Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in the United States and, indeed, worldwide. It has become a global public health issue. In the United States, the prevalence in the general population is estimated at ∼20%, while that in the morbidly obese population at ∼75-92% and in the pediatric population at ∼13–14%. The progressive form of NAFLD, nonalcoholic steatohepatitis, is estimated at ∼3–5%, with ∼3–5% of these having progressed to cirrhosis. Thus, the numbers of individuals at risk for end-stage liver disease and development of primary liver cancer is large. NAFLD is an independent risk factor for cardiovascular disease, leads to increased all-cause mortality, and to increased liver-related mortality. This review focuses on recent advances in our understanding of the NAFLD disease spectrum, including etiology, diagnosis, treatment, and genetic and environmental risk factors and suggests future directions for research in this important area.
Keywords: steatohepatitis, fibrosis, cirrhosis, liver tumors, cardiovascular disease, obesity, diabetes
Nonalcoholic fatty liver disease (NAFLD) is rapidly becoming a worldwide public health problem. It is the most common liver disease in the United States and, indeed, worldwide. Current estimates are that ∼20% of the general US population has NAFLD. The prevalence in the morbidly obese population has been estimated as 75–92%, while that in the pediatric population as 13–14%. At present, it is estimated that ∼6 million individuals in the US general population have progressed to nonalcoholic steatohepatitis (NASH) and ∼600,000 to NAFLD-related cirrhosis. Thus, the number of individuals at risk for end-stage liver disease and development of primary liver cancer and those potentially eligible for liver transplant is large. Prevalence of NAFLD appears to be increasing, in part due to the increasing numbers of adult and pediatric individuals who are obese or overweight or have metabolic syndrome or type 2 diabetes, all major risk factors for development of NAFLD.
Ludwig et al. (1) were the first to name the disease in 1980 and to describe a series of patients with NASH. Since that time, the disease has been recognized in populations worldwide [for recent reviews on the epidemiology of NAFLD, see (2, 3)].
NAFLD represents a spectrum of diseases ranging from “simple steatosis,” which is considered relatively benign, to NASH and NAFLD-associated cirrhosis and end-stage liver disease. NAFLD has become a common cause of liver transplant. It also has been identified as an important risk factor for development of primary liver cancer (4, 5), mostly due to NAFLD-associated cirrhosis.
In addition to higher prevalence of NAFLD in patients with obesity, metabolic syndrome, and type 2 diabetes, NAFLD also can be induced by a variety of drugs and toxins (6). NAFLD and insulin resistance are interrelated in a complex fashion and may be synergistic to some degree [for recent review, see (7)].
Cooccurrence of NAFLD with Hepatitis C or HIV worsens their prognosis (8–10). NAFLD is reported to be an independent risk factor for cardiovascular disease (11–13). This may reflect similar risk factors, such as dyslipidemia or immune dysregulation; however, to date, common mechanisms have not been identified. NAFLD is associated with increased all-cause mortality and increased liver-related mortality (14, 15).
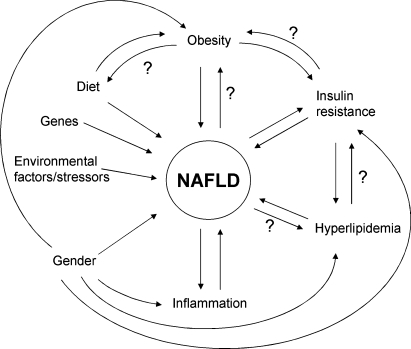
To date, major gaps remain in our understanding of the etiology of NAFLD and why it progresses. It is generally agreed that dysregulation of lipid metabolism is involved. Furthermore, it seems likely that dysregulation of the immune response plays an important role, particularly in progression. Clearly, NAFLD is a complex disease with many interacting metabolic pathways that appear to be regulated by the interplay of genetic predisposition and environmental factors. It seems likely that many of the molecular mechanisms involved in the development and progression of NAFLD will share similarities with those for development of obesity, and development and progression of metabolic syndrome, type 2 diabetes, cardiovascular disease, and malignancy. It also is clear that both development and progression of NAFLD involve unique genetic and environmental interactions. Some of the factors associated with NAFLD and how they might interact are depicted in Fig. 1.
Fig. 1.
Factors that may impact NAFLD. Any or all metabolic pathways may play a role in NAFLD and its progression dependent on an individual's cohort of genes and genetic and epigenetic interactions. The question mark indicates that little evidence is available supporting an influence, but that, hypothetically, one may exist.
DIAGNOSIS
Diagnosis and staging of NAFLD remains complex. Currently, the only accepted way to diagnose NAFLD is by a careful medical history combined with liver biopsy. To assess NAFLD stage, the only unequivocal way at present is by liver biopsy, despite its limitations; it is recognized that a biopsy can over- or underestimate the degree of disease (16–18). Given the invasive nature of liver biopsy and other caveats with this technique, it is clear that noninvasive, reliable methods for diagnosing and staging NAFLD are urgently needed. A number have been proposed, but to date, none are in routine clinical use (19, 20) [for recent overview, (21)].
Most individuals with NAFLD have been diagnosed after abnormal liver function tests and/or ultrasound or computed tomography scans indicated a fatty liver. These tests usually were ordered for reasons other than suspected liver disease. It is important to note that it is possible to have NAFLD, especially the chronic, progressive form, in the setting of apparently normal liver function tests and a minimal fatty liver (22, 23). It also is important to note that NAFLD is not obligatorily associated with obesity, metabolic syndrome, or type 2 diabetes. Individuals without these conditions can, and do, develop NAFLD, and certainly not all individuals who are obese or have metabolic syndrome or type 2 diabetes develop progressive NAFLD.
ETIOLOGY
The etiology of NAFLD and its progression is complex and remains incompletely understood. It is clearly multifactorial. Many cases are related to a “Western lifestyle,” i.e., nutrient abundance coupled with a sedentary lifestyle; however, it is likely that genetic predisposition plays an important, if not decisive, role in determining which individuals are at increased risk for development of NAFLD and for its progression.
The first recognized stage of NAFLD, “simple” benign steatosis, can be viewed as indicative that adipose tissue fat storage capacity has been exceeded, particularly in the case of visceral adiposity, a major risk factor for NAFLD and its progression. Adipose tissue, particularly visceral adipose tissue, has been recognized as an endocrine organ; it secretes a variety of hormones, cytokines. and chemokines, both pro- and anti-inflammatory [for recent review, see (24)], some of which have been suggested to play a role in progression of NAFLD to its less benign stages.
Many of the molecular pathways implicated in NAFLD and its progression appear similar to those found in other “injured” organs and tissues, regardless of original insult. In addition to dysregulation in lipid metabolism, the innate immune system is likely to play an important role in the initial response of the liver to insult/injury [for recent review, see (25)]. A fibrotic response also is likely to have similar molecular mechanisms [for recent review, see (26)]. It is likely that liver-specific modes of regulation are involved in the development of NAFLD and its progression as well as host factors.
ROLE OF GENETICS
A number of studies over the years have implicated genetic predisposition in NAFLD. For example, it was noted that NAFLD appears to have a familial component (27, 28). For a recent review related to genetics in NAFLD, see (29).
As data have accumulated, it is clear that ethnic differences play a role in susceptibility to NAFLD, especially progressive NAFLD, that cannot be explained simply on the basis of diet or socioeconomic differences. Recent examples include the higher incidence of NASH in US populations of Hispanic origin relative to whites and a lower incidence in African Americans, despite a higher rate of obesity (30–33). Because of the mixed racial heritage of such populations in the US, it would be useful in the future to identify them more precisely using accepted racial origin genetic markers. This is of particular importance when accumulating data that may ultimately be used to set public policy regarding population screening and/or public health intervention strategies.
Other populations at high risk for development of NAFLD include the South Asian Indian population (34–36). This population also is at high risk for development of metabolic syndrome, type 2 diabetes, and cardiovascular disease, suggesting the possibility of gene variants that impact shared molecular pathways among these clinical entities. NAFLD also is common in other Asia-Pacific populations (35, 36). Furthermore, not surprisingly, given the high incidence of metabolic syndrome in Amerindian populations, NAFLD also is common throughout Latin America (2).
Recently, as technical and financial constraints have eased, samples from populations with well-defined NAFLD have begun to be used for genome scans to discover gene variants that are more common in NAFLD patients than in a control population. Recently, using this technique, a study from the Dallas group (37) identified ethnic differences in variants of a gene, PNPLA3, that are associated with different propensity to NAFLD and its progression.
Other groups have examined single nucleotide polymorphism (SNP) variants in candidate genes chosen for their known implication in regulation of lipid metabolism or relationship with risk factors for NAFLD [for recent review, see (29)]. In addition, gene/environment interactions are beginning to be explored. A recent example is the association of two common SNP variants in the adiponectin gene that were previously associated with cardiometabolic risk and with the response to dietary fat in NAFLD patients (38).
We and others have begun looking for chromosomal regions harboring gene variants that affect the onset of NAFLD and its progression using classic mouse genetic approaches. Knowledge of the identity of such chromosomal regions discovered in the mouse, and the genes harbored therein, can be translated to the human genome to define areas with high probability of relationship with human NAFLD and its progression.
ROLE OF ENVIRONMENTAL FACTORS
It seems clear that environmental factors can play a major role in the etiology of NAFLD, especially in genetically susceptible populations. Foremost among these are nutrient abundance, especially the typical Western-style diet rich in simple carbohydrates, saturated fat, and highly processed food stuffs. When this is coupled with a Western sedentary lifestyle, caloric imbalance can occur, resulting in increased weight gain in most individuals. In both adult and pediatric populations, increasing prevalence of overweight/obesity is associated with increased prevalence of NAFLD.
Other factors that have been implicated are the composition of the intestinal microflora, clearly in part a reflection of diet [for discussion, see (39)].
ANIMAL MODELS
One of the major problems that has hampered basic research in the NAFLD area is the lack of animal models that recapitulate the human disease. This is a controversial area [for recent overview, see (40)]. Most models have been genetically altered rodents and/or rodents fed high-fat diets or diets deficient in methionine and choline. Most develop a fatty liver and many develop aspects of steatohepatitis. However, rarely do they spontaneously develop fibrosis. Because it is highly unlikely that NAFLD in the human population is monogenic, study of animals with deletion or overexpression of a single gene may not mimic etiology of the human disease at the molecular level. Likewise, choice of experimental diet may not mimic the human diets associated with development of NAFLD. To date, very little work appears to have been reported with other animal models.
We recently serendipitously discovered a mixed genetic background line of mice that when fed a Western diet, spontaneously developed the entire spectrum of human NAFLD over time, including bridging fibrosis and increased incidence of primary hepatoma (unpublished observations).
TREATMENTS
Currently, the only accepted treatment for NAFLD regardless of stage is lifestyle modifications [for recent review, see (41)]. These include weight loss by a combination of decreased caloric intake and increased physical activity. Of potential importance is choice of diet, for example, low fat/high carbohydrate versus high fat/low carbohydrate. To date, this has not been investigated rigorously, but some studies suggest that this is an important consideration to prevent progression or worsening of NAFLD disease stage [for review of this area, see (42)]. Another option, generally available only for the morbidly obese, is bariatric surgery [for recent review, see (43)]. Evidence has accumulated that both approaches can reverse NASH and to some extent fibrosis, at least in some individuals. An important caveat for both treatment approaches is that rapid weight loss by any means is to be avoided because it can cause NAFLD progression.
Over the years, a number of small clinical trials assessing treatments for NAFLD have been reported. A variety of drugs such as the statins [for review see (44)], the glitazones [(45, 46) and references therein], and other modalities [for recent comprehensive review, see (47)] have been studied. These treatments were chosen largely based on the identification of risk factors often associated with NAFLD. In general, efficacy has been relatively modest, benefit has not occurred in all individuals (in some, disease has progressed), results have not been verified in other populations, or results have been widely disparate from study to study. In some trials, NAFLD and its stage have not been rigorously defined. Often the definition of NAFLD has varied from trial to trial. It is hoped that with use of uniform diagnostic and staging criteria that the efficacy of treatments can be tested more rigorously in the future.
An important question at present regarding treatment paradigms for NAFLD is whether to treat “simple” steatosis by other than lifestyle changes. Given that not all such patients will progress to chronic steatohepatitis, cirrhosis, or end-stage liver disease, and given the sheer number of patients with simple steatosis, it may not be cost effective to treat all patients with this stage of NAFLD with drugs for which the risk/benefit ratio may not be optimal.
Based on results to date, as with most complex metabolic diseases, subgroups of NAFLD patients will be resistant to a given treatment, while others will benefit and still others will show worsening of disease. Thus, it is critical to identify characteristics of these subgroups of patients to target appropriate groups of patients in the future for a given treatment modality to maximize risk-to-benefit ratio and cost effectiveness. As for most complex diseases, it is likely that in future, NAFLD will be a target for “personalized” medical intervention based in part on an individual's genetics.
SUMMARY AND FUTURE DIRECTIONS
NAFLD is a “young” disease entity whose magnitude and seriousness have been appreciated increasingly over the past ∼30 years. Although medical and scientific interest and basic, translational, and clinical research efforts in this area have grown exponentially, key questions and problems remain that must be addressed to decrease morbidity and mortality from the entire spectrum of NAFLD. Among these are 1) the availability of uniform diagnostics, preferably noninvasive, for identification and staging of disease. Attainment of this goal is important not only for NAFLD diagnosis and staging in the clinic but also for the success of clinical trials assessing potential prevention or treatment paradigms and for genetic studies. 2) Effective treatments for prevention of NAFLD or to reverse or prevent its progression are needed. This area is critically dependent on uniform diagnostic criteria and better noninvasive diagnostic and staging methods. Because major gaps remain in our understanding of the etiology and progression of NAFLD at the basic level, most potential treatments studied to date have been directed at associated known risk factors rather than at NAFLD-specific molecular targets. Thus, there is an urgent need for support of basic research in the NAFLD arena to identify such targets. 3) Appropriate animal models need to be developed that more closely mimic the human disease. This will enable more rapid advancement in basic research at the molecular level and provide preclinical models for testing potential treatment or prevention modalities. 4) Individuals with genetic predisposition to NAFLD and/or its progression need to be identified. To attain this goal, carefully designed genetic studies in both humans and appropriate animal models are required. Such studies will lead to improved clinical diagnosis and management of NAFLD patients and also will help identify new molecular targets for potential drug development. 5) Strategies are needed to modulate known risk factors associated with onset of NAFLD and/or its progression to end-stage liver disease. For example, it is clear that lifestyle can play an important role, regardless of the area of the world. To date, it is not clear how to institute lifestyle changes effectively at both individual and population-wide levels. Clearly this is a difficult and complex area. To address it will require a dedicated multidisciplinary approach [see (48) for an example of a recent effort in this direction].
Acknowledgments
The author thanks Drs. Nathan Bass, Jaquelyn Maher, Arun Sanyal, and Richard Green for valuable discussions of NAFLD over the years and Dr. William Duane for review of the manuscript. The author takes full responsibility for this review and apologizes to anyone whose work was inadvertently overlooked.
The author's work is supported by a Merit Award from the Department of Veterans Affairs and by DK-072187 from the National Institutes of Health.
Published, JLR Papers in Press, December 12, 2008.
References
- 1.Ludwig J., T. R. Viggiano, D. B. Mc, and B. J. O. H. Gill. 1980. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 55 434–438. [PubMed] [Google Scholar]
- 2.Lazo M., and J. M. Clark. 2008. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin. Liver Dis. 28 339–350. [DOI] [PubMed] [Google Scholar]
- 3.Ong J. P., and Z. M. Younossi. 2007. Epidemiology and natural history of NAFLD and NASH. Clin. Liver Dis. 11 1–16. [DOI] [PubMed] [Google Scholar]
- 4.Marrero J. A., R. J. Fontana, G. L. Su, H. S. Conjeevaram, D. M. Emick, and A. S. Lok. 2002. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 36 1349–1354. [DOI] [PubMed] [Google Scholar]
- 5.Bullock R. E., A. M. Zaitoun, G. P. Aithal, S. D. Ryder, I. J. Beckingham, and D. N. Lobo. 2004. Association of non-alcoholic steatohepatitis without significant fibrosis with hepatocellular carcinoma. J. Hepatol. 41 685–686. [DOI] [PubMed] [Google Scholar]
- 6.Chitturi S., and G. C. Farrell. 2001. Etiopathogenesis of nonalcoholic steatohepatitis. Semin. Liver Dis. 21 27–41. [DOI] [PubMed] [Google Scholar]
- 7.Utzschneider K. M., and S. E. Kahn. 2006. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 91 4753–4761. [DOI] [PubMed] [Google Scholar]
- 8.Ramesh S., and A. J. Sanyal. 2004. Hepatitis C and nonalcoholic fatty liver disease. Semin. Liver Dis. 24 399–413. [DOI] [PubMed] [Google Scholar]
- 9.Guaraldi G., N. Squillace, C. Sentarelli, G. Orlando, R. D'Amico, G. Ligabue, F. Fiocchi, S. Zona, P. Loria, R. Esposito, et al. 2008. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin. Infect. Dis. 47 250–257. [DOI] [PubMed] [Google Scholar]
- 10.Merriman R. B. 2006. Nonalcoholic fatty liver disease and HIV infection. Curr. HIV/AIDS Rep. 3 113–117. [DOI] [PubMed] [Google Scholar]
- 11.Targher G., L. Bertolani, R. Padovani, S. Rodella, G. Zoppini, L. Zenari, M. Cigolini, G. Falezza, and G. Arcaro. 2006. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 29 1325–1330. [DOI] [PubMed] [Google Scholar]
- 12.Aygun C., O. Kocaman, T. Sahin, S. Uraz, A. T. Eminler, A. Celebi, O. Senturk, and S. Hulager. 2008. Evaluation of metabolic syndrome frequency and carotid artery intima-media thickness as risk factors for atherosclerosis in patients with nonalcoholic fatty liver disease. Dig. Dis. Sci. 53 1352–1357. [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein E., J. E. Levine, and J. B. Schwimmer. 2008. Hepatic, cardiovascular and endocrine outcomes of the histological subphenotypes of nonalcoholic fatty liver disease. Semin. Liver Dis. 28 380–385. [DOI] [PubMed] [Google Scholar]
- 14.Ong J. P., A. Pitts, and Z. M. Younossi. 2008. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 49 608–612. [DOI] [PubMed] [Google Scholar]
- 15.Dunn W., R. Xu, D. L. Wingard, C. Rogers, P. Angulo, Z. M. Younossi, and J. B. Schwimmer. 2008. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am. J. Gastroenterol. 103 2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratziu V., F. Charlotte, A. Heurtier, S. Gombert, P. Giral, E. Bruckert, A. Grimaldi, F. Capron, and T. Poynard; LIDO Study Group. 2005. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 128 1898–1906. [DOI] [PubMed] [Google Scholar]
- 17.Yeh M. M., and E. M. Brunt. 2007. Pathology of nonalcoholic fatty liver disease. Am. J. Clin. Pathol. 128 837–847. [DOI] [PubMed] [Google Scholar]
- 18.Merriman R. B., L. D. Ferrell, M. G. Patti, S. R. Weston, M. S. Pabst, B. E. Aouizerat, and N. M. Bass. 2006. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 44 874–880. [DOI] [PubMed] [Google Scholar]
- 19.Angulo P., J. M. Hui, G. Marchesini, E. Bugianesi, J. George, G. C. Farrell, F. Enders, S. Saksesa, A. D. Burt, J. P. Bida, et al. 2007. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 45 846–854. [DOI] [PubMed] [Google Scholar]
- 20.Munteanu M., V. Ratziu, R. Morra, D. Messous, F. Imbert-Bismut, and T. Poynard. 2008. Noninvasive biomarkers for the screening of fibrosis, steatosis and steatohepatitis in patients with metabolic risk factors: Fibro Test-Fibro-Max™ Experience. J. Gastrointestin. Liver Dis. 17 187–191. [PubMed] [Google Scholar]
- 21.Wieckowska A., and A. E. Feldstein. 2008. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin. Liver Dis. 28 386–395. [DOI] [PubMed] [Google Scholar]
- 22.Mofrad P., M. J. Contos, M. Haque, C. Sargeant, R. A. Fisher, V. A. Lukethia, R. K. Sterling, M. L. Schiffman, R. T. Stravitz, and A. J. Sanyal. 2003. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 37 1286–1292. [DOI] [PubMed] [Google Scholar]
- 23.Schwimmer J. B. 2007. Definitive diagnosis and assessment of risk for nonalcoholic fatty liver disease in children and adolescents. Semin. Liver Dis. 27 312–318. [DOI] [PubMed] [Google Scholar]
- 24.Kershaw E. E., and J. S. Flier. 2004. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89 2548–2556. [DOI] [PubMed] [Google Scholar]
- 25.Maher J. J., P. Leon, and J. C. Ryan. 2008. Beyond insulin resistance: innate immunity in nonalcoholic steatohepatitis. Hepatology. 48 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jou J., S. S. Choi, and A. M. Diehl. 2008. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin. Liver Dis. 28 370–379. [DOI] [PubMed] [Google Scholar]
- 27.Willner I. R., B. Waters, S. R. Patil, A. Reuben, J. Morelli, and C. A. Riely. 2001. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency and severity of disease. Am. J. Gastroenterol. 96 2957–2961. [DOI] [PubMed] [Google Scholar]
- 28.Struben V. M., E. E. Hespenheide, and S. H. Caldwell. 2000. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am. J. Med. 108 9–13. [DOI] [PubMed] [Google Scholar]
- 29.Osterreicher C. H., and D. A. Brenner. 2007. The genetics of nonalcoholic fatty liver disease. Ann. Hepatol. 6 83–88. [PubMed] [Google Scholar]
- 30.Caldwell S. H., D. M. Harris, J. T. Patrie, and E. E. Hespenheide. 2002. Is NASH underdiagnosed among African Americans? Am. J. Gastroenterol. 97 1496–1500. [DOI] [PubMed] [Google Scholar]
- 31.Browning J. D., L. S. Szczepaniak, R. Dobbins, P. Nuremberg, J. D. Horton, J. C. Cohen, S. M. Grundy, and H. H. Hobbs. 2004. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 40 1387–1395. [DOI] [PubMed] [Google Scholar]
- 32.Browning J. D., K. S. Kumar, M. H. Saboorian, and D. L. Thiele. 2004. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am. J. Gastroenterol. 99 292–298. [DOI] [PubMed] [Google Scholar]
- 33.Weston S. R., W. Leyden, R. Murphy, N. M. Bass, B. P. Bell, M. M. Manos, and N. A. Terrault. 2005. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 41 372–379. [DOI] [PubMed] [Google Scholar]
- 34.Petersen K. F., S. Dufour, J. Feng, D. Befroy, J. Dziura, C. D. Man, C. Cobelli, and G. I. Shulman. 2006. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc. Natl. Acad. Sci. USA. 103 18273–18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amarapurkar D. N., E. Hashimoto, L. A. Lesmana, J. D. Sollano, P. J. Chen, and K. L. Goh. 2007. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J. Gastroenterol. Hepatol. 22 788–793. [DOI] [PubMed] [Google Scholar]
- 36.Fan J. G., T. Saibara, S. Chitturi, B. I. Kim, J. J. Sung, and A. Chutaputti; Asia-Pacific Working Party for NAFLD. 2007. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J. Gastroenterol. Hepatol. 22 794–800. [DOI] [PubMed] [Google Scholar]
- 37.Romeo S., J. Kozlitina, C. Xing, A. Pertsemlidis, D. Cox, L. A. Pennacchio, E. Boerwinkle, J. C. Cohen, and H. H. Hobbs. 2008. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musso G., R. Gambino, F. De Michieli, M. Durazzo, G. Pagano, and M. Cassader. 2008. Adiponectin gene polymorphisms modulate acute adiponectin response to dietary fat: possible pathogenetic role in NASH. Hepatology. 47 1167–1177. [DOI] [PubMed] [Google Scholar]
- 39.Solga S. F., and A. M. Diehl. 2003. Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics. J. Hepatol. 38 681–687. [DOI] [PubMed] [Google Scholar]
- 40.Anstee Q. M., and R. D. Goldin. 2006. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 87 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rafiq N., and Z. M. Younossi. 2008. Effects of weight loss on nonalcoholic fatty liver disease. Semin. Liver Dis. 28 427–433. [DOI] [PubMed] [Google Scholar]
- 42.Zivkovic A. M., J. B. German, and A. J. Sanyal. 2007. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 86 285–300. [DOI] [PubMed] [Google Scholar]
- 43.Verna E. C., and P. D. Berk. 2008. Role of fatty acids in the pathogenesis of obesity and fatty liver: impact of bariatric surgery. Semin. Liver Dis. 28 407–426. [DOI] [PubMed] [Google Scholar]
- 44.Browning J. D. 2006. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology. 44 466–471. [DOI] [PubMed] [Google Scholar]
- 45.Ratziu V., P. Giral, S. Jacqueminet, F. Charlotte, A. Hartemann-Heurtier, L. Serfaty, P. Podevin, J. M. Lacorte, C. Bernhardt, E. Bruckert, et al; LIDO Study Group. 2008. Rosiglitazone for nonalcoholic steatohepatitis: one year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) trial. Gastroenterology. 135 100–110. [DOI] [PubMed] [Google Scholar]
- 46.Guruprasad P. A., J. A. Thomas, P. V. Kaye, A. Lawson, S. D. Ryder, I. Spendlove, A. S. Austin, J. G. Freeman, L. Morgan, and J. Webber. 2008. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 135 1176–1184. [DOI] [PubMed] [Google Scholar]
- 47.Kashi M. R., D. W. Torres, and S. A. Harrison. 2008. Current and emerging therapies in nonalcoholic fatty liver disease. Semin. Liver Dis. 28 396–406. [DOI] [PubMed] [Google Scholar]
- 48.Bellentani S., R. Della Grave, A. Suppini, and G. Marchesini; Fatty Liver Italian Network. 2008. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology. 47 746–754. [DOI] [PubMed] [Google Scholar]