Abstract
There is increasing evidence that oxidized phospholipids (OxPLs) play an important role in atherosclerosis. These phospholipids accumulate in human and mouse lesions. Specific OxPLs have been identified as major regulators of many cell types present in the vessel wall. In endothelial cells, >1,000 genes are regulated. Some of these genes are pro-atherogenic and others anti-atherogenic. The anti-atherogenic effects are likely important in slowing the atherogenic process. Several receptors and signaling pathways associated with OxPL action have been identified and shown to be upregulated in human lesions. A structural model of the mechanism by which specific OxPLs serve as CD36 ligands has been identified. Specific oxidized phospholipids are also present in plasma and associated with Lp(a) particles. In humans, OxPL/apolipoprotein B has been shown to be a prognostic indicator and a separate risk factor for coronary events. Levels of OxPL in plasma have been shown to be correlated with platelet activation. The results of these studies suggest an important role for OxPL in all stages of atherosclerosis.
Keywords: endothelial, macrophage, smooth muscle cells, clinical trials
CHEMISTRY OF OXIDIZED PHOSPHOLIPIDS
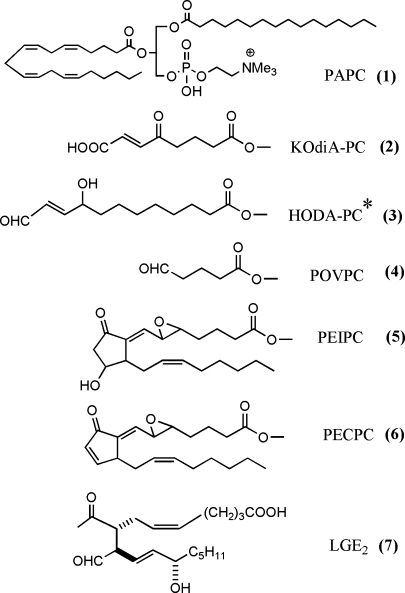
Several reviews and original articles have identified the structure of bioactive oxidized phospholipids that are formed from PUFAs at the sn-2 position (1–3). The number of oxidation products from each PUFA is likely at least 50, and effects of all of these products have not been examined. For the purposes of this review, we will only include structures of several well-studied molecules (Fig. 1). Bioactive oxidized phospholipids may contain fragmentation products of PUFA, such as 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phoshorylcholine and 9-keto-10-dodecendioic acid ester of 2-lyso-phosphatidyl choline (KOdiA-PC); prostaglandins, such as 15 deoxy-delta12,14 prostaglandin J2 (PGJ2) and 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphoryl choline (PEIPC); and levuglandins. These molecules exhibit different biological activities. An important recent development is a better understanding of the initial interaction of oxidized phospholipids with cells. Receptors have been identified, including CD36, SRB1, EP2, VEGFR2, and the PAF receptor (1, 4). An interaction with TLR4 has also been suggested in some studies but not others (1, 5). A possible mechanism by which cells recognize oxidized phospholipids has been suggested involving the novel confirmation of these lipids in membranes (6). These studies demonstrated that, when present in vesicles, truncated oxidized fatty acids at the sn-2 position move from the hydrophobic interior to the aqueous exterior of the vesicle. This would allow their recognition by cell surface receptors. An earlier model of isoprostane-containing phospholipids suggests that they are highly twisted and may distort membrane areas in which they are present (7). These studies and others (8) suggest that phospholipid oxidation products can integrate into lipid membranes of cells and lipoproteins; they then can either act as ligands or may cause local membrane disruption. In addition, strong evidence has been presented for the ability of oxidized phospholipids to form protein adducts. Probably the most well characterized are the levuglandins that have been studied mainly in the retina but also are associated with atherosclerosis. These molecules are formed on phospholipids and are then hydrolyzed to the free levuglandins, which form adducts (3). In addition, studies have been performed employing biotinylated phospholipid oxidation products of arachidonate and linoleate (9, 10). These demonstrate covalent binding of the unhydrolyzed oxidized phospholipids (OxPLs) to both endothelial cell and plasma proteins, one prominent protein being Apo A1. Using this method, the OxPL bound different amino acids from those bound by hydroxynonenal (a fatty acid oxidation product), suggesting that the phospholipid backbone may alter the pocket in which binding occurs.
Fig. 1.
Structure of selected bioactive phospholipid oxidation products. All are derived from palmitoyl arachidonyl phosphatidyl choline (PAPC) except that indicated with an asterisk, which is derived from palmitoyl linoleolyl phosphatidyl choline. The full molecule is shown for PAPC and the sn-2 position only for all of the other phospholipids.
OxPLs REGULATE VASCULAR CELL FUNCTION
Endothelial cells
The multiple effects of OxPLs on endothelial cells have been recently reviewed (1, 11). After 4 h of treatment with 50 μg/ml of Ox-PAPC, ∼1,000 genes are regulated: ∼600 are upregulated and 400 downregulated (12). In addition, a major difference in responsiveness to specific effects of Ox-PAPC of endothelial cells from different human donors has been documented (12). Upregulation of some of these same genes was seen in vivo in mice (13). Atherogenic pathways upregulated include inflammation, cholesterol synthesis, coagulation, and a decrease in cell division. There was also evidence of oxidative stress responses, including an increase in the anti-atherogenic redox genes and genes associated with the UPR response (12, 14, 15). Though Ox-PAPC inhibits cells division at 4–6 h after treatment, the same concentration of Ox-PAPC increases angiogenesis seen at 72 h after treatment (16). The lipid most strongly stimulating expression of many of the genes discussed above is PEIPC, which is active at 0.5–1 μg/ml. These and other studies provide evidence for differential effectiveness of the different lipids. Even where the same lipid affects more than one function, there is evidence for different signaling pathways being important in gene expression (4, 16). For example, there is evidence that VEGFR2 activation increases IL-8 and MCP-1 synthesis but does not effect redox signaling. EP2 activation increases monocyte binding but does not affect angiogenesis.
In addition to gene regulation, important effects of Ox-PAPC on endothelial cell function independent of gene regulation have been reported. Ox-PAPC was shown to increase monocyte but not neutrophil binding by activating β-1 integrin (11, 13). Ox-PAPC at 10 μg/ml was demonstrated to stabilize adherens junctions (17). However, higher concentrations have been reported to cause protein influx into the lung, suggesting decreased cell contact (18). These latter two studies point out the strong importance of the level of Ox-PAPC employed as a determinant of cell function.
Thus, the effects of OxPLs are complex, with some being pro-atherogenic and others anti-atherogenic. Since different pathways may regulate these effects, it may be possible to preserve the anti-atherogenic effects while inhibiting the pro-atherogenic effects; HDL in fact was shown to be an agent fulfilling these requirements (19).
Macrophages
Many of the effects of OxPL are mediated by its interaction with CD36. Several groups have shown that LDL supplemented with Ox-PAPC or vesicles supplemented with fragmented α/β unsaturated fatty acids at the sn-2 position, such as KOdiA or HODA PC, bind to CD36 (2, 20). In these experiments, as little as 0.3 mol% of HDdiA PC was sufficient to cause CD36 binding. In one of these studies, this binding resulted in foam cell formation, whereas in the other study, it resulted in lipid accumulation but no buildup of cholesterol ester. Another important phagocytic function of macrophages is the uptake of apoptotic cells, which are abundant in atherosclerotic plaques. OxPLs, including oxidized phosphatidyl serine and phosphatidyl choline derivatives, were shown to serve as ligands for macrophage uptake of apoptotic cells (21, 22). Vesicles containing oxidized CD36 ligands or antibodies to CD36 ligands could block apoptotic cell uptake. In CD36 null mice, apoptotic cells accumulate (22). In addition to CD36, OxPLs also interact with and bind to other pattern recognition receptors in macrophages, including TLRs CD14, LPS binding protein, and C-reactive protein competing with native ligands (1, 23–26). Thus, the formation of OxPL during inflammation may represent an important feedback mechanism to limit further tissue damage.
OxPLs have also been shown to activate macrophages. Recently, it was shown that Ox-PAPC was involved in inducing lung injury and cytokine production by lung macrophages (18). Binding of OxPL to CD36 was also demonstrated to activate signaling pathways that caused foam cell formation and simple binding was not sufficient (27). At high concentrations, OxPL leads to activation of pro-apoptotic signaling in various cells, including smooth muscle cells (SMCs) (28) and macrophages (29). Mechanisms of induction of pro-apoptotic pathways by OxPL have been reviewed recently (28). However, in atherosclerotic lesions, where macrophages are constantly exposed to high levels of OxPLs, macrophages survive for surprisingly long periods of time, despite the toxic environment, implying the upregulation of survival mechanisms, such as antioxidant pathways and phase II detoxification genes, some of which have been observed (12, 20).
Dendritic cells
OxPLs have been shown to modulate the maturation process of dendritic cells (DCs), thereby influencing decisive steps of the adaptive immune response. Ox-PAPC was shown to block LPS-induced expression of costimulatory molecules CD40 and CD83 and inhibit secretion of proinflammatory cytokines. This led to a dampening of T-cell proliferation and interferon-γ secretion by T-cells (30). These results strongly indicated a specific block of the Th1-orientated immune response by Ox-PAPC. Studies in apolipoprotein E (apoE)-deficient mice demonstrated a severely compromised immune response, accompanied by a depressed contact- and delayed-type hypersensitivity reaction (31). In apoE-deficient mice on a high-fat diet, it was shown that decreased DC activation led to impaired Th1 and enhanced Th2 responses (32). A recent study shows that OxPLs regulate innate immunity in human leprosy (33). PEIPC accumulated in macrophages infected with live mycobacteria and inhibited expression of CD1b on differentiating DCs leading to decreased antigen presentation. These effects could be reversed by HDL. In addition to effects on DCs, OxPLs were shown to directly affect and induce anergy in T-cells (34). These results show that a dyslipidemic microenvironment can directly interfere with DC and T-cell responses to pathogen-derived signals and skew the development of T-cell-mediated immunity.
SMCs
Phenotypic switching of SMCs involving increased proliferation, enhanced migration, and downregulation of SMC differentiation marker genes plays a critical role in atherogenesis. In addition, smooth muscle cell apoptosis contributes to plaque instability. Several studies have reported that OxPLs stimulated differentiation and increased cell division of SMCs (35, 36), while others showed activation of apoptotic signaling pathways (28). It is clear from these studies that apoptosis depends on the conditions of culture and the concentration of oxidized lipid. Recently, it was shown that Ox-PAPC caused phenotypic switching in SMCs by suppressing the expression of several Krüppel-like transcription factor 4-dependent differentiation markers (36). In unpublished studies, 0.1 μg/ml of PEIPC showed significant effects on SMC differentiation. OxPLs also potently affect connexin expression and function in endothelial cells and SMCs (37), which may have significant effects on the progression of atherosclerotic lesions by enhancing smooth muscle cell interaction with the endothelium in the fibrous plaque (38). The in vitro effects of Ox-PAPC led to connexin changes seen in atherosclerotic lesions.
Platelets
Studies in mouse and humans have demonstrated that platelets can be activated by the interaction of OxPL with CD36 (39). Several studies in thrombosis models in mice showed that that clotting time was decreased in apoE null mice on a Western diet but not in CD36 null mice. CD36 OxPL ligands, including KOdiA-PC and HODA PC, were increased in the plasma of susceptible strains. Studies of human plasma suggest that the levels of OxPC CD36 control thrombogenicity in humans. Enhanced activation of platelets was observed in human plasmas containing the highest amounts of OxPC CD36. These studies demonstrate that specific OxPLs regulate platelet function by binding to CD36.
SUMMARY OF THE EFFECTS OF OxPLs ON VASCULAR WALL CELLS
OxPLs affect the function of all vascular wall cells. One phospholipid oxidation product, PEIPC, has been shown to be active at concentrations (100–500 ng/ml) ∼10-fold lower than those measured in the vessel wall. OxPLs isolated from the vessel wall and diluted 10-fold were able to stimulate an inflammatory response. Furthermore, the concentration of OxPLs in plasma of mice and humans with increased platelet reactivity precisely overlap with levels that modulate platelet aggregation. These studies suggest that levels of OxPLs in vivo are sufficient to regulate atherosclerosis and thrombosis.
EVIDENCE FOR A ROLE OF OxPLs IN HUMAN ATHEROSCLEROSIS
Another chapter in this series addresses the current status of the oxidation hypothesis in atherosclerosis to which the reader is referred. However, we think it is important to point out the increasing evidence that OxPLs play a role in human atherosclerosis. These include 1) the observation that the plasma levels of OxPC CD36 correlate with platelet response to agonists (39); and 2) signal transduction pathways, SREBP, UPR, STAT3, and CNX 43, activated by Ox-PAPC in vitro are also activated in human lesions (11, 37). Perhaps the strongest evidence comes from 3) the prognostic value of OxPLs on apolipoprotein B-100 particles (OxPL/apoB) measured with antibody E06. E06 recognizes PC present in OxPL but not native PL. It recognizes particular OxPLs, such as 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phoshorylcholine, present as a lipid, or if covalently attached to a variety of different peptides, irrespective of amino acid sequence (40).
Prognostic studies on OxPL/apoB
A number of studies support a role for OxPL/apoB levels as a prognostic indicator of atherosclerosis and a separate risk factor. In a series of clinical studies (now encompassing >25,000 subjects), it has been consistently documented that a strong correlation is present between OxPL/apoB and lipoprotein (a) [Lp(a)] levels (41–43). E06 was used to detect OxPL and does not react with native Lp(a). The strong correlation is due to the fact that, where OxPL is present, most E06 recognizable epitopes are on Lp(a) rather than other apoB-containing lipoproteins. It is interesting that this strong correlation of OxPL/apoB and Lp(a) is dependent on the genetically determined apo(a) isoform size, with the strength of this correlation being weakest for the largest isoforms and strongest with the lowest number of kringle IV repeats (44). Multiple cross-sectional and prospective studies have demonstrated that Lp(a) is an independent risk factor for cardiovascular death, myocardial infarction, stroke, and peripheral arterial disease (45). These observations have suggested the hypothesis that E06-detectable OxPL, which is present primarily on LDL containing Lp(a), may confer unique atherothrombotic properties to Lp(a) (41, 43). Supporting this connection, normal mice, LDLR−/− and apoE−/− mice, which do not have Lp(a), have low OxPL/apoB levels even when markedly hypercholesterolemic. By contrast, Lp(a) transgenic mice without hypercholesterolemia or atherosclerosis have very high OxPL/apoB levels that reflect the Lp(a) levels (43).
In a series of studies beginning in 2003, it has been determined that OxPL/apoB levels are elevated in patients with coronary, carotid, and femoral artery disease (44, 46), acute coronary syndromes, and following percutaneous coronary intervention (43). For example, OxPL/apoB levels strongly correlated with both the presence and extent of angiographically determined coronary artery disease measured in 504 patients undergoing coronary angiography (46). Patients in the highest quartile of OxPL/apoB had the most advanced angiographically detected coronary artery disease. In the overall cohort, this association was independent of all clinical and lipid risk factors (including C-reactive protein), except for Lp(a). However, in patients <60 years old, OxPL/apoB levels were independent even of Lp(a), suggesting that OxPL may mediate atherogenicity above and beyond its association with Lp(a), perhaps through additional proinflammatory mechanisms. These data were corroborated in peripheral arterial disease in the Bruneck population consisting of an unselected community cohort of men and women strongly and significantly associated with the presence, extent, and interim development of carotid and femoral atherosclerosis (44). OxPL/apoB and Lp(a) also predicted the presence of symptomatic cardiovascular disease at entry into the study and also predicted new cardiovascular events over a 10-year period, independent of other risk factors, and also provided additional prognostic information within each Framingham Risk Score tertile (47). This study has subsequently been confirmed in a case-control study of ∼2,700 subjects in the EPIC-Norfolk population, where elevated levels of OxPL/apoB were independently associated with increased risk for cardiovascular events (48).
RELATIONSHIP OF OxPL/apoB TO THERAPEUTIC INTERVENTIONS
All studies performed to date with low-fat vegetarian or step II American Heart Association diet, or different doses and different types of statins, show an increase rather than a decrease in OxPL/apoB, along with a concomitant increase in Lp(a) (49). Although the mechanistic underpinnings of this observation are not fully determined, dietary atherosclerosis regression studies in animals with or without Lp(a) have shown similar increases in OxPL/apoB and, importantly, with concomitant removal of OxPL from atherosclerotic lesions (49). This suggests the hypothesis that mobilization of OxPL from the vessel wall or other inflammatory sites and transfer to lipoproteins, and particularly Lp(a) in humans, may occur during atherosclerosis regression. These observations suggest the possibility that the change in OxPL/apoB following therapeutic interventions may represent an early index of plaque stabilization and/or atherosclerosis regression, as changes are noted as early as 4 months after therapy. This hypothesis is currently being tested in large clinical trials.
FUTURE DIRECTIONS
The studies above provide strong evidence that OxPLs play an important role in atherosclerosis. The next challenge will be to determine if therapeutic inhibition of the OxPL interaction with vessel wall cells can inhibit atherosclerosis. It will be important to identify the lipid oxidation products that activate each response in the various cell types and the receptors or binding molecules and signal transduction pathways activated by these lipids. It will also be important to identify, in various stages of atherogenesis, the OxPL species that accumulate at sufficient levels to affect vascular cell function. The OxPL signal is generally more sustained than that of cytokines, and it will be important to understand how this is controlled. Studies comparing genetic and epigenetic responses to OxPL among human donors may identify novel regulatory pathways. It will be important to identify the molecules that degrade OxPL, one of which is PAF-AH type VII (as discussed in another chapter), and determine the comparative effects of the liberated oxidized fatty acids to that of the OxPL. It will also be important to identify the molecules that control production of OxPL. Myeloperoxidase has already been identified, but other candidates, for example, paraoxonase and HDL, will likely also contribute to this regulation. It will be important to gain a better understanding of the role of Lp(a)-bound OxPL in vessel wall regulation and to gain an understanding of how treatments like statins cause OxPL removal from the vessel wall. If the change in OxPL/apoB can be demonstrated to predict the clinical usefulness of therapeutic agents, particularly those related to lipoproteins and oxidation (e.g., statins and HDL mimetics, such as D-4F, etc.), then it may find a unique niche in clinical medicine. Compared with cytokines, OxPLs are recently discovered regulators of vascular function and much remains to be learned.
Abbreviations
apoB, apolipoprotein B
apoE, apolipoprotein E
DC, dendritic cell
KOdiA-PC, 9-keto-10-dodecendioic acid ester of 2-lyso-phosphatidyl choline
Lp(a), lipoprotein (a)
OxPL, oxidized phospholipid
PAPC, palmitoyl arachidonyl phosphatidyl choline
PGJ2, 15 deoxy-delta12,14 prostaglandin J2
PEIPC, 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphoryl choline
SMC, smooth muscle cell
This work was supported by National Institutes of Health Grants P01 HL30568 (J.B.), R01 HL064731 (J.B.), and R01 HL084422 (N.L.), by the American Heart Association (N.L.), and by The Fondation Leducq (S.T.).
Published, JLR Papers in Press, December 4, 2008.
References
- 1.Bochkov V. N. 2007. Inflammatory profile of oxidized phospholipids. Thromb. Haemost. 97 348–354. [PubMed] [Google Scholar]
- 2.Podrez E. A., E. Poliakov, Z. Shen, R. Zhang, Y. Deng, M. Sun, P. J. Finton, L. Shan, M. Febbraio, D. P. Hajjar, et al. 2002. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 277 38517–38523. [DOI] [PubMed] [Google Scholar]
- 3.Salomon R. G. 2005. Isolevuglandins, oxidatively truncated phospholipids, and atherosclerosis. Ann. N. Y. Acad. Sci. 1043 327–342. [DOI] [PubMed] [Google Scholar]
- 4.Zimman A., K. P. Mouillesseaux, T. Le, N. M. Gharavi, A. Ryvkin, T. G. Graeber, T. T. Chen, A. D. Watson, and J. A. Berliner. 2007. Vascular endothelial growth factor receptor 2 plays a role in the activation of aortic endothelial cells by oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 27 332–338. [DOI] [PubMed] [Google Scholar]
- 5.Erridge C., D. J. Webb, and C. M. Spickett. 2007. Toll-like receptor 4 signalling is neither sufficient nor required for oxidised phospholipid mediated induction of interleukin-8 expression. Atherosclerosis. 193 77–85. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg M. E., X. M. Li, B. G. Gugiu, X. Gu, J. Qin, R. G. Salomon, and S. L. Hazen. 2008. The lipid whisker model of the structure of oxidized cell membranes. J. Biol. Chem. 283 2385–2396. [DOI] [PubMed] [Google Scholar]
- 7.Morrow J. D., J. A. Awad, H. J. Boss, I. A. Blair, and L. J. Roberts 2nd. 1992. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA. 89 10721–10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moumtzi A., M. Trenker, K. Flicker, E. Zenzmaier, R. Saf, and A. Hermetter. 2007. Import and fate of fluorescent analogs of oxidized phospholipids in vascular smooth muscle cells. J. Lipid Res. 48 565–582. [DOI] [PubMed] [Google Scholar]
- 9.Gugiu B. G., K. Mouillesseaux, V. Duong, T. Herzog, A. Hekimian, L. Koroniak, T. M. Vondriska, and A. D. Watson. 2008. Protein targets of oxidized phospholipids in endothelial cells. J. Lipid Res. 49 510–520. [DOI] [PubMed] [Google Scholar]
- 10.Szapacs M. E., H. Y. Kim, N. A. Porter, and D. C. Liebler. 2008. Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe. J. Proteome Res. 7 4237–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berliner J. A., and N. M. Gharavi. 2008. Endothelial cell regulation by phospholipid oxidation products. Free Radic. Biol. Med. 45 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gargalovic P. S., M. Imura, B. Zhang, N. M. Gharavi, M. J. Clark, J. Pagnon, W. P. Yang, A. He, A. Truong, S. Patel, et al. 2006. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc. Natl. Acad. Sci. USA. 103 12741–12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitinger N. 2005. Oxidized phospholipids as triggers of inflammation in atherosclerosis. Mol. Nutr. Food Res. 49 1063–1071. [DOI] [PubMed] [Google Scholar]
- 14.Lee S., R. Li, B. Kim, R. Palvolgyi, T. Ho, Q. Z. Yang, J. Xu, W. L. Szeto, H. Honda, and J. A. Berliner. 2008. Ox-PAPC activation of plasma membrane electron transport (PMET) system increases expression of heme oxygenase 1 (HO-1) in human aortic endothelial cell (HAEC). J Lipid Res. 50 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouhanizadeh M., J. Hwang, R. E. Clempus, L. Marcu, B. Lassegue, A. Sevanian, and T. K. Hsiai. 2005. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: implication of NADPH oxidase. Free Radic. Biol. Med. 39 1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bochkov V. N., M. Philippova, O. Oskolkova, A. Kadl, A. Furnkranz, E. Karabeg, T. Afonyushkin, F. Gruber, J. Breuss, A. Minchenko, et al. 2006. Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ. Res. 99 900–908. [DOI] [PubMed] [Google Scholar]
- 17.Birukov K. G., V. N. Bochkov, A. A. Birukova, K. Kawkitinarong, A. Rios, A. Leitner, A. D. Verin, G. M. Bokoch, N. Leitinger, and J. G. Garcia. 2004. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ. Res. 95 892–901. [DOI] [PubMed] [Google Scholar]
- 18.Imai Y., K. Kuba, G. G. Neely, R. Yaghubian-Malhami, T. Perkmann, G. van Loo, M. Ermolaeva, R. Veldhuizen, Y. H. Leung, H. Wang, et al. 2008. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 133 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gharavi N. M., P. S. Gargalovic, I. Chang, J. A. Araujo, M. J. Clark, W. L. Szeto, A. D. Watson, A. J. Lusis, and J. A. Berliner. 2007. High-density lipoprotein modulates oxidized phospholipid signaling in human endothelial cells from proinflammatory to anti-inflammatory. Arterioscler. Thromb. Vasc. Biol. 27 1346–1353. [DOI] [PubMed] [Google Scholar]
- 20.Groeneweg M., M. N. Vergouwe, P. G. Scheffer, H. P. Vermue, M. D. Sollewijn Gelpke, A. M. Sijbers, N. Leitinger, M. H. Hofker, and M. P. de Winther. 2008. Modification of LDL with oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (oxPAPC) results in a novel form of minimally modified LDL that modulates gene expression in macrophages. Biochim. Biophys. Acta. 1781 336–343. [DOI] [PubMed] [Google Scholar]
- 21.Chou M. Y., K. Hartvigsen, L. F. Hansen, L. Fogelstrand, P. X. Shaw, A. Boullier, C. J. Binder, and J. L. Witztum. 2008. Oxidation-specific epitopes are important targets of innate immunity. J. Intern. Med. 263 479–488. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg M. E., M. Sun, R. Zhang, M. Febbraio, R. Silverstein, and S. L. Hazen. 2006. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 203 2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bochkov V. N., A. Kadl, J. Huber, F. Gruber, B. R. Binder, and N. Leitinger. 2002. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 419 77–81. [DOI] [PubMed] [Google Scholar]
- 24.Erridge C., S. Kennedy, C. M. Spickett, and D. J. Webb. 2008. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 283 24748–24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller Y. I., S. Viriyakosol, C. J. Binder, J. R. Feramisco, T. N. Kirkland, and J. L. Witztum. 2003. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 278 1561–1568. [DOI] [PubMed] [Google Scholar]
- 26.Walton K. A., A. L. Cole, M. Yeh, G. Subbanagounder, S. R. Krutzik, R. L. Modlin, R. M. Lucas, J. Nakai, E. J. Smart, D. K. Vora, et al. 2003. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler. Thromb. Vasc. Biol. 23 1197–1203. [DOI] [PubMed] [Google Scholar]
- 27.Rahaman S. O., D. J. Lennon, M. Febbraio, E. A. Podrez, S. L. Hazen, and R. L. Silverstein. 2006. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 4 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fruhwirth G. O., and A. Hermetter. 2008. Mediation of apoptosis by oxidized phospholipids. Subcell. Biochem. 49 351–367. [DOI] [PubMed] [Google Scholar]
- 29.Chen R., L. Yang, and T. M. McIntyre. 2007. Cytotoxic phospholipid oxidation products. Cell death from mitochondrial damage and the intrinsic caspase cascade. J. Biol. Chem. 282 24842–24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bluml S., S. Kirchberger, V. N. Bochkov, G. Kronke, K. Stuhlmeier, O. Majdic, G. J. Zlabinger, W. Knapp, B. R. Binder, J. Stockl, et al. 2005. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J. Immunol. 175 501–508. [DOI] [PubMed] [Google Scholar]
- 31.Angeli V., J. Llodra, J. X. Rong, K. Satoh, S. Ishii, T. Shimizu, E. A. Fisher, and G. J. Randolph. 2004. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 21 561–574. [DOI] [PubMed] [Google Scholar]
- 32.Shamshiev A. T., F. Ampenberger, B. Ernst, L. Rohrer, B. J. Marsland, and M. Kopf. 2007. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J. Exp. Med. 204 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz D., A. D. Watson, C. S. Miller, D. Montoya, M. T. Ochoa, P. A. Sieling, M. A. Gutierrez, M. Navab, S. T. Reddy, J. L. Witztum, et al. 2008. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J. Clin. Invest. 118 2917–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seyerl M., S. Bluml, S. Kirchberger, V. N. Bochkov, O. Oskolkova, O. Majdic, and J. Stockl. 2008. Oxidized phospholipids induce energy in human peripheral blood T cells. Eur. J. Immunol. 38 778–787. [DOI] [PubMed] [Google Scholar]
- 35.Heery J. M., M. Kozak, D. M. Stafforini, D. A. Jones, G. A. Zimmerman, T. M. McIntyre, and S. M. Prescott. 1995. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J. Clin. Invest. 96 2322–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pidkovka N. A., O. A. Cherepanova, T. Yoshida, M. R. Alexander, R. A. Deaton, J. A. Thomas, N. Leitinger, and G. K. Owens. 2007. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ. Res. 101 792–801. [DOI] [PubMed] [Google Scholar]
- 37.Isakson B. E., G. Kronke, A. Kadl, N. Leitinger, and B. R. Duling. 2006. Oxidized phospholipids alter vascular connexin expression, phosphorylation, and heterocellular communication. Arterioscler. Thromb. Vasc. Biol. 26 2216–2221. [DOI] [PubMed] [Google Scholar]
- 38.Kwak B. R., N. Veillard, G. Pelli, F. Mulhaupt, R. W. James, M. Chanson, and F. Mach. 2003. Reduced connexin43 expression inhibits atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Circulation. 107 1033–1039. [DOI] [PubMed] [Google Scholar]
- 39.Podrez E. A., T. V. Byzova, M. Febbraio, R. G. Salomon, Y. Ma, M. Valiyaveettil, E. Poliakov, M. Sun, P. J. Finton, B. R. Curtis, et al. 2007. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 13 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman P., S. Horkko, D. Steinberg, J. L. Witztum, and E. A. Dennis. 2002. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J. Biol. Chem. 277 7010–7020. [DOI] [PubMed] [Google Scholar]
- 41.Bergmark C., A. Dewan, A. Orsoni, E. Merki, E. R. Miller, M. J. Shin, C. J. Binder, S. Horkko, R. M. Krauss, M. J. Chapman, et al. 2008. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49 2230–2239. [DOI] [PubMed] [Google Scholar]
- 42.Choi S. H., A. Chae, E. Miller, M. Messig, F. Ntanios, A. N. DeMaria, S. E. Nissen, J. L. Witztum, and S. Tsimikas. 2008. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J. Am. Coll. Cardiol. 52 24–32. [DOI] [PubMed] [Google Scholar]
- 43.Tsimikas S., and J. L. Witztum. 2008. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr. Opin. Lipidol. 19 369–377. [DOI] [PubMed] [Google Scholar]
- 44.Tsimikas S., S. Kiechl, J. Willeit, M. Mayr, E. R. Miller, F. Kronenberg, Q. Xu, C. Bergmark, S. Weger, F. Oberhollenzer, et al. 2006. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J. Am. Coll. Cardiol. 47 2219–2228. [DOI] [PubMed] [Google Scholar]
- 45.Anuurad E., M. B. Boffa, M. L. Koschinsky, and L. Berglund. 2006. Lipoprotein(a): a unique risk factor for cardiovascular disease. Clin. Lab. Med. 26 751–772. [DOI] [PubMed] [Google Scholar]
- 46.Tsimikas S., E. S. Brilakis, E. R. Miller, J. P. McConnell, R. J. Lennon, K. S. Kornman, J. L. Witztum, and P. B. Berger. 2005. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 353 46–57. [DOI] [PubMed] [Google Scholar]
- 47.Kiechl S., J. Willeit, M. Mayr, B. Viehweider, M. Oberhollenzer, F. Kronenberg, C. J. Wiedermann, S. Oberthaler, Q. Xu, J. L. Witztum, et al. 2007. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler. Thromb. Vasc. Biol. 27 1788–1795. [DOI] [PubMed] [Google Scholar]
- 48.Tsimikas, S., Z. Mallat, P. J. Talmud, J. J. Kastelein, E. D. Warner, E. R. Miller, J. L. Witztum, K. T. Khaw, and S. M. Boekholdt. 2008. Elevated oxidized phospholipids on apolipoprotein B-100 particles are associated with future cardiovascular events in the EPIC-Norfolk Study: Potentiation of risk with lipoprotein-associated (Lp-PLA2) and secretory phospholipase A2 (sPLA2) activity. Circulation. In press.
- 49.Tsimikas S. 2008. In vivo markers of oxidative stress and therapeutic interventions. Am. J. Cardiol. 101 34D–42D. [DOI] [PubMed] [Google Scholar]