276 2752–2757. [DOI] [PubMed] [Google Scholar]
- 24.Bunney T. D., R. Harris, N. L. Gandarillas, M. B. Josephs, S. M. Roe, S. C. Sorli, H. F. Paterson, F. Rodrigues-Lima, D. Esposito, C. P. Ponting, et al. 2006. Structural and mechanistic insights into Ras association domains of phospholipase C ɛ. Mol. Cell. 21 495–507. [DOI] [PubMed] [Google Scholar]
- 25.Song C., T. Satoh, H. Edamatsu, D. Wu, M. Tadano, X. Gao, and T. Kataoka. 2002. Differential roles of Ras and Rap1 in growth factor-dependent activation of phospholipase C ɛ. Oncogene. 21 8105–8113. [DOI] [PubMed] [Google Scholar]
- 26.Kelley G. G., S. E. Reks, and A. V. Smrcka. 2004. Hormonal regulation of phospholipase C-ɛ through distinct and overlapping pathways involving G12 and Ras family G-proteins. Biochem. J. 378 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunney T. D., and M. Katan. 2006. Phospholipase C epsilon: linking second messengers and small GTPases. Trends Cell Biol. 16 640–648. [DOI] [PubMed] [Google Scholar]
- 28.Hains M. D., M. R. Wing, S. Maddileti, D. P. Siderovski, and T. K. Harden. 2006. Gα12/13- and Rho-dependent activation of phospholipase C-ɛ by lysophosphatidic acid and thrombin receptors. Mol. Pharmacol. 69 2068–2075. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M., S. Evellin, P. A. Weernink, F. von Dorp, H. Rehmann, J. W. Lomasney, and K. H. Jakobs. 2001. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat. Cell Biol. 3 1020–1024. [DOI] [PubMed] [Google Scholar]
- 30.Tadano M., H. Edamatsu, S. Minamisawa, U. Yokoyama, Y. Ishikawa, N. Suzuki, H. Saito, D. Wu, M. Masago-Toda, Y. Yamawaki-Kataoka, et al. 2005. Congenital semilunar valvulogenesis defect in mice deficient in phospholipase C ɛ. Mol. Cell. Biol. 25 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., E. A. Oestreich, N. Maekawa, T. A. Bullard, K. L. Vikstrom, R. T. Dirksen, G. G. Kelley, B. C. Blaxall, and A. V. Smrcka. 2005. Phospholipase Cɛ modulates β-adrenergic receptor-dependent cardiac contraction and inhibits cardiac hypertrophy. Circ. Res. 97 1305–1313. [DOI] [PubMed] [Google Scholar]
- 32.Oestreich E. A., H. Wang, S. Malik, K. A. Kaproth-Joslin, B. C. Blaxall, G. G. Kelley, R. T. Dirksen, and A. V. Smrcka. 2007. Epac-mediated activation of phospholipase C-ɛ plays a critical role in β-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J. Biol. Chem. 282 5488–5495. [DOI] [PubMed] [Google Scholar]
- 33.Bai Y., H. Edamatsu, S. Maeda, H. Saito, N. Suzuki, T. Satoh, and T. Kataoka. 2004. Crucial role of phospholipase Cɛ in chemical carcinogen-induced skin tumor development. Cancer Res. 64 8808–8810. [DOI] [PubMed] [Google Scholar]
- 34.Ikuta S., H. Edamatsu, M. Li, L. Hu, and T. Kataoka. 2008. Crucial role of phospholipase Cɛ in skin inflammation induced by tumor-promoting phorbol ester. Cancer Res. 68 64–72. [DOI] [PubMed] [Google Scholar]
- 35.Wing M. R., J. T. Snyder, J. Sondek, and T. K. Harden. 2003. Direct activation of phospholipase C-ɛ by Rho. J. Biol. Chem. 278 41253–41258. [DOI] [PubMed] [Google Scholar]
- 36.Seifert J. P., M. R. Wing, J. T. Snyder, S. Gershburg, J. Sondek, and T. K. Harden. 2004. RhoA activates purified phospholipase C-ɛ by a guanine nucleotide-dependent mechanism. J. Biol. Chem. 279 47992–47997. [DOI] [PubMed] [Google Scholar]
- 37.Seifert J. P., Y. Zhou, S. N. Hicks, J. Sondek, and T. K. Harden. 2008. Dual activation of phospholipase C-ɛ by Rho and Ras GTPases. J. Biol. Chem. 283 29690–29698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wing M. R., D. Houston, G. G. Kelley, C. J. Der, D. P. Siderovski, and T. K. Harden. 2001. Activation of phospholipase C-ɛ by heterotrimeric G protein βγ-subunits. J. Biol. Chem. 276 48257–48261. [DOI] [PubMed] [Google Scholar]
- 39.Kelley G. G., K. A. Kaproth-Joslin, S. E. Reks, A. V. Smrcka, and R. J. Wojcikiewicz. 2006. G-protein-coupled receptor agonists activate endogenous phospholipase Cɛ and phospholipase Cβ3 in a temporally distinct manner. J. Biol. Chem. 281 2639–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Citro S., S. Malik, E. A. Oestreich, J. Radeff-Huang, G. G. Kelley, A. V. Smrcka, and J. H. Brown. 2007. Phospholipase Cɛ is a nexus for Rho and Rap-mediated G protein-coupled receptor-induced astrocyte proliferation. Proc. Natl. Acad. Sci. USA. 104 15543–15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piechulek T., T. Rehlen, C. Walliser, P. Vatter, B. Moepps, and P. Gierschik. 2005. Isozyme-specific stimulation of phospholipase C-γ2 by Rac GTPases. J. Biol. Chem. 280 38923–38931. [DOI] [PubMed] [Google Scholar]
- 42.Walliser C., M. Retlich, R. Harris, K. L. Everett, M. B. Josephs, P. Vatter, D. Esposito, P. C. Driscoll, M. Katan, P. Gierschik, et al. 2008. Rac regulates its effector phospholipase Cγ2 through interaction with a split pleckstrin homology domain. J. Biol. Chem. 283 30351–30362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hicks S. N., M. R. Jezyk, S. Gershburg, J. P. Seifert, T. K. Harden, and J. Sondek. 2008. General and versatile autoinhibition of PLC isozymes. Mol. Cell. 31 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]