Abstract
The prostanoids are a family of lipid mediators generated by the action of cyclooxygenase on a 20-carbon unsaturated fatty acid, arachidonic acid. Prostanoids are generated widely in response to diverse stimuli and, acting in a paracrine or autocrine manner, play important roles in normal physiology and disease. This review summarizes the current knowledge on prostanoid generation and the roles of individual mediators, their biosynthetic pathways, and their receptors in health and disease.
Keywords: prostaglandins, lipids, autacoids
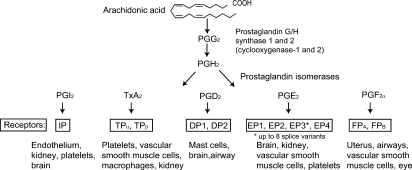
Arachidonic acid (AA), a 20-carbon unsaturated fatty acid, is the predominant precursor for a family of lipid mediators, the eicosanoids. Eicosanoid biosynthesis begins with release of AA, esterified in the sn-2 domain of membrane phospholipids, by the action of phospholipase A2, particularly group IVA cytosolic cPLA2. Three major groups of enzymes, prostaglandin G/H synthases, lipoxygenases, or epoxygenases, then catalyze the formation of the prostaglandins (PGs) and thromboxane A2 (TxA2), the leukotrienes, or the epoxyeicosatrienoic acids, respectively. A parallel family of free radical catalyzed isomers, the isoeicosanoids, is formed by nonenzymatic peroxidation of AA in situ (1). This review will focus on the PGs and TxA2, collectively termed the prostanoids (Fig. 1), in normal physiology and disease.
Fig. 1.
The prostanoid biosynthesis response pathway.
PROSTANOID BIOSYNTHESIS
Prostanoids are formed by the action of prostaglandin G/H synthase, or cyclooxygenase (COX), on AA. COX, an evolutionarily conserved (2) bisfunctional enzyme, exists as two distinct isoforms, COX-1 or COX-2. COX-1, expressed constitutively in most cells, is the dominant (but not exclusive) source of prostanoids for housekeeping functions, such as gastric epithelial cytoprotection and hemostasis. COX-2, induced by cytokines, shear stress, and tumor promoters, is the more important source of prostanoid formation in inflammation and perhaps cancer. However, both enzymes contribute to the generation of autoregulatory and homeostatic prostanoids, and both can contribute to prostanoid formation during inflammation.
COX-1 or COX-2 function as homodimers, and perhaps heterodimers (3), inserted into the endoplasmic reticular membrane, to transform AA into the unstable cyclic endoperoxides PGG2 and PGH2 (Fig. 1). Downstream isomerases and synthases complete the biosynthesis of TxA2 and D, E, F, and I series PGs. COX-1 and COX-2 are closely related in their amino acid sequence and crystal structure (4). Isoform-specific preference for downstream enzymes has been reported in heterologous expression systems, although the biological relevance is unknown. COX-1 couples preferentially, but not exclusively, with thromboxane synthase, prostaglandin F synthase, and the cytosolic prostaglandin E synthase (PGES) isozymes. COX-2 prefers prostaglandin I synthase (PGIS) and the microsomal (m) PGES isozymes, both of which are induced by cytokines and tumor promoters. Two forms of prostaglandin D and F synthase have been identified, underscoring the diversity of the isomerases and synthases.
PROSTANOID RECEPTORS
Prostanoids activate membrane receptors at, or close to, the site of their formation. Specific G-protein-coupled receptors have been cloned for all the prostanoids (5). A single gene product has been identified for prostacyclin [the I prostanoid (IP) receptor], PGF2α [the F prostanoid receptor (FP)], and TxA2 [the T prostanoid receptor (TP)], while four distinct PGE2 receptors (the EP1–4) and two PGD2 receptors (DP1 and DP2) have been cloned. Two additional isoforms of the human TP and FP and eight EP3 variants are generated through differential splicing. EP2, EP4, IP, and DP1 receptors activate adenylyl cyclase via Gs, increasing intracellular cAMP. EP1, FP, and TP activate phosphatidylinositol metabolism, leading to the formation of inositol trisphosphate with mobilization of intracellular Ca2+ stores. TP couples to multiple G proteins, including G12/13 and G16, to stimulate small G protein signaling pathways and may activate or inhibit adenylyl cyclase. EP3 isoforms can couple via Gi or G12 to elevation of intracellular Ca2+, inhibition of cAMP generation, and activation of the small G protein Rho. The DP2 (also known as the chemoattractant receptor homologous molecule expressed on Th2 cells) couples to a Gi-type G protein to inhibit cAMP synthesis and elevate intracellular Ca2+.
PROSTANOIDS IN HEALTH AND DISEASE
Inflammation
Prostanoid biosynthesis is significantly increased in inflamed tissue. Nonselective COX-1/COX-2 inhibitors, aspirin and the traditional (t) nonsteroidal anti-inflammatory drugs (NSAIDs), are widely used anti-inflammatory agents, highlighting the pro-inflammatory role of the prostanoids. The marked induction of COX-2 in inflammatory cells and tissues provided a rationale for development of selective COX-2 inhibitors. However, although COX-2 is a major source of pro-inflammatory prostanoids, an inflammatory role for COX-1 is also evident (6). Indeed, COX-1 accounts for ∼10–15% of the prostanoid formation induced by lipopolysaccharide in humans, and both isozymes are expressed in circulating inflammatory cells ex vivo. Impaired inflammatory responses have been reported in both COX-1- and COX-2- deficient mice, although they diverge in time course and intensity. Human data indicate that COX-1-derived products drive the initial phase of an acute inflammation, with COX-2 upregulation occurring within several hours.
PGE2 and PGI2 are the predominant pro-inflammatory prostanoids. Both markedly enhance edema formation and leukocyte infiltration by promoting blood flow. PGE2 and PGI2, through activation of the EP2 and IP, respectively, also increase vascular permeability and leukocyte infiltration (5). The chemotactic function of PGD2, a major product of mast cells, contributes to inflammation in allergic responses, particularly in the lung. Activation of DP1 increases blood flow and vascular permeability and promotes T-cell polarization to the Th2 phenotype. PGD2 can also promote inflammation via DP2 through activation of Th2 cells and eosinophils (7). DP2 antagonists may prove useful in treatment of airway inflammation; however, conflicting results with respect to airway function in DP2-deficient mice suggest a complex context- and tissue-dependent inflammatory role for PGD2.
Cyclooxygenase inhibitors have a long history as antipyretic drugs. The early phase of the pyretic response to IL-1β, an endogenous pyrogen that mediates the febrile response to a variety of exogenous stimuli, is likely mediated by ceramide, but the late response is mediated by central induction of PGE2 synthesis. IL-1β and other cytokines induce the coordinate expression of COX-2 and mPGES-1 in endothelial cells of the highly vascularized midline of the preoptic area. PGE2 can cross the blood-brain barrier to act on the EP3, and perhaps EP1, expressed on thermosensitive neurons (8).
Pain
PGE2 and PGI2 reduce the threshold of nociceptor sensory neurons to stimulation. This “peripheral sensitization” potentiates the pain-producing activity of bradykinin and other autocoids. COX-1 and COX-2, expressed in the spinal cord, release PGs in response to peripheral pain stimuli. PGE2, and perhaps also PGD2, PGI2, and PGF2α, contribute to “central sensitization” (9), an increase in spinal dorsal horn neuron excitability that augments pain intensity, widens the perception area, and results in pain from innocuous stimuli.
COX-1-deficient mice show increased tolerance to pain stimuli. COX-1 is the predominant isoform expressed in neurons of dorsal root ganglia, and prolonged expression has been observed in spinal microglia during central sensitization (10). COX-2, expressed basally in both neurons and glia, contributes to central sensitization in the early phase of peripheral inflammation. Within a few hours, it is upregulated widely in the spinal chord, contributing to prolonged central sensitization. Activation of EP1 and IP play a more pronounced role during early phases of inflammatory pain, while activation of EP2 may be more relevant in chronic events (11).
Renal function
Renal PGs, especially PGE2 and PGI2, but also PGF2α and TxA2, perform complex and intricate functions in the kidney (12, 13). Both COXs are expressed in renal tissue; COX-1 mainly in the cortical and medullary collecting ducts, mesangial cells, arteriolar endothelium, and the epithelium of Bowman's capsule and COX-2 in medullary interstitial cells, the macula densa, and the cortical thick ascending limb.
COX inhibition is associated with an increased risk of peripheral edema and sodium retention (14). COX-2-derived prostanoids increase medullary blood flow and inhibit tubular sodium reabsorption. Indeed, expression of medullary COX-2 and mPGES-1 is increased by high salt intake, and COX-1-derived products promote salt excretion in the collecting ducts. Loss of these effects may underlie the systemic or salt-sensitive hypertension often associated with COX inhibition. However, COX-1 and COX-2 products may have contrasting renal effects; COX-1 deletion or inhibition reduces the hypertensive response to angiotensin (Ang) II in rodents, while COX-2 deletion or inhibition reduces medullary blood flow and enhances the hypertensive response to Ang II. Therefore, the renal side effects of selective and nonselective NSAIDs may not be identical, a notion that is supported by a large meta-analysis showing that selective inhibition of COX-2 elevates blood pressure more than nonselective inhibition (15).
Renal-cortex-derived prostanoids modulate systemic blood pressure in a manner that is distinct from the medullary enzyme. Cortical COX-2-derived PGE2 and PGI2 increase renal blood flow and glomerular filtration by local vasodilation, actions that may be particularly relevant in marginally functioning kidneys and volume-contracted states. Cortical COX-2 expression is increased by low dietary salt intake, which, through the action of PGE2, and possibly PGI2, increases renin release, leading to sodium retention and elevated blood pressure. Concordantly, COX-2 inhibition or disruption decreases plasma renin following administration of a low-salt diet (12).
The regulated expression patterns of renal prostanoid receptors, particularly the EPs, contributes substantially to this complexity (12). EP1, expressed exclusively in collecting ducts, mediates PGE2-dependent inhibition of salt and water absorption. EP1-deficient mice have a urine concentration defect due to decreased vasopressin release, resulting in hypotension (16). EP2 mRNA is present at low levels in the descending thin limb of Henle and the vasa recta of the outer medulla; mice deficient in EP2 develop salt-sensitive hypertension. EP3 is abundant in the thick ascending limb and collecting ducts and also in glomeruli. EP4 is expressed primarily in the glomerulus and outer medullary vasa recta, mediating PGE2-dependent dilation of the renal microcirculation as well as renin release. TP is localized to glomeruli and the renal vasculature, corresponding to TxA2's potent vasoconstrictor effects that reduce renal blood flow and glomerular filtration rate. The FP is highly expressed in the distal convoluted tubules and the cortical collecting duct. In vitro studies suggest that activation of the FP modulates water absorption through inhibition of vasopressin action (17), while infusion of PGF2α causes both natriuresis and diuresis. Mice with FP deficiency exhibit hypotension, probably due to decreased renin-angiotensin activity (unpublished observations). The IP is predominantly expressed in renal interlobular, vasa recta, and glomerular afferent arterioles. Similar to PGE2, PGI2 dilates the glomerular microvasculature and can regulate renin release.
Cardiovascular
The importance of prostanoids in maintaining cardiovascular homeostasis is highlighted by clinical experience with NSAIDs. Despite their superior gastrointestinal safety, compared with nonselective tNSAIDs, COX-2 selective inhibitors increase the risks of myocardial infarction, stroke, systemic and pulmonary hypertension, thrombosis, congestive heart failure, and sudden cardiac death (18). Seven randomized controlled trials, involving three structurally distinct COX-2 inhibitors (rofecoxib, celecoxib, and valdecoxib), provide compelling evidence for this cardiovascular hazard (18, 19). This extends to some older drugs, such as diclofenac, meloxicam, and nimesulide, which also show some COX-2 selectivity.
Because of their short half-life, prostanoids do not circulate and do not impact directly systemic vascular tone. However, they may modulate local vascular tone at the site of their formation and affect systemic blood pressure through the kidney. Upon infusion, PGE2, PGD2, and PGI2 elicit vasodilation in most vascular beds. Vasoconstriction can result from activation of EP1 and EP3 by PGE2. We and others reported that, unlike interruption of COX-2 activity, deletion of mPGES-1, which substantially reduced PGE2 biosynthesis, fails to alter blood pressure in mice fed a normal or high-salt diet (20). Hypertension induced by Ang II infusion in hyperlipidemic mice is also uninfluenced (21), although the hypertensive response to a more intensive salt load, and to Ang II infusion, in normolipidemic mice was augmented (22). This discrepancy may reflect differences in mouse strain genetic background and/or dosing regimen.
Polymorphisms in PGIS and the IP have been associated with essential hypertension (23), although studies argue against a role for PGI2 in the homeostatic maintenance of vascular tone. PGI2 limits pulmonary hypertension induced by hypoxia and systemic hypertension induced by Ang II. PGI2, and its stable analogs, have been used successfully to treat pulmonary arterial hypertension (24). Local subcutaneous release of PGD2 causes dilation of skin vasculature contributing to the facial flushing associated with niacin treatment in humans. PGF2α is a potent constrictor of both pulmonary arteries and veins in humans, while TxA2 is a vasoconstrictor in the whole animal and in isolated vascular beds.
Hypertension and congestive cardiac failure have been observed in placebo-controlled trials of NSAIDs (18). We recently found that selective deletion of cardiomyocyte COX-2 resulted in heart failure and cardiac fibrosis in mice (unpublished observations), demonstrating a direct role of COX-2-derived prostanoids in cardiac function. Acting on the IP or the EP3, respectively, PGI2 and PGE2 protect against oxidative injury in cardiac tissue. Global deletion of the IP augments ischemia/reperfusion injury (25), while both global deletion of mPGES-1 (26) and cardiomyocyte-specific deletion of the EP4 (27) exacerbate the decline in cardiac function after myocardial infarction. In an alternative heart failure model, COX-2-derived TXA2 contributed to oxidant stress and isoprostane generation to increase cardiomyocyte apoptosis and fibrosis (28). A compensatory rise in PGI2 biosynthesis reflects the apparent role of this PG in subserving a homeostatic cardioprotective function.
Platelets
Activated platelets synthesize TxA2, amplifying further platelet activation and recruitment (29). The total biosynthesis of TxA2 is augmented in clinical syndromes of platelet activation, including unstable angina, myocardial infarction and stroke. Mature platelets express only COX-1, although megakaryocytes and immature platelet forms also express COX-2 (30). While most NSAIDs inhibit COX reversibly, aspirin acetylates Ser-529 in COX-1 (Ser-516 in COX-2) covalently, inhibiting its enzymatic activity irreversibly. This unique feature, which sustains inhibition of platelet TxA2 biosynthesis, together with the limited capacity of platelets for de novo protein synthesis, underlies aspirin's position as the only COX inhibitor with proven cardioprotective activity (31).
In platelets, the TP couples predominantly to Gq and G12/13. Gq activates protein kinase C-dependent pathways, which facilitate platelet aggregation, whereas G12/G13-mediated Rho/Rho-kinase-dependent regulation of myosin light chain phosphorylation contributes to platelet shape change. Endogenous inhibitors of platelet function, including nitric oxide and prostacyclin (PGI2), serve to limit platelet activation in vivo (18).
PGI2 is the major platelet inhibitory prostanoid. It is synthesized by COX-2, and to a lesser degree by COX-1, in vascular endothelial and smooth muscle cells. Following activation of the IP-Gs-adenylyl cyclase pathway, cAMP activates PKA deactivating myosin light chain kinase to reduce myosin phosphorylation and decrease platelet aggregation (32). PKA also targets the vasodilator-stimulated phosphoprotein, which may regulate fibrinogen binding by modulating inside-out signaling of the integrin αIIbβ3. The IP may also modify specifically the response to TxA2 through formation of an IPTP heterodimer (33). Augmented PGI2 biosynthesis in syndromes of platelet activation serves to constrain the effects of platelet agonists, vasoconstrictors, and stimuli of platelet activation (34). The increased incidence of myocardial infarction and stroke associated with selective COX-2 inhibition, which is most parsimoniously explained by inhibition of COX-2-dependent PGI2 formation (18), supports this concept.
The role of PGE2 in platelet function in vivo remains less clear. In vitro, high PGE2 concentrations (>10 mM), acting via the IP or, theoretically, the EP2 or EP4, inhibit platelet function (35). Low concentrations of PGE2, acting via the EP3, augment submaximal platelet stimulation by other agonists; mice lacking EP3 have an increased bleeding tendency and decreased susceptibility to thromboembolism. Deletion of mPGES-1 does not affect thrombogenesis in mice, probably because of substrate rediversion and augmented PGI2 formation (20).
Atherothrombosis
In atherosclerosis, an inflammatory cardiovascular disease, unstable or ruptured plaques can result in intravascular thrombosis leading to severe clinical complications. Increased atherogenesis may be fundamental to evolution of a cardiovascular risk in patients taking COX-2-selective NSAIDs chronically (18). However, studies examining the impact of selective COX-2 inhibition on atherogenesis have yielded conflicting results. COX-2 inhibitors have been shown variously to retard, accelerate, or leave unaltered atherogenesis in mouse models (36). This may result from differences in drug specificity or dosing strategy but may also reflect the contrasting effect of local prostanoids, elaborated in a cell-specific manner, over the course of the disease.
Individual prostanoids are associated with contrasting effects in atherothrombosis. Suppression of TxA2 biosynthesis, as well as TP antagonism or deletion, retards atherogenesis in mice (37, 38). Conversely, PGI2 appears atheroprotective (38, 39). As mentioned above, the cardiovascular hazard associated with selective inhibition of COX-2 is mechanistically explicable by suppression of cardioprotective COX-2-derived prostanoids, especially PGI2 (18, 23). In addition to its action as a general restraint on platelet activation (see platelet section, above) and its potential role in retarding atherogenesis, PGI2 limits vascular proliferation, remodeling, and hypertension (20, 34, 38, 39). These mechanisms, primarily elucidated in rodents, would be expected to alter the cardiovascular risk of humans. Indeed, an Arg-212-to-Cys substitution in IP, which disrupts its signaling, cosegregated with increased cardiovascular risk in a recent study (23).
PGE2 effects on atherothrombosis are more complex. mPGES-1 colocalizes, in some settings, with COX-2, and both enzymes are subject to regulation during inflammation. Deletion or inhibition of mPGES-1 markedly reduces inflammatory responses, in several mouse models, and mPGES-1 deletion reduces atherogenesis in fat fed hyperlipidemic mice (40). Interestingly, in addition to the expected depression of PGE2 production, deletion of mPGES-1 augments biosynthesis of PGI2, presumably through rediversion of PGH2 to PGIS. It is currently unclear, however, whether elevated PGI2 contributes to the cardiovascular profile of mPGES-1 deletion and whether small molecule inhibitors of mPGES-1 will have the same metabolic and functional effects as gene deletion.
Aortic aneurysm
Abdominal aortic aneurysm (AAA), also an inflammatory cardiovascular disease, is a major cause of morbidity and mortality in humans. Nonsurgical treatments that retard aneurysm development, or induce its regression, remain to be identified. Human AAA biopsies stain strongly for COX-2, and there is evidence for the potential efficacy of NSAIDs, particularly COX-2 selective inhibitors, for treatment of aneurysmal growth. In mouse models, deletion or selective inhibition of COX-2 or mPGES-1, but not inhibition of COX-1, decreases AAA formation (21). Augmented biosynthesis of both PGI2 and PGD2 coincides with mPGES-1 deficiency, suggesting a contribution of substrate rediversion to reduced AAA formation and to the depression of attendant oxidant stress. These studies raise the possibility that specific mPGES-1 inhibitors might prevent or retard human AAA formation. However, mPGES-1 deletion is also associated with eccentric cardiac myocyte hypertrophy, left ventricular dilation, and impaired left ventricular contractile function, after myocardial infarction (26). The extent to which these observations may be translated to clinical indications of mPGES-1 inhibitors is unclear and necessitates further investigation.
Lung
COX-1- and COX-2-deficient mice, or mice treated with COX inhibitors, display exaggerated inflammatory airway responses and bronchocontrictor hyperresponsiveness in murine models of asthma (41). PGE2 can have anti-inflammatory and antiasthmatic effects via activation of EP3 (42). IP deletion exaggerates features of acute and chronic experimental asthma, including increased bronchial hyperresponsiveness, while inhaled iloprost (a PGI2 analog) suppresses the cardinal features of asthma in mice (43). The role of PGD2 in allergic asthma is complicated (7). Deletion of DP1 in mice sharply reduces allergen-induced infiltration of lymphocytes and eosinophils and airway hyperreactivity. However, activation of DP1 on bone-marrow-derived cells inhibits airway inflammation, and activation of DP1 on lung dendritic cells may also control the extent of airway inflammation (44). In vitro studies strongly support a pro-inflammatory role for PGD2 acting at the DP2. However, the surprising increase in the allergic asthmatic responses in DP2-deficient mice suggests a more complex function for PGD2 than previously thought.
Cancer
Pharmacological inhibition, or genetic deletion of COX-2, reduces tumor formation in experimental animal models of colon, breast, lung, and other cancers (45, 46). In mice, targeted overexpression of COX-2 in the mammary epithelium is sufficient to induce tumorogenesis. Large epidemiologic studies concur, reporting significant reductions in relative risk for developing these, and other, cancers with the incidental use of NSAIDs (47). COX inhibitors significantly decrease polyp formation, in patients with familial polyposis coli, while a polymorphism in COX-2 has been associated with increased risk of colon cancer. Three randomized controlled trials, the Adenoma Prevention with Celecoxib trial, the Prevention of Sporadic Adenomatous Polyps trial, and the Adenomatous Polyp Prevention on Vioxx trial, reported a significant reduction in the reoccurrence of adenomas in patients receiving either celecoxib or rofecoxib (48). However, this coincided with an increase in risk of cardiovascular events, undermining the risk-to-benefit ratio. Although celecoxib remains approved for use in patients with familial polyposis coli, none of these trials included the cheaper COX-1/COX-2 nonselective tNSAIDs as comparators, despite evidence in rodent models implicating both enzymes in polyp formation.
Most data indicate that PGE2 is the a primary pro-oncogenic prostanoid, at least in part via transactivation of epidermal growth factor receptor (46). PGE2 facilitates tumor initiation, progression, and metastasis through multiple biological effects, including increased proliferation and angiogenesis, decreased apoptosis, augmented cellular invasiveness, and modified immunosuppression. Mice lacking EP1, EP2, or EP4 display reduced disease in multiple carcinogenesis models. By contrast, EP3 may play a protective role in some cancers. The pro- and anti-oncogenic roles of other prostanoids remain under investigation, with TxA2 emerging as another likely COX-2-derived procarcinogenic mediator (45).
CONCLUSIONS
Deletion of prostanoid receptors in mice has revealed a remarkably diverse and contrasting biology mediated by this family of lipids. The therapeutic potential of this pathway is illustrated by the clinical efficacy of aspirin and the NSAIDs. Although NSAIDs selective for inhibition of COX-2 confer a risk of cardiovascular events, this appears to involve only 1–2% of exposed patients, and it would seem logical to investigate these drugs further to seek a scientific basis for risk management. In the meantime, other targets in the pathway, such as mPGES-1 and many of the receptors, have emerged as drug targets. It is likely that the harvest from this pathway will continue to yield therapeutic benefit in the years to come.
Acknowledgments
G.A.F. is the McNeil Professor of Translational Medicine and Therapeutics.
Abbreviations
AA, arachidonic acid
AAA, abdominal aortic aneurysm
Ang, angiotensin
COX, cyclooxygenase
IP, I prostanoid
NSAID, nonsteroidal anti-inflammatory drug
PG, prostaglandin
TxA2, thromboxane A2
This work was supported by grants from the National Institutes of Health (HL-83799, HL-62250, HL-081012, HL-066233, and RR-023567), the American Heart Association (Jon Holden DeHaan Foundation 0730314N, 0430148N, and 0735397N), and the University of Pennsylvania's Institute for Translational Medicine and Therapeutics' Transdisciplinary Program in Translational Medicine and Therapeutics (UL1RR024134).
Published, JLR Papers in Press, December 17, 2008.
References
- 1.Fam S. S., and J. D. Morrow. 2003. The isoprostanes: unique products of arachidonic acid oxidation-a review. Curr. Med. Chem. 10 1723–1740. [DOI] [PubMed] [Google Scholar]
- 2.Grosser T., S. Yusuff, E. Cheskis, M. A. Pack, and G. A. FitzGerald. 2002. Developmental expression of functional cyclooxygenases in zebrafish. Proc. Natl. Acad. Sci. USA. 99 8418–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu Y., J. Fan, X. S. Chen, D. Wang, A. J. Klein-Szanto, R. L. Campbell, G. A. FitzGerald, and C. D. Funk. 2006. Genetic model of selective COX2 inhibition reveals novel heterodimer signaling. Nat. Med. 12 699–704. [DOI] [PubMed] [Google Scholar]
- 4.FitzGerald G. A., and P. Loll. 2001. COX in a crystal ball: current status and future promise of prostaglandin research. J. Clin. Invest. 107 1335–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hata A. N., and R. M. Breyer. 2004. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 103 147–166. [DOI] [PubMed] [Google Scholar]
- 6.McAdam B. F., I. A. Mardini, A. Habib, A. Burke, J. A. Lawson, S. Kapoor, and G. A. FitzGerald. 2000. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J. Clin. Invest. 105 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettipher R., T. T. Hansel, and R. Armer. 2007. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat. Rev. Drug Discov. 6 313–325. [DOI] [PubMed] [Google Scholar]
- 8.Engblom D., S. Saha, L. Engstrom, M. Westman, L. P. Audoly, P. J. Jakobsson, and A. Blomqvist. 2003. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat. Neurosci. 6 1137–1138. [DOI] [PubMed] [Google Scholar]
- 9.Minami T., H. Nakano, T. Kobayashi, Y. Sugimoto, F. Ushikubi, A. Ichikawa, S. Narumiya, and S. Ito. 2001. Characterization of EP receptor subtypes responsible for prostaglandin E2-induced pain responses by use of EP1 and EP3 receptor knockout mice. Br. J. Pharmacol. 133 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F. Y., Y. Wan, Z. K. Zhang, A. R. Light, and K. Y. Fu. 2007. Peripheral formalin injection induces long-lasting increases in cyclooxygenase 1 expression by microglia in the spinal cord. J. Pain. 8 110–117. [DOI] [PubMed] [Google Scholar]
- 11.Zeilhofer H. U. 2007. Prostanoids in nociception and pain. Biochem. Pharmacol. 73 165–174. [DOI] [PubMed] [Google Scholar]
- 12.Breyer M. D., and R. M. Breyer. 2000. Prostaglandin receptors: their role in regulating renal function. Curr. Opin. Nephrol. Hypertens. 9 23–29. [DOI] [PubMed] [Google Scholar]
- 13.Hao C. M., and M. D. Breyer. 2007. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 71 1105–1115. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J., E. L. Ding, and Y. Song. 2006. Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: meta-analysis of randomized trials. JAMA. 296 1619–1632. [DOI] [PubMed] [Google Scholar]
- 15.Aw T. J., S. J. Haas, D. Liew, and H. Krum. 2005. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch. Intern. Med. 165 490–496. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy C. R., H. Xiong, S. Rahal, J. Vanderluit, R. S. Slack, Y. Zhang, Y. Guan, M. D. Breyer, and R. L. Hebert. 2007. Urine concentrating defect in prostaglandin EP1-deficient mice. Am. J. Physiol. Renal Physiol. 292 F868–F875. [DOI] [PubMed] [Google Scholar]
- 17.Hebert R. L., M. Carmosino, O. Saito, G. Yang, C. A. Jackson, Z. Qi, R. M. Breyer, C. Natarajan, A. N. Hata, Y. Zhang, et al. 2005. Characterization of a rabbit kidney prostaglandin F(2{alpha}) receptor exhibiting G(i)-restricted signaling that inhibits water absorption in the collecting duct. J. Biol. Chem. 280 35028–35037. [DOI] [PubMed] [Google Scholar]
- 18.Grosser T., S. Fries, and G. A. Fitzgerald. 2006. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J. Clin. Invest. 116 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia Rodriguez L. A., S. Tacconelli, and P. Patrignani. 2008. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J. Am. Coll. Cardiol. 52 1628–1636. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y., M. Wang, Y. Yu, J. Lawson, C. D. Funk, and G. A. Fitzgerald. 2006. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J. Clin. Invest. 116 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M., E. Lee, W. Song, E. Ricciotti, D. J. Rader, J. A. Lawson, E. Puré, and G. A. Fitzgerald. 2008. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II–induced abdominal aortic aneurysm formation. Circulation. 117 1302–1309. [DOI] [PubMed] [Google Scholar]
- 22.Jia Z., A. Zhang, H. Zhang, Z. Dong, and T. Yang. 2006. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ. Res. 99 1243–1251. [DOI] [PubMed] [Google Scholar]
- 23.Arehart E., J. Stitham, F. W. Asselbergs, K. Douville, T. MacKenzie, K. M. Fetalvero, S. Gleim, Z. Kasza, Y. Rao, L. Martel, et al. 2008. Acceleration of cardiovascular disease by a dysfunctional prostacyclin receptor mutation: potential implications for cyclooxygenase-2 inhibition. Circ. Res. 102 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steiropoulos P., G. Trakada, and D. Bouros. 2008. Current pharmacological treatment of pulmonary arterial hypertension. Curr. Clin. Pharmacol. 3 11–19. [DOI] [PubMed] [Google Scholar]
- 25.Xiao C. Y., A. Hara, K. Yuhki, T. Fujino, H. Ma, Y. Okada, O. Takahata, T. Yamada, T. Murata, S. Narumiya, et al. 2001. Roles of prostaglandin I2 and thromboxane A2 in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 104 2210–2215. [DOI] [PubMed] [Google Scholar]
- 26.Degousee N., S. Fazel, D. Angoulvant, E. Stefanski, S. C. Pawelzik, M. Korotkova, S. Arab, P. Liu, T. F. Lindsay, S. Zhuo, et al. 2008. Microsomal prostaglandin E2 synthase-1 deletion leads to adverse left ventricular remodeling after myocardial infarction. Circulation. 117 1701–1710. [DOI] [PubMed] [Google Scholar]
- 27.Qian J. Y., P. Harding, Y. Liu, E. Shesely, X. P. Yang, and M. C. LaPointe. 2008. Reduced cardiac remodeling and function in cardiac-specific EP4 receptor knockout mice with myocardial infarction. Hypertension. 51 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z., R. Vezza, T. Plappert, P. McNamara, J. A. Lawson, S. Austin, D. Pratico, M. S. Sutton, and G. A. FitzGerald. 2003. COX-2-dependent cardiac failure in Gh/tTG transgenic mice. Circ. Res. 92 1153–1161. [DOI] [PubMed] [Google Scholar]
- 29.FitzGerald G. A. 1991. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am. J. Cardiol. 68 11B–15B. [DOI] [PubMed] [Google Scholar]
- 30.Rocca B., P. Secchiero, G. Ciabattoni, F. O. Ranelletti, L. Catani, L. Guidotti, E. Melloni, N. Maggiano, G. Zauli, and C. Patrono. 2002. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proc. Natl. Acad. Sci. USA. 99 7634–7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrono C., B. Coller, J. E. Dalen, G. A. FitzGerald, V. Fuster, M. Gent, J. Hirsh, and G. Roth. 2001. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest. 119 39S–63S. [DOI] [PubMed] [Google Scholar]
- 32.Jin R. C., B. Voetsch, and J. Loscalzo. 2005. Endogenous mechanisms of inhibition of platelet function. Microcirculation. 12 247–258. [DOI] [PubMed] [Google Scholar]
- 33.Wilson S. J., A. M. Roche, E. Kostetskaia, and E. M. Smyth. 2004. Dimerization of the human receptors for prostacyclin and thromboxane facilitates thromboxane receptor-mediated cAMP generation. J. Biol. Chem. 279 53036–53047. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y., S. C. Austin, B. Rocca, B. H. Koller, T. M. Coffman, T. Grosser, J. A. Lawson, and G. A. FitzGerald. 2002. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 296 539–541. [DOI] [PubMed] [Google Scholar]
- 35.Fabre J. E., M. Nguyen, K. Athirakul, K. Coggins, J. D. McNeish, S. Austin, L. K. Parise, G. A. FitzGerald, T. M. Coffman, and B. H. Koller. 2001. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. J. Clin. Invest. 107 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linton M. F., and S. Fazio. 2004. Cyclooxygenase-2 and inflammation in atherosclerosis. Curr. Opin. Pharmacol. 4 116–123. [DOI] [PubMed] [Google Scholar]
- 37.Egan K. M., M. Wang, M. B. Lucitt, A. M. Zukas, E. Pure, J. A. Lawson, and G. A. FitzGerald. 2005. Cyclooxygenases, thromboxane, and atherosclerosis: plaque destabilization by cyclooxygenase-2 inhibition combined with thromboxane receptor antagonism. Circulation. 111 334–342. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi T., Y. Tahara, M. Matsumoto, M. Iguchi, H. Sano, T. Murayama, H. Arai, H. Oida, T. Yurugi-Kobayashi, J. K. Yamashita, et al. 2004. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J. Clin. Invest. 114 784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egan K. M., J. A. Lawson, S. Fries, B. Koller, D. J. Rader, E. M. Smyth, and G. A. Fitzgerald. 2004. COX-2 derived prostacyclin confers atheroprotection on female mice. Science. 306 1954–1957. [DOI] [PubMed] [Google Scholar]
- 40.Wang M., A. M. Zukas, Y. Hui, E. Ricciotti, E. Pure, and G. A. FitzGerald. 2006. Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proc. Natl. Acad. Sci. USA. 103 14507–14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peebles R. S., and T. V. Hartert. 2002. Highlights from the annual scientific assembly: patient-centered approaches to asthma management: strategies for treatment and management of asthma. South. Med. J. 95 775–779. [PubMed] [Google Scholar]
- 42.Kunikata T., H. Yamane, E. Segi, T. Matsuoka, Y. Sugimoto, S. Tanaka, H. Tanaka, H. Nagai, A. Ichikawa, and S. Narumiya. 2005. Suppression of allergic inflammation by the prostaglandin E receptor subtype EP3. Nat. Immunol. 6 524–531. [DOI] [PubMed] [Google Scholar]
- 43.Idzko M., H. Hammad, M. van Nimwegen, M. Kool, N. Vos, H. C. Hoogsteden, and B. N. Lambrecht. 2007. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J. Clin. Invest. 117 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammad H., M. Kool, T. Soullie, S. Narumiya, F. Trottein, H. C. Hoogsteden, and B. N. Lambrecht. 2007. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J. Exp. Med. 204 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M. T., K. V. Honn, and D. Nie. 2007. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 26 525–534. [DOI] [PubMed] [Google Scholar]
- 46.Wang D., and R. N. Dubois. 2006. Prostaglandins and cancer. Gut. 55 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris R. E., J. Beebe-Donk, H. Doss, and D. Burr Doss. 2005. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade. Oncol. Rep. 13 559–583. [PubMed] [Google Scholar]
- 48.Bertagnolli M. M. 2007. Chemoprevention of colorectal cancer with cyclooxygenase-2 inhibitors: two steps forward, one step back. Lancet Oncol. 8 439–443. [DOI] [PubMed] [Google Scholar]