Abstract
During an epidemiological survey made in São Paulo (Brazil), fecal specimens were periodically collected from 100 randomly chosen babies from birth to the age of 18 months. The stools, routinely collected each month and also collected each time a child presented any sign of disease, were screened for the presence of adenoviruses. Sixteen adenovirus strains, isolated from the stools of healthy and ill children, were characterized by restriction enzyme analysis. Five isolates were from subgenus A, five were from subgenus B, four were from subgenus C, and two were from subgenus D. All but two showed some restriction patterns different from those of the 42 human adenovirus prototypes and all the genome types described up to now. No fastidious adenovirus (subgenus F, serotypes 40 and 41) was encountered in the stools examined. We report here the restriction enzyme analysis of isolates of subgenera B and C. The following new designation genome types are proposed: Ad3e1 (subgenus B) and Ad1d, Ad5a1, and Ad5a2 (subgenus C).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T., Best B., Wigand R. A proposal for naming adenovirus genome types, exemplified by adenovirus type 6. J Gen Virol. 1985 Dec;66(Pt 12):2685–2691. doi: 10.1099/0022-1317-66-12-2685. [DOI] [PubMed] [Google Scholar]
- Adrian T., Wadell G., Hierholzer J. C., Wigand R. DNA restriction analysis of adenovirus prototypes 1 to 41. Arch Virol. 1986;91(3-4):277–290. doi: 10.1007/BF01314287. [DOI] [PubMed] [Google Scholar]
- Arens M., Dilworth V. Remarkably homogeneous population of adenovirus type 3 and 7 genome types. J Clin Microbiol. 1988 Aug;26(8):1604–1608. doi: 10.1128/jcm.26.8.1604-1608.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. S., Richmond S. J. Genetic heterogeneity of recent isolates of adenovirus types 3, 4, and 7. J Clin Microbiol. 1986 Jul;24(1):30–35. doi: 10.1128/jcm.24.1.30-35.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Jeffries B. C., Pyles G., Christmas E. E., Reid J. L., Chanock R. M., Parrott R. H. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. II. Variation in adenovirus infections by year and season. Am J Epidemiol. 1972 Mar;95(3):218–227. doi: 10.1093/oxfordjournals.aje.a121389. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Stallings E. P., Lewis C., Miles A. J., Gardner M. K., Parrott R. H. Adenoviruses and pediatric gastroenteritis. J Infect Dis. 1985 Mar;151(3):437–443. doi: 10.1093/infdis/151.3.437. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Vargosko A. J., Jeffries B. C., Arrobio J. O., Rindge B., Parrott R. H., Chanock R. M. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am J Epidemiol. 1969 Dec;90(6):484–500. doi: 10.1093/oxfordjournals.aje.a121094. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Yolken R. H., Kapikian A. Z., Arrobio J. O., Rodriguez W. J., Wyatt R. G., Chanock R. M., Parrott R. H. Comparative epidemiology of two rotavirus serotypes and other viral agents associated with pediatric gastroenteritis. Am J Epidemiol. 1979 Sep;110(3):243–254. doi: 10.1093/oxfordjournals.aje.a112809. [DOI] [PubMed] [Google Scholar]
- Brůcková M., Wadell G., Sundell G., Syrůcek L., Kunzová L. An outbreak of respiratory disease due to a type 5 adenovirus identified as genome type 5a. Acta Virol. 1980 May;24(3):161–165. [PubMed] [Google Scholar]
- Dekker B. M., van Ormondt H. The nucleotide sequence of fragment HindIII-C of human adenovirus type 5 DNA (map positions 17.1-31.7). Gene. 1984 Jan;27(1):115–120. doi: 10.1016/0378-1119(84)90244-0. [DOI] [PubMed] [Google Scholar]
- Fife K. H., Ashley R., Corey L. Isolation and characterization of six new genome types of human adenovirus types 1 and 2. J Clin Microbiol. 1985 Jan;21(1):20–23. doi: 10.1128/jcm.21.1.20-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. P., Brandt C. D., Wassermann F. E., Hall C. E., Spigland I., Kogon A., Elveback L. R. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969 Jan;89(1):25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- GARDNER P. S., McGREGOR C. B., DICK K. Association between diarrhoea and adenovirus type 7. Br Med J. 1960 Jan 9;1(5166):91–93. doi: 10.1136/bmj.1.5166.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith N. S. A survey of enteroviruses and adenoviruses in the faeces of normal children aged 0-4 years. A report of the Public Health Laboratory Service and the Society of Medical Officers of Health. J Hyg (Lond) 1965 Dec;63(4):441–455. doi: 10.1017/s0022172400045344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Mauthe G., Joshua J., Hannan C. K. Examination of uncommon clinical isolates of human adenoviruses by restriction endonuclease analysis. J Clin Microbiol. 1985 Apr;21(4):611–616. doi: 10.1128/jcm.21.4.611-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
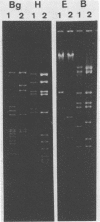
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Leite J. P., Pereira H. G., Azeredo R. S., Schatzmayr H. G. Adenoviruses in faeces of children with acute gastroenteritis in Rio de Janeiro, Brazil. J Med Virol. 1985 Feb;15(2):203–209. doi: 10.1002/jmv.1890150213. [DOI] [PubMed] [Google Scholar]
- Li Q. G., Wadell G. Analysis of 15 different genome types of adenovirus type 7 isolated on five continents. J Virol. 1986 Oct;60(1):331–335. doi: 10.1128/jvi.60.1.331-335.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. G., Wadell G. Comparison of 17 genome types of adenovirus type 3 identified among strains recovered from six continents. J Clin Microbiol. 1988 May;26(5):1009–1015. doi: 10.1128/jcm.26.5.1009-1015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffet H. L., Shulenberger H. K., Burkholder E. R. Epidemiology and etiology of severe infantile diarrhea. J Pediatr. 1968 Jan;72(1):1–14. doi: 10.1016/s0022-3476(68)80394-4. [DOI] [PubMed] [Google Scholar]
- O'Donnell B., Bell E., Payne S. B., Mautner V., Desselberger U. Genome analysis of species 3 adenoviruses isolated during summer outbreaks of conjunctivitis and pharyngoconjunctival fever in the Glasgow and London areas in 1981. J Med Virol. 1986 Mar;18(3):213–227. doi: 10.1002/jmv.1890180303. [DOI] [PubMed] [Google Scholar]
- Pereira H. G., Azeredo R. S., Leite J. P., Andrade Z. P., De Castro L. A combined enzyme immunoassay for rotavirus and adenovirus (EIARA). J Virol Methods. 1985 Jan;10(1):21–28. doi: 10.1016/0166-0934(85)90084-9. [DOI] [PubMed] [Google Scholar]
- Wadell G., Cooney M. K., da Costa Linhares A., de Silva L., Kennett M. L., Kono R., Gui-Fang R., Lindman K., Nascimento J. P., Schoub B. D. Molecular epidemiology of adenoviruses: global distribution of adenovirus 7 genome types. J Clin Microbiol. 1985 Mar;21(3):403–408. doi: 10.1128/jcm.21.3.403-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., Varsányi T. M., Lord A., Sutton R. N. Epidemic outbreaks of adenovirus 7 with special reference to the pathogenicity of adenovirus genome type 7b. Am J Epidemiol. 1980 Nov;112(5):619–628. doi: 10.1093/oxfordjournals.aje.a113034. [DOI] [PubMed] [Google Scholar]
- Wadell G., de Jong J. C. Restriction endonucleases in identification of a genome type of adenovirus 19 associated with keratoconjunctivitis. Infect Immun. 1980 Feb;27(2):292–296. doi: 10.1128/iai.27.2.292-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. H., Shields A. F., Fife K. H. Genomic variation of adenovirus type 5 isolates recovered from bone marrow transplant recipients. J Clin Microbiol. 1987 Feb;25(2):305–308. doi: 10.1128/jcm.25.2.305-308.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigand R., Adrian T., Bricout F. A new human adenovirus of subgenus D: candidate adenovirus type 42. Arch Virol. 1987;94(3-4):283–286. doi: 10.1007/BF01310720. [DOI] [PubMed] [Google Scholar]
- Yow M. D., Melnick J. L., Blattner R. J., Stephenson W. B., Robinson N. M., Burkhardt M. A. The association of viruses and bacteria with infantile diarrhea. Am J Epidemiol. 1970 Jul;92(1):33–39. doi: 10.1093/oxfordjournals.aje.a121177. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Kidd A. H., Wadell G., Kapsenberg J. G., Muzerie C. J., Wermenbol A. G., Firtzlaff R. G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]
- de Jong T. A., Wermenbol A. G., van der Avoort H. G., Wigand R. Genome type analysis of adenovirus 37 isolates. J Med Virol. 1988 May;25(1):77–83. doi: 10.1002/jmv.1890250111. [DOI] [PubMed] [Google Scholar]