Abstract
Clinicians have traditionally regarded the complications of atherosclerosis as a consequence of progressive arterial stenosis leading to critical narrowings that impede blood flow. Our contemporary understanding of the thrombotic complications of atherosclerosis has undergone a transformation based on a body of observations by pathologists and clinicians. In the late 1980s, clinicians had to confront the counterintuitive notion that plaques that cause acute myocardial infarction often do not produce high-grade stenoses (Smith, S. C., Jr. 1996. Risk-reduction therapy: the challenge to change. Circulation. 93: 2205–2211.). Observations from serial angiographic studies and on culprit lesions of acute myocardial infarction postthrombolysis highlighted this apparent paradox. These contrarian clinical findings prompted cardiologists to consider more carefully the findings of generations of pathologists that plaques that cause fatal coronary thrombi often result from a physical disruption of the atheromatous plaque that may not indeed cause critical arterial narrowing. This convergence of clinical and pathological observations highlighted the importance of understanding the mechanisms of disruption of plaques that can precipitate thromboses.
Keywords: plaque rupture, atheroma, acute coronary syndromes
PLAQUE DISRUPTION: A FREQUENT CAUSE OF THROMBOSIS COMPLICATING ATHEROMA
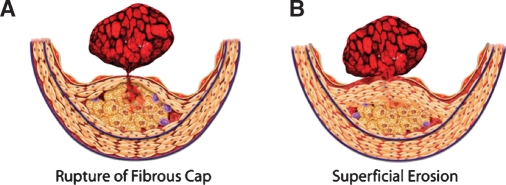
Autopsy studies have indicated that a fracture of the plaque's fibrous cap precipitates most fatal acute myocardial infarctions. Superficial erosion of the intima accounts for a substantial minority of such thrombi (Fig. 1). Other mechanisms of plaque disruption, including intraplaque hemorrhage and erosion of calcified nodules, account for a small proportion of fatal coronary thrombi (1).
Fig. 1.
Fibrous cap rupture and superficial erosion. Rupture of the fibrous cap (A) triggers two-thirds to three-quarters of all fatal coronary thromboses. Superficial erosion (B) occurs in one-fifth to one-quarter of fatal coronary thromboses. Certain populations, such as diabetics and women, seem to have superficial erosion more often as a mechanism of plaque disruption and thrombosis (42).
These various observations clearly identified the capital importance of understanding the molecular and cellular mechanisms of plaque disruption to master the biology of the thrombotic complications of atherosclerosis. Quantitative morphometric studies by pathologists highlighted features of plaques that had caused fatal thrombi, often referred to in clinical shorthand as “vulnerable” plaques. The characteristics of plaques that caused fatal coronary thrombi include a thin fibrous cap, a large lipid pool, many inflammatory cells, and, curiously, a paucity of vascular smooth muscle cells (2). These morphological characteristics prompted our group to investigate the biological determinants of these characteristics of plaques that caused fatal thrombi (3).
DISORDERED COLLAGEN METABOLISM PREDISPOSES TO PLAQUE DISRUPTION
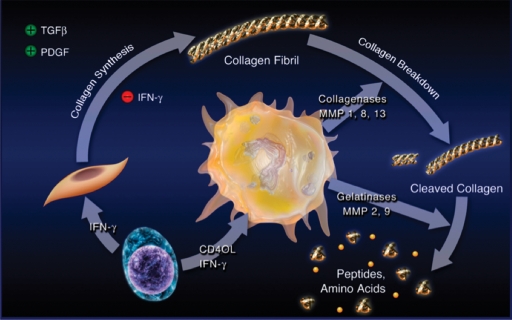
We first focused on the metabolism of interstitial collagen that lends strength to the plaque's fibrous cap and protects it from rupture and, hence, thrombosis. Previous studies established the arterial smooth muscle cell as the source of much of the extracellular matrix of the atheroma. We hypothesized that molecular mediators associated with atherogenesis could alter collagen metabolism in ways that could thin or weaken the plaque's fibrous cap (Fig. 2). Studies of human vascular smooth muscle cells in culture showed that the inflammatory mediator interferon-γ (IFN-γ) strikingly inhibited the ability of the smooth muscle cell to express the genes encoding procollagens (4). Ample evidence existed that IFN-γ operated in human atherosclerotic plaques. These data indicated an important mechanistic link between inflammation and impaired synthesis of collagen in atheromata.
Fig. 2.
Inflammation regulates metabolism of fibrillar collagen, which may induce atherosclerotic plaque disruption. The T-lymphocyte releases proinflammatory cytokines, including IFN-γ (lower left), that prevent smooth muscle cells from generating the new collagen needed to lay down the collagenous matrix of the plaque's fibrous cap, which protects the plaque from rupture. The T-cell-derived cytokine CD40L stimulates mononuclear phagocytes (center) to elaborate interstitial collagenases, such as MMP-1, MMP-8, and MMP-13, which catalyze the initial proteolytic cleavage of the intact collagen fibril. The cleaved collagen can then experience additional degradation by gelatinases such as MMP-9. In this way, inflammation can threaten the stability of atherosclerotic plaques; increase their propensity to rupture; and, as a result, cause thromboses that trigger most acute coronary syndromes. TGFβ, transforming growth factor β (43).
We further investigated the hypothesis that collagen catabolism controlled by inflammation could also influence remodeling of the extracellular matrix in atherosclerosis. Indeed, human atherosclerotic plaques contain interstitial collagenases of the matrix metalloproteinase (MMP) family. Early studies showed overexpression of MMP-1 by plaque macrophages in smooth muscle cells themselves (5–7). Follow-on studies identified MMP-13 in plaques and provided evidence for collagenolysis in situ in human atherosclerotic plaques (8). Surprisingly, plaques also overexpressed a form of interstitial collagenase (MMP-8) previously associated with neutrophils, leukocytes not abundant in undisrupted atheromata (9). These in vitro studies and observations on human and experimental atherosclerotic plaques strongly supported a link between inflammation and impaired ability of smooth muscle cells to repair and maintain the plaque's fibrous cap due to decreased collagen synthesis. Pro-inflammatory cytokines augment production of all three of these interstitial collagenases (MMPs 1, 13, and 8) by vascular wall cells and monocyte/macrophages (5, 10, 11). Thus, inflammation directs a dual defect in collagen metabolism: decreased synthesis and increased breakdown. These findings furnish a molecular explanation for the thinning of the fibrous cap associated with disrupted plaques.
Subsequent studies in genetically altered mice buttress this mechanism. Observations on compound mutant mice susceptible to atherosclerosis due to inactivation of apolipoprotein E (Apo−/−) also bearing a “knock-in” of a collagenase-resistant form of procollagen supported this notion. Atherosclerotic plaques in these collagenase-resistant mice accumulated more collagen than those with wild-type collagen (12). Mature mice do not express an ortholog of MMP-1 but do express MMP-13. Compound mutant animals deficient in both apolipoprotein E and MMP-13 also showed accumulation of collagen compared with MMP-13 wild-type counterparts (13). MMP-14, a membrane-associated MMP, also appears to contribute to collagenolysis in experimental atherosclerosis (14). MMP-14 may act to process the zymogen form of MMP-13 to the active enzyme. Molecular imaging studies have shown overexpression of MMP activity in regions of atherosclerotic plaques in mice (15). These convergent lines of evidence all suggest that inflammation tightly regulates the steady-state level of interstitial collagen in the plaque. When inflammation prevails, these mechanisms can render a plaque more likely to rupture and hence cause thrombosis.
DEATH OF SMOOTH MUSCLE CELLS MAY FAVOR PLAQUE DISRUPTION
Among other characteristics of plaques that caused fatal thrombosis, morphometric histopathologic studies identified relative paucity of smooth muscle cells (16). In 1995, we hypothesized that death of smooth muscle cells related to inflammation might provide a mechanism for the relative lack of smooth muscle cells in regions of plaques that had failed mechanically, ruptured, and provoked thrombosis (3). In particular, because smooth muscle cells produce most of the interstitial collagen in blood vessels, we reasoned that scarcity of smooth muscle cells might constitute one factor contributing to decreased collagen and thinning of the plaque's fibrous cap. We and others found evidence for programmed cell death of smooth muscle cells in atherosclerotic plaques (17). Our initial work reported colocalization of apoptotic cells in atheromata with caspase-1, the prototype of the family of proteinases that characterize apoptosis (18). Caspase-1 links to inflammation as it processes pro-interleukin-1-β and pro-interleukin-18 to their active, pro-inflammatory forms.
Subsequent work showed that exposure of human smooth muscle cells to mixtures of pro-inflammatory cytokines sensitized them to apoptosis (19). We also implicated fas-fas ligand signaling in programmed cell death within atheromata (20). These various observations tightened the link between inflammation and cell death in atheromata, a potential contributor to collagen lack and plaque fragility. In addition to smooth muscle cells, plaque macrophages die by apoptosis, as we hypothesized in 1992 (21). Apoptotic bodies elaborated by dying macrophages can contain the potent procoagulant tissue factor (22). Embolization of tissue factors when plaques disrupt or undergo percutaneous intervention may precipitate distal thrombosis of smaller coronary vessels leading to “no-reflow” (23).
INFLAMMATION REGULATES THE THROMBOGENICITY OF PLAQUES
Indeed, tissue factor expression by plaque macrophages appears primordial in triggering thrombosis that complicates plaque disruption. Although expression of tissue factor by a subset of plaque macrophages was well established, the stimulus to tissue factor expression in these phagocytes remained uncertain (24, 25). The soluble cytokines associated with atherosclerotic plaques, including interleukin-1 and tumor necrosis factor isoforms, poorly stimulate tissue factor gene expression by human macrophages. CD40-ligand (CD154), a cell-surface-associated inflammatory cytokine, readily elicits tissue factor production by human monocyte/macrophages (10). Thus, inflammation controls not just the propensity of plaques to rupture by altering collagen levels by the mechanisms described above, but also can heighten the thrombogenicity of the plaque's interior, favoring thrombus formation when a plaque fractures.
SUPERFICIAL EROSION AND CORONARY THROMBOSIS
Fracture of the plaque's fibrous cap accounts for some two-thirds to three-quarters of fatal acute myocardial infarctions. Around a quarter or a fifth of these events result not from a frank fracture of the plaque's fibrous cap but from a superficial erosion of the intima. Superficial erosion appears particularly important in individuals with dyslipidemia and in women (1). Understanding of the molecular pathogenesis of superficial erosion has lagged that of plaque rupture. Apoptosis and desquamation of luminal endothelial cells exposing platelets and coagulation factors to the basement membrane provide one possible mechanism for superficial erosion complicated by thrombosis. In this regard, production of hypochlorous acid by myeloperoxidase, an enzyme localized in plaques and associated with acute myocardial infarction, can kill endothelial cells (26). Hypochlorous acid also appears to induce agonal tissue factor production by endothelial cells. Endothelial cells activated by inflammatory mediators also produce enzymes capable of degrading extracellular matrix, including MMPs. Overproduction of active forms of the matrix-degrading enzymes could sever the tethers between endothelial cells and the subjacent basement membrane, facilitating their sloughing and consequent local thrombosis (11). In these manners, inflammation may promote superficial erosion and rupture of the plaque's fibrous cap.
DOES LIPID LOWERING MODIFY ASPECTS OF PLAQUE BIOLOGY RELATED TO THROMBOSIS
The studies described above provided a mechanistic basis for understanding plaque disruption and thrombosis. Could these features of plaques associated with thrombotic complications ameliorate with risk factor reduction? In particular, clinical data indicate that lipid-lowering therapies, particularly the statins, can prevent myocardial infarction, and in appropriately powered studies show prolongation of life in broad categories of individuals with or without established atherosclerotic disease. We know from numerous angiographic trials that reduction in the degree of stenosis of fixed lesions, while statistically significant, appears far smaller in magnitude than the reductions in risk of cardiovascular events. We thus hypothesized that lipid lowering might mitigate inflammation within plaques and alter features of plaques associated with thrombotic complications in humans.
We initially undertook to test this hypothesis in rabbits with experimental atherosclerosis, some continuing an atherogenic diet and others with restricted cholesterol and saturated fat in the diet. These studies showed a striking decrease in accumulation of inflammatory cells, markers of inflammatory activation, such as vascular cell adhesion molecule-1 expression, reactive oxygen species production, oxidized LDL accumulation, and, importantly, in levels of interstitial collagenase and other matrix metalloproteinases (27–29). Quantitative histomorphometric studies documented an increase in fibrillar collagen levels reciprocal with collagenase reductions. Hand in hand with the reduced inflammation and reinforcement of the plaque's fibrous skeleton, tissue factor expression in the intima declined as did levels of CD40 and CD40-ligand, putative triggers of tissue factor gene expression (30). Thus, lipid lowering produces coordinated changes in plaque biology reflected by reduced inflammation and oxidative stress as well as reinforcement of the plaque's collagenous extracellular matrix.
These initial rabbit studies used extreme dietary manipulation to modify plasma lipid levels. We therefore undertook follow-on experiments in rabbits with genetically induced dyslipidemia treated with statins rather than dietary manipulation. In Watanabe rabbits, statin treatment lowered LDL cholesterol levels far less than in the diet-induced atherosclerosis in our initial series of rabbit experiments. Nonetheless, statin treatment produced changes in plaque biology highly congruent with the effects of lipid lowering by diet, substantiating the view that statins can lower inflammation and reinforce the fibrillar collagen network in atheromata (31, 32). These studies provided a basis for understanding the reduction in thrombotic events, both coronary and cerebrovascular, produced by lipid-lowering therapy, particular the statin family of drugs.
The clinical extension of the concept that lipid lowering and statins can reduce inflammation emerged from work that used the biomarker C-reactive protein (CRP) as a gauge of inflammatory status. In seminal studies, Dr. Paul Ridker documented decreases in CRP measured with a high-sensitivity assay in patients enrolled in the Cholesterol and Recurrent Events study (33). Subsequent studies performed throughout the world confirmed these initial observations and indicated that statins as a class reduced inflammation. These consistent observations left unanswered the question of whether the anti-inflammatory effect produced by statins derived from LDL lowering or whether the statins might decrease inflammation independent of effects on cholesterol. Numerous in vitro studies indicated that statins had “pleiotropic” effects due to interference with prenylation of small G proteins involved in intracellular signaling or by induction of Kruppel-like factor-2, a transcription factor that can regulate several genes that may protect against atherosclerosis and thrombosis (34–38).
Further analyses of large-scale clinical trials provided insight in this regard. Dr. Ridker collaborated with the leaders of a large clinical trial known as TIMI-22/PROVE-IT. This study randomized survivors of acute coronary syndromes to high-intensity or standard statin treatment and followed them for several years for recurrent events. A prespecified analysis of this study tracked recurrent events in participants categorized by achieving above or below median LDL levels or above or below median CRP levels 30 days after enrollment, a time chosen to permit equilibration of the participants' on-study drug and waning of the acute phase response due to the coronary syndrome (39). In this study, as in several others, the individual drop in CRP correlated poorly with the individual drop in LDL. Individuals with above median LDL and above median CRP after 30 days suffered the most recurrent events. Those who achieved below median LDL and below median CRP had the best outcome in this analysis. The provocative and intriguing observation was that those who achieved either variable above median but below median for the other had recurrent events at an intermediate and similar rate. This pattern of partitioning of recurrent events depending on achieved LDL or CRP also occurred in a post hoc analysis of an independent study of acute coronary syndrome patients treated with a different statin. These results indicated strongly that clinical benefits of statins result from a combination of LDL reduction and lowering of CRP that appears independent of LDL reduction. In a similar analysis of a primary prevention study (AFCAPS/TexCAPS), the administration of a statin drug showed clear benefit in individuals with below median LDL during the trial (40). Those who had below median LDL and below median CRP showed no benefit with statin treatment. In the provocative part of this analysis, those with below median LDL but above median CRP benefited from statin treatment as much as the two above median LDL groups.
These observations spawned the hypothesis that individuals with inflammation indicated by slight elevations in CRP but with “average” LDL levels might benefit from statin therapy. The JUPITER trial tested this conjecture. JUPITER enrolled individuals without known cardiovascular disease with LDL levels below 131 mg/dl, thus fulfilling no criteria for statin treatment according to current guidelines. For enrollment in JUPITER, individuals needed to have a CRP >2 mg/l. The enrolled population had a median LDL level of 108 mg/dl and a CRP of 4.3 mg/l. This study enrolled >17, 000 individuals, including almost 40% women and 25% underrepresented minorities (blacks and Hispanics). On recommendation of the independent data safety monitoring committee, the study terminated some 2 years prematurely because of overwhelming benefit. Targeting of statin treatment based on inflammatory status produced a >40% decline in the primary endpoint of cardiovascular events, reduced myocardial infarction and stroke by some 47%, and reduced overall mortality by a statistically significant 20% (41). The design of JUPITER did not address the mechanism of benefit of the statin therapy. LDL lowering to 55 mg/dl, the median level achieved, lower than in previous large statin trials, doubtless contributed to event reduction. Forthcoming analyses of JUPITER reporting the relationship of LDL and CRP levels in trial to event reduction may provide insight into the possible contribution of reduction of inflammation to the clinical benefit but cannot establish a causal relationship in this regard.
CONCLUSIONS
Based on autopsy studies and clinical results, plaque disruption has come to the fore as a mechanism of fatal coronary thrombosis. We now possess data that shed light on the molecular and cellular mechanisms that underlie plaque disruption and thrombosis. Lipid lowering and statins, perhaps due in part to direct anti-inflammatory effects, likely improve outcomes by modifying the biology of plaques that promote disruption and thrombosis. Inflammation provides a common mechanistic link that transduces numerous risk factors for events to altered plaque biology. Interventions that have succeeded in reducing thrombotic complications of atherosclerosis also seem to reduce inflammation, certainly in the case of statins. Inflammatory biomarkers appear to play a role in predicting risk in individuals who may not have traditional risk factors for cardiovascular events. A combination of the tried-and-true traditional risk factors, such as LDL, low HDL, triglycerides, blood pressure, age, and sex, together with inflammatory biomarkers may provide us an opportunity for sharpening our risk prediction and successfully targeting therapy to prevent events going forward. The emergence of novel imaging modalities that can gauge inflammation may provide a tool for the translation of pathophysiologic hypotheses to humans. Methods that permit the imaging of inflammation should speed the development of novel therapies by aiding dose selection and providing early signals of biological effect, allowing prioritization of approaches to be tested in large endpoint trials. Understanding the pathophysiology of plaque disruption, the thrombotic complications of atherosclerosis, and the role inflammation biology plays in these processes should continue to provide advances in basic clinical science of atherosclerosis in years to come.
Acknowledgments
The author thanks long-term colleagues and collaborators, including Drs. Masanori Aikawa and Paul M. Ridker, for their insights and contribution to the concepts and experiments described in this review.
Abbreviations
CRP, C-reactive protein
IFN-γ, inflammatory mediator interferon-γ
MMP, matrix metalloproteinase
The author receives support from the National Heart, Lung, and Blood Institute, the Donald W. Reynolds Foundation, and Fondation Leducq. The author serves as an unpaid consultant for AstraZeneca.
Published, JLR Papers in Press, December 18, 2008.
References
- 1.Virmani R., A. P. Burke, A. Farb, and F. D. Kolodgie. 2006. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 47 C13–C18. [DOI] [PubMed] [Google Scholar]
- 2.Davies M. J. 1996. Stability and instability: the two faces of coronary atherosclerosis. The Paul Dudley White Lecture, 1995. Circulation. 94 2013–2020. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. 1995. The molecular bases of the acute coronary syndromes. Circulation. 91 2844–2850. [DOI] [PubMed] [Google Scholar]
- 4.Amento E. P., N. Ehsani, H. Palmer, and P. Libby. 1991. Cytokines and growth factors positively and negatively regulate intersitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 11 1223–1230. [DOI] [PubMed] [Google Scholar]
- 5.Galis Z. S., M. Muszynski, G. Sukhova, E. Simon-Morrisey, E. Unemori, M. Lark, E. Amento, and P. Libby. 1994. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ. Res. 75 181–189. [DOI] [PubMed] [Google Scholar]
- 6.Nikkari S. T., K. D. O'Brien, M. Ferguson, T. Hatsukami, H. G. Welgus, C. E. Alpers, and A. W. Clowes. 1995. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis. Circulation. 92 1393–1398. [DOI] [PubMed] [Google Scholar]
- 7.Galis Z. S., G. Sukhova, R. Kranzhöfer, S. Clark, and P. Libby. 1995. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc. Natl. Acad. Sci. USA. 92 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukhova G. K., U. Schonbeck, E. Rabkin, F. J. Schoen, A. R. Poole, R. C. Billinghurst, and P. Libby. 1999. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 99 2503–2509. [DOI] [PubMed] [Google Scholar]
- 9.Herman M. P., G. K. Sukhova, P. Libby, N. Gerdes, N. Tang, D. B. Horton, M. Kilbride, R. E. Breitbart, M. Chun, and U. Schonbeck. 2001. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 104 1899–1904. [DOI] [PubMed] [Google Scholar]
- 10.Mach F., U. Schoenbeck, J-Y. Bonnefoy, J. Pober, and P. Libby. 1997. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40. Induction of collagenase, stromelysin, and tissue factor. Circulation. 96 396–399. [DOI] [PubMed] [Google Scholar]
- 11.Dollery C. M., and P. Libby. 2006. Atherosclerosis and proteinase activation. Cardiovasc. Res. 69 625–635. [DOI] [PubMed] [Google Scholar]
- 12.Fukumoto Y., J. O. Deguchi, P. Libby, E. Rabkin-Aikawa, Y. Sakata, M. T. Chin, C. C. Hill, P. R. Lawler, N. Varo, F. J. Schoen, et al. 2004. Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation. 110 1953–1959. [DOI] [PubMed] [Google Scholar]
- 13.Deguchi J. O., E. Aikawa, P. Libby, J. R. Vachon, M. Inada, S. M. Krane, P. Whittaker, and M. Aikawa. 2005. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 112 2708–2715. [DOI] [PubMed] [Google Scholar]
- 14.Schneider F., G. K. Sukhova, M. Aikawa, J. Canner, N. Gerdes, S. M. Tang, G. P. Shi, S. S. Apte, and P. Libby. 2008. Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation. 117 931–939. [DOI] [PubMed] [Google Scholar]
- 15.Deguchi J. O., M. Aikawa, C-H. Tung, E. Aikawa, D-E. Kim, V. Ntziachristos, R. Weissleder, and P. Libby. 2006. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 114 55–62. [DOI] [PubMed] [Google Scholar]
- 16.Davies M. J., P. D. Richardson, N. Woolf, D. R. Katz, and J. Mann. 1993. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br. Heart J. 69 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng Y. J., and P. Libby. 2002. Progression of atheroma: a struggle between death and procreation. Arterioscler. Thromb. Vasc. Biol. 22 1370–1380. [DOI] [PubMed] [Google Scholar]
- 18.Geng Y-J., and P. Libby. 1995. Evidence for apoptosis in advanced human atheroma. Co-localization with interleukin-1 β-converting enzyme. Am. J. Pathol. 147 251–266. [PMC free article] [PubMed] [Google Scholar]
- 19.Geng Y-J., Q. Wu, M. Muszynski, G. Hansson, and P. Libby. 1996. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1-beta. Arterioscler. Thromb. Vasc. Biol. 16 19–27. [DOI] [PubMed] [Google Scholar]
- 20.Henderson E. L., Y. J. Geng, G. K. Sukhova, A. D. Whittemore, J. Knox, and P. Libby. 1999. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 99 96–104. [DOI] [PubMed] [Google Scholar]
- 21.Libby P., and S. K. Clinton. 1992. Cytokines as mediators of vascular pathology. Nouv. Rev. Fr. Hématol. 34 S47–S53. [PubMed] [Google Scholar]
- 22.Rauch U., and Y. Nemerson. 2000. Circulating tissue factor and thrombosis. Curr. Opin. Hematol. 7 273–277. [DOI] [PubMed] [Google Scholar]
- 23.Bonderman D., A. Teml, J. Jakowitsch, C. Adlbrecht, M. Gyongyosi, W. Sperker, H. Lass, W. Mosgoeller, D. H. Glogar, P. Probst, et al. 2002. Coronary no-reflow is caused by shedding of active tissue factor from dissected atherosclerotic plaque. Blood. 99 2794–2800. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox J. N., K. M. Smith, S. M. Schwartz, and D. Gordon. 1989. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc. Natl. Acad. Sci. USA. 86 2839–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgington T. S. 2007. Surfing tissue factor. From the predicted to the discovery and elucidation of unanticipated functions and biology of potential significance. Thromb. Haemost. 98 36–37. [PubMed] [Google Scholar]
- 26.Sugiyama S., K. Kugiyama, M. Aikawa, S. Nakamura, H. Ogawa, and P. Libby. 2004. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler. Thromb. Vasc. Biol. 24 1309–1314. [DOI] [PubMed] [Google Scholar]
- 27.Aikawa M., E. Rabkin, Y. Okada, S. J. Voglic, S. K. Clinton, C. E. Brinckerhoff, G. K. Sukhova, and P. Libby. 1998. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 97 2433–2444. [DOI] [PubMed] [Google Scholar]
- 28.Aikawa M., S. Sugiyama, C. Hill, S. Voglic, E. Rabkin, Y. Fukumoto, F. Schoen, J. L. Witztum, and P. Libby. 2002. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 106 1390–1396. [DOI] [PubMed] [Google Scholar]
- 29.Aikawa M., and P. Libby. 2004. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc. Pathol. 13 125–138. [DOI] [PubMed] [Google Scholar]
- 30.Aikawa M., S. J. Voglic, S. Sugiyama, E. Rabkin, M. B. Taubman, J. T. Fallon, and P. Libby. 1999. Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation. 100 1215–1222. [DOI] [PubMed] [Google Scholar]
- 31.Aikawa M., E. Rabkin, S. Sugiyama, S. J. Voglic, Y. Fukumoto, Y. Furukawa, M. Shiomi, F. J. Schoen, and P. Libby. 2001. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 103 276–283. [DOI] [PubMed] [Google Scholar]
- 32.Fukumoto Y., P. Libby, E. Rabkin, C. C. Hill, M. Enomoto, Y. Hirouchi, M. Shiomi, and M. Aikawa. 2001. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation. 103 993–999. [DOI] [PubMed] [Google Scholar]
- 33.Ridker P. M., N. Rifai, M. A. Pfeffer, F. Sacks, and E. Braunwald. 1999. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) investigators. Circulation. 100 230–235. [DOI] [PubMed] [Google Scholar]
- 34.Schonbeck U., and P. Libby. 2004. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 109 II18–II26. [DOI] [PubMed] [Google Scholar]
- 35.Ray K. K., and C. P. Cannon. 2005. Early time to benefit with intensive statin treatment: could it be the pleiotropic effects? Am. J. Cardiol. 96 54F–60F. [DOI] [PubMed] [Google Scholar]
- 36.Libby P., M. Aikawa, and M. K. Jain. 2006. Vascular endothelium and atherosclerosis. Handb. Exp. Pharmacol. 176 285–306. [DOI] [PubMed] [Google Scholar]
- 37.Wang C. Y., P. Y. Liu, and J. K. Liao. 2008. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol. Med. 14 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arslan F., G. Pasterkamp, and D. P. de Kleijn. 2008. Unraveling pleiotropic effects of statins: bit by bit, a slow case with perspective. Circ. Res. 103 334–336. [DOI] [PubMed] [Google Scholar]
- 39.Ridker P. M., C. P. Cannon, D. Morrow, N. Rifai, L. M. Rose, C. H. McCabe, M. A. Pfeffer, and E. Braunwald. 2005. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352 20–28. [DOI] [PubMed] [Google Scholar]
- 40.Ridker P. M., N. Rifai, M. Clearfield, J. R. Downs, S. E. Weis, J. S. Miles, and A. M. Gotto, Jr. 2001. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 344 1959–1965. [DOI] [PubMed] [Google Scholar]
- 41.Ridker P. M., E. Danielson, F. A. Fonseca, J. Genest, A. M. Gotto, Jr., J. J. Kastelein, W. Koenig, P. Libby, A. J. Lorenzatti, J. G. Macfadyen, et al. 2008. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359 2195–2207. [DOI] [PubMed] [Google Scholar]
- 42.Libby P., and P. Theroux. 2005. Pathophysiology of coronary artery disease. Circulation. 111 3481–3488. [DOI] [PubMed] [Google Scholar]
- 43.Libby, P. Inflammation in cardiovascular disease: the biological basis of inflammatory biomarkers. Chapter in: Morrow DA, editor. Cardiovascular Biomarkers: Pathophysiology and Disease Management. Totowa: Humana Press Inc; 2006. p. 205–220.