Abstract
Given the multiple differences between mice and men, it was once thought that mice could not be used to model atherosclerosis, principally a human disease. Apolipoprotein E-deficient (apoEKO) mice have convincingly changed this view, and the ability to model human-like plaques in these mice has provided scientists a platform to study multiple facets of atherogenesis and to explore potential therapeutic interventions. In addition to its well-established role in lipoprotein metabolism, recent observations of reduced adiposity and improved glucose homeostasis in apoEKO mice suggest that apoE may also play a key role in energy metabolism in peripheral organs, including adipose tissue. Finally, along with apoEKO mice, knockin mice expressing human apoE isoforms in place of endogenous mouse apoE have provided insights into how quantitative and qualitative genetic alterations interact with the environment in the pathogenesis of complex human diseases.
Keywords: apolipoprotein E isoforms, diabetes, adipose tissue
Apolipoprotein E (apoE) plays a central role in lipoprotein metabolism and is required for the efficient clearance of diet-derived chylomicrons and liver-derived VLDL remnants by the liver (1). Consequently, mice lacking apoE (apoEKO) provided the first practical model of hyperlipidemia and atherosclerosis. In this review, we revisit the primary features of lipoprotein metabolism and atherosclerosis in apoEKO mice and the contributions of human apoE isoforms using the apoE knock-in mice. We then extend our discussion to more recent observations that suggest an important role for apoE in peripheral energy metabolism and consequently in metabolic syndrome (MetS) and its components, mainly obesity and diabetes.
LIPOPROTEIN METABOLISM IN APOEKO MICE
Plasma cholesterol in wild-type mice on a regular chow diet is ∼80 mg/dl, primarily carried in HDL particles. Mice have a small amount of LDL and other atherogenic lipoproteins, such as VLDL remnants. This high HDL-to-LDL ratio is maintained even when mice are fed diets similar to those consumed by humans in Western society. This is in marked contrast with humans who carry the majority of their plasma cholesterol in LDL (110 mg/dl) (2). It is well established in humans that a low ratio of HDL to LDL cholesterol confers a high risk of atherosclerosis and subsequent cardiovascular disease (3). Thus, the natural atheroprotective lipoprotein profile in mice could account for the absence of these pathologic conditions.
Despite the different plasma lipid profiles, cholesterol transport and metabolism are sufficiently similar in the two species, suggesting that inducing suitable disturbances in plasma lipoprotein metabolism would also lead to atherosclerosis in mice. Gene targeting in embryonic stem cells developed during the 1980s (4–6) opened the door to test this concept, and mice homozygous for a defective apoE gene were produced by us and independently by Plump et al. in 1992 (7–9).
Although extremely rare, humans lacking apoE are reported to have elevated remnant cholesterol in plasma (10). Similar to these individuals, apoEKO mice accumulate cholesterol-rich remnant particles with plasma cholesterol levels reaching 400 mg/dl, even when fed a regular low-fat, low-cholesterol diet.
ATHEROSCLEROSIS IN apoEKO MICE
Although atherosclerosis is not a distinguishing feature described in apoE-deficient humans (10), apoE deficiency alone proved to be sufficient for aortic atherosclerotic plaques to develop in mice. In addition, diets high in fat and cholesterol markedly accelerate plaque development in these mice. Thus, apoEKO mice, and subsequently mice lacking the LDL receptor (LDLR) that develop severe atherosclerosis on a Western-type diet (11), have demonstrated that hyperlipidemia is an essential prerequisite for the development of atherosclerotic lesions.
The lesion development and plaque composition in apoEKO mice is also similar to that in humans, establishing it as an excellent animal model for studying the pathogenesis of atherosclerosis. A small collection of foam cells that are tightly adhered to the aortic valve begin to appear in mice at about 2 months of age. With time the complexity of the lesion increases to have fibrous caps, a necrotic core of foam cell debris, cholesterol crystals, and calcifications. Large advanced plaques are often associated with the thickening of medial and adventitial tissue, accompanied by chronic inflammation. Lesions with spontaneous hemorrhage and rupture, the features associated with clinical symptoms of human atherosclerosis, have been observed in some studies of older, cholesterol-fed mice (12, 13). However, the occurrence of these events in apoEKO mice is not sufficiently reliable, leaving room for improvement in studying the plaque rupture process.
ApoEKO mice have been used extensively for several years to study the impact of various genetic and environmental risk factors on atherosclerotic susceptibility and resistance and to evaluate the effects of various therapeutic means. These studies have been comprehensively reviewed elsewhere (14).
MICE WITH HUMAN apoE ISOFORMS
While the apoEKO mouse has been established as an excellent model of atherosclerosis, the lack of apoE is extremely rare in the human population. However, apoE is polymorphic in humans, and plasma LDL cholesterol levels and atherosclerosis risk are both strongly associated with the three common apoE isoforms in the order of apoE4 > apoE3 > apoE2. This association is rather counterintuitive because apoE4 (Arg-112 and Arg-158) binds to LDLR with a slightly higher affinity than apoE3 (Cys-112 and Arg-158), while apoE2 (Cys-112 and Cys-158) binds to the receptor with much reduced affinity (15).
Unlike in humans, the plasma lipoprotein profiles in apoE knockin mice expressing the human apoE proteins in place of mouse apoE are reflective of their different LDLR affinities. Thus, mice with apoE3 and apoE4 are normolipidemic and do not develop atherosclerosis even on a Western-type diet (15). In contrast, mice with human apoE2, which binds to LDLR with less affinity, accumulate plasma remnants with high cholesterol and triglycerides (TG) and develop atherosclerosis (16).
Surprisingly, however, mice with human apoE2, E3, or E4 recapitulate the lipoprotein profiles of their human counterparts when they also express a high amount of the human LDLR and are fed a Western-type diet (17). Thus, an increased expression of LDLR in mice with human apoE4 causes an accumulation of cholesterol-rich, apoE-poor remnants in plasma, a reduction of HDL, and severe atherosclerosis. In marked contrast, the same increase in LDLR in apoE2 mice ameliorates their hyperlipidemia and diet-induced atherosclerosis. These results raise the possibility that apoE4, by binding strongly to excess LDLR, is prevented from transferring to nascent lipoproteins, a step necessary for their subsequent clearance. This in turn leads to an increase in the plasma concentration of apoE-poor remnants. Indeed, we found that primary hepatocytes from apoE4 mice secrete less apoE into the medium than hepatocytes from apoE2 mice. Increased LDLR expression leads to a localization of apoE4 on the hepatocyte surface and enhances sequestration of apoE-deficient VLDL remnants injected into apoE4 mice. However, these surface-bound VLDL were poorly internalized compared with apoE2 mice (18).
ApoE isoform-dependent changes in cholesterol uptake and efflux from macrophages have been reported (19, 20). Cholesterol delivery to macrophages in culture increases as LDLR expression increases, and the effect was more prominent in apoE4 macrophages than those with apoE3 (21). Conversely, increased LDLR expression reduces cholesterol efflux from macrophages expressing apoE4 but not apoE3 (22). Consequently, in mice with human apoE4 that lack the LDLR (LDLRKO), the replacement of bone marrow cells with cells expressing LDLR increased atherosclerosis in a dose-dependent manner compared with mice transplanted with LDLRKO cells. In contrast, atherosclerosis in LDLRKO mice expressing human apoE3 was not affected by the bone marrow with varying levels of LDLR expression (22). Although further tests are required to extrapolate these findings in mice to humans, interactions between apoE isoforms and LDLR in macrophages likely contribute to the association of apoE4 with an increased cardiovascular risk in humans.
ApoEKO MICE AND ADIPOSE TISSUE BIOLOGY
In addition to its primary site of synthesis in the liver, apoE is also synthesized in peripheral tissues, including adipose tissue (23, 24). Recent studies indicate that apoE may be a crucial player in peripheral lipid uptake and energy homeostasis and consequently in the development of MetS. MetS is a combination of several conditions, including obesity, hyperglycemia, hyperinsulinemia, dyslipidemia, hypertension, and a prothrombotic, proinflammatory state (25). Obesity is a prominent aspect of MetS, and adipose tissue is now considered to be an important regulator of energy metabolism. Expression of apoE in adipocytes decreases in response to obesity and tumor necrosis factor-α but increases with fasting and weight loss (26, 27). ApoE expression is regulated by nuclear receptors, such as liver X receptor and peroxisome proliferator-activated receptor γ (PPARγ), which is vital for adipocyte differentiation (28).
ApoEKO mice are leaner than wild-type mice (29–31). Absence of apoE also reduces body weight and some of their obesity-associated metabolic complications, including impaired glucose tolerance and insulin resistance in obese models, such as ob/ob and Ay/+ mice (30, 32, 33). Impaired delivery of liver-derived VLDL to adipocytes could partly account for the suppressed body weight gain and fat accumulation in apoEKO mice. In the adipocyte, apoE interacts with the VLDL receptor, which facilitates hydrolysis of TG by LPL (34). Indeed, mice lacking the VLDL receptor are protected from obesity (35). Chiba et al. (30) showed that wild-type VLDL, but not VLDL from apoEKO mice, induces differentiation of bone marrow stromal cells and 3T3-L1 cells into adipocytes. However, LPL inhibition did not alter the adipogenic activity of VLDL, suggesting that apoE-mediated VLDL uptake, but not hydrolysis of VLDL, plays a major role in adipogenesis.
Modulation of adiposity and tissue insulin sensitivity by adipose-derived apoE is suggested by the work of Huang et al. (36), who showed that apoE synthesized by adipocytes promotes TG uptake in culture and that the lack of endogenous apoE led to a marked defect in TG uptake from exogenous VLDL even when the VLDL contained apoE. The uptake was restored by adenoviral expression of apoE. The authors further showed that a PPARγ agonist increased apoE expression and TG accumulation in wild-type adipocytes, but the same PPARγ stimulation produced significantly less TG synthesis and TG accumulation in apoEKO adipocytes. Thus, adipose-derived apoE may play a role in intracellular lipid storage in an autocrine and/or paracrine fashion. Further studies are necessary to determine the relative roles of lipoprotein-associated, circulating apoE and apoE synthesized by adipose tissue in metabolic homeostasis.
HUMAN APOE ISOFORMS AND ADIPOSITY
Epidemiological studies have suggested that the apoE polymorphism modifies a long recognized association between increased body fat, particularly abdominal fat, and increased plasma VLDL in humans (37). For example, in the Heritage Family Study population, a pleiotropic quantitative trait locus for TG and adiposity was found on ch19q13 where APOE is located (38). ApoE isoforms were associated with body mass index in the order of apoE4 < apoE3 < apoE2 in 15,000 individuals from the Atherosclerosis Risk in Communities study (39).
Similar to their human counterparts, mice expressing human apoE3 gain more body weight and adipose tissue mass than mice with apoE4 when fed a Western-type diet. Despite being leaner, apoE4 mice begin to show impairment of glucose tolerance earlier than apoE3 mice, mainly because adipocytes expressing apoE4 fail to buffer postprandial lipids and glucose completely (40). Adenoviral expression of apoE3 in cultured apoE-null adipocytes induced mRNA expression for adiponectin in a dose-dependent manner, but the induction was significantly blunted in cells expressing apoE4. ApoE4 expression increased mRNA for Glut1, but not Glut4, in adipocytes, suggesting that apoE4 may be interfering with insulin-regulated pathways. These apoE isoform-dependent effects on body fat are a reminder that in addition to total fat mass, the functionality of fat cells is also an important determinant of disease risk.
ApoE IN DIABETES AND BEYOND
Cardiovascular incidents as a consequence of atherosclerosis are significantly increased in diabetic patients. Multiple diabetic atherosclerosis studies have employed apoEKO mice to induce diabetes with streptozotocin injection, which ablates the insulin producing β-cells in the pancreas. The resulting type 1 diabetes accelerated atherosclerosis development in apoEKO mice in association with an increase in plasma cholesterol levels (41). However, the diabetes-induced enhancement of atherosclerosis is attenuated, without a significant change in plasma lipid levels, by the administration of the soluble receptor for advanced glycation end products (42), rosiglitazone (43), or lipoic acid (44), among others. Thus, the increased oxidative stress and inflammation consequent to the high plasma glucose are major contributors to accelerated atherosclerosis in diabetes.
Type 2 diabetes is more common than type 1 diabetes in humans. In contrast with the consistently observed increase in atherosclerosis by the streptozotocin-induced type 1 diabetes, data are conflicting regarding the effects of type 2 diabetes on atherosclerosis development in apoEKO mice. For example, insulin resistance due to the lack of the insulin receptor substrate 2 gene was shown to accelerate atherosclerosis development in apoEKO mice (45). By contrast, a reduction of atherosclerosis has been reported in apoEKO mice treated with gold thioglucose to destroy the hypothalamic satiety center and to produce type 2 diabetes (46). Similarly, while leptin receptor deficiency (db/db) induces key features of type 2 diabetes in apoEKO mice, including hyperglycemia, hyperinsulinemia, dyslipidemia, and accelerated atherosclerosis (47), deficiency in the leptin receptor protects LDLRKO mice from atherosclerosis (48). Conflicting results have been reported in apoEKO mice with leptin deficiency (ob/ob): an increased atherosclerosis in chow-fed mice by Gruen et al. (49) and smaller plaques in mice fed an atherogenic diet by Chiba et al. (50). While different experimental conditions, such as diet and methods employed for atherosclerosis evaluation, may be contributing to these different outcomes, reconciliation of these differences is of great interest because hyperleptinemia and insulin resistance frequently occur together in MetS patients.
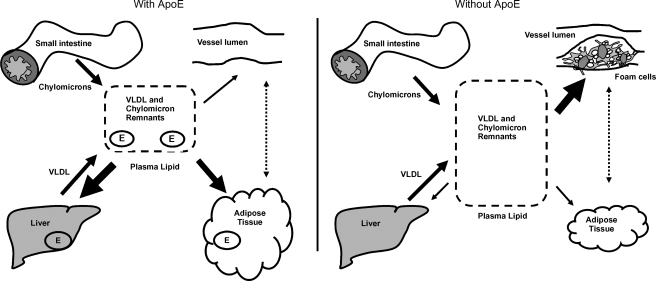
Figure 1 illustrates the metabolic roles of apoE in vivo. ApoE-mediated lipoprotein uptake in liver and adipose tissue lowers plasma lipids (left panel). By contrast, impaired lipoprotein uptake by the liver, in the absence of apoE, leads to an increase in VLDL and chylomicron remnants in the plasma and increased formation of foam cells in the vessel wall and atherosclerosis (right panel). On the other hand, apoE deficiency also reduces adiposity and improves insulin sensitivity, which may have an atheroprotective potential. In the studies of diabetic atherosclerosis, such as those described above, apoEKO mice have provided an effective sensitizer for atherosclerosis, but the possibility that apoE may also have direct roles in the pathogenesis of diabetes and its complications has not been addressed. The potential roles of apoE isoforms in MetS and the development and progression of diabetic complications highlight the future use of the apoE knockin mice in elucidating the mechanisms by which apoE exerts isoform-specific effects.
Fig. 1.
ApoE-mediated TG uptake by liver and adipose tissue contributes to maintaining normal plasma lipid levels (left panel). Impaired TG uptake in liver and adipose tissue in the absence of apoE contributes to the accumulation of VLDL and chylomicron remnants in the plasma and foam cell accumulation in the vessel wall (right panel). The arrows represent changes in lipid accumulation in different tissues in the presence and absence of apoE. ApoE is important in peripheral energy metabolism and may have an effect on plaques in addition to its established role in lipoprotein trafficking.
Our understanding of apoE in energy metabolism beyond its role in lipoprotein metabolism is still far from complete. The impairments in metabolic disorders have a potential to modulate atherosclerosis and cardiovascular disease progression. Similar to their contributions toward the understanding of lipoprotein metabolism and pathogenesis of atherosclerosis, apoEKO mice and mice with humanized apoE will be invaluable in elucidating these roles in future studies.
Acknowledgments
The authors thank Drs. J. Homeister, C.J. Edgell, S. Lord, and H. Tomita for discussion.
Abbreviations
apoE, apolipoprotein E
apoEKO, apolipoprotein E deficient
LDLR, LDL receptor
TG, triglycerides
MetS, metabolic syndrome
PPARγ, peroxisome proliferator-activated receptor γ
This work was supported by National Institutes of Health Grant HL-042630.
Published, JLR Papers in Press, December 5, 2008.
References
- 1.Mahley R. W. 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 240 622–630. [DOI] [PubMed] [Google Scholar]
- 2.Havel, R. J., and J. P. Kane. 1989. Introduction: structure and metabolism of plasma lipoproteins. In The Metabolic Basis of Inherited Disease. C. S. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle, editors. McGraw-Hill, New York. 1129–1138.
- 3.Miller N. E. 1982. Coronary atherosclerosis and plasma lipoproteins: epidemiology and pathophysiologic consideons. J. Cardiovasc. Pharmacol. 4 S190–S195. [PubMed] [Google Scholar]
- 4.Smithies O., R. G. Gregg, S. S. Boggs, M. A. Koralewski, and R. S. Kucherlapati. 1985. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 317 230–234. [DOI] [PubMed] [Google Scholar]
- 5.Thomas K. R., and M. R. Capecchi. 1987. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 51 503–512. [DOI] [PubMed] [Google Scholar]
- 6.Evans M. J., and M. H. Kauffman. 1981. Establishment in culture of pleuripotent cells from mouse embryos. Nature. 292 154–156. [DOI] [PubMed] [Google Scholar]
- 7.Piedrahita J. A., S. H. Zhang, J. R. Hagaman, P. M. Oliver, and N. Maeda. 1992. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 89 4471–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plump A. S., J. D. Smith, T. Hayek, K. Aalto-Setala, A. Walsh, J. G. Verstuyft, E. M. Rubin, and J. L. Breslow. 1992. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 71 343–353. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S. H., R. L. Reddick, J. A. Piedrahita, and N. Maeda. 1992. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 258 468–471. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer E. J., R. E. Gregg, G. Ghiselli, T. M. Forte, J. M. Ordovas, L. A. Zech, and H. B. Brewer, Jr. 1986. Familial apolipoprotein E deficiency. J. Clin. Invest. 78 1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishibashi S., M. S. Brown, J. L. Goldstein, R. D. Gerard, R. E. Hammer, and J. Herz. 1993. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld M. E., P. Polinsky, R. Virmani, K. Kauser, G. Rubanyi, and S. M. Schwartz. 2000. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler. Thromb. Vasc. Biol. 20 2587–2592. [DOI] [PubMed] [Google Scholar]
- 13.Williams H., J. L. Johnson, K. G. Carson, and C. L. Jackson. 2002. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 22 788–792. [DOI] [PubMed] [Google Scholar]
- 14.Maeda, N., R. C. Givens, and R. L. Reddick. 2007. Cardiovascular disease: mouse models of atherosclerosis. In The Mouse in Medical Research. 2nd edition. J. G. Fox, S. W. Barthold, M. T. Davisson, C. E. Newcomer, F. W. Quimby, and A. L. Smith, editors. Academic Press, Burlington, MA. 535–563.
- 15.Knouff C., M. E. Hinsdale, H. Mezdour, M. K. Altenburg, M. Watanabe, S. H. Quarfordt, P. M. Sullivan, and N. Maeda. 1999. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J. Clin. Invest. 103 1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan P. M., H. Mezdour, S. H. Quarfordt, and N. Maeda. 1998. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J. Clin. Invest. 102 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malloy S. I., M. K. Altenburg, C. Knouff, L. Lanningham-Foster, J. S. Parks, and N. Maeda. 2004. Harmful effects of increased LDLR expression in mice with human APOE*4 but not APOE*3. Arterioscler. Thromb. Vasc. Biol. 24 91–97. [DOI] [PubMed] [Google Scholar]
- 18.Altenburg M., J. Arbones-Mainar, L. Johnson, J. Wilder, and N. Maeda. 2008. Human LDL receptor enhances sequestration of ApoE4 and VLDL remnants on the surface of hepatocytes but not their internalization in mice. Arterioscler. Thromb. Vasc. Biol. 28 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullen P., A. Cignarella, B. Brennhausen, S. Mohr, G. Assmann, and A. von Eckardstein. 1998. Phenotype-dependent differences in apolipoprotein E metabolism and in cholesterol homeostasis in human monocyte-derived macrophages. J. Clin. Invest. 101 1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara M., T. Matsushima, H. Satoh, N. Iso-o, H. Noto, M. Togo, S. Kimura, Y. Hashimoto, and K. Tsukamoto. 2003. Isoform-dependent cholesterol efflux from macrophages by apolipoprotein E is modulated by cell surface proteoglycans. Arterioscler. Thromb. Vasc. Biol. 23 269–274. [DOI] [PubMed] [Google Scholar]
- 21.Lucic D., Z. H. Huang, S. Gu de, M. K. Altenburg, N. Maeda, and T. Mazzone. 2007. Regulation of macrophage apoE secretion and sterol efflux by the LDL receptor. J. Lipid Res. 48 366–372. [DOI] [PubMed] [Google Scholar]
- 22.Altenburg M., L. Johnson, J. Wilder, and N. Maeda. 2007. Apolipoprotein E4 in macrophages enhances atherogenesis in a low density lipoprotein receptor-dependent manner. J. Biol. Chem. 282 7817–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driscoll D. M., and G. S. Getz. 1984. Extrahepatic synthesis of apolipoprotein E. J. Lipid Res. 25 1368–1379. [PubMed] [Google Scholar]
- 24.Zechner R., R. Moser, T. C. Newman, S. K. Fried, and J. L. Breslow. 1991. Apolipoprotein E gene expression in mouse 3T3–L1 adipocytes and human adipose tissue and its regulation by differentiation and lipid content. J. Biol. Chem. 266 10583–10588. [PubMed] [Google Scholar]
- 25.Grundy S. M., H. B. Brewer, Jr., J. I. Cleeman, S. C. Smith, Jr., and C. Lenfant. 2004. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler. Thromb. Vasc. Biol. 24 e13–e18. [DOI] [PubMed] [Google Scholar]
- 26.Yue L., N. Rasouli, G. Ranganathan, P. A. Kern, and T. Mazzone. 2004. Divergent effects of peroxisome proliferator-activated receptor gamma agonists and tumor necrosis factor alpha on adipocyte ApoE expression. J. Biol. Chem. 279 47626–47632. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z. H., R. M. Luque, R. D. Kineman, and T. Mazzone. 2007. Nutritional regulation of adipose tissue apolipoprotein E expression. Am. J. Physiol. Endocrinol. Metab. 293 E203–E209. [DOI] [PubMed] [Google Scholar]
- 28.Rosen E. D., C. H. Hsu, X. Wang, S. Sakai, M. W. Freeman, F. J. Gonzalez, and B. M. Spiegelman. 2002. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 16 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreyer S. A., C. Vick, T. C. Lystig, P. Mystkowski, and R. C. LeBoeuf. 2002. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am. J. Physiol. Endocrinol. Metab. 282 E207–E214. [DOI] [PubMed] [Google Scholar]
- 30.Chiba T., T. Nakazawa, K. Yui, E. Kaneko, and K. Shimokado. 2003. VLDL induces adipocyte differentiation in ApoE-dependent manner. Arterioscler. Thromb. Vasc. Biol. 23 1423–1429. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann S. M., D. Perez-Tilve, T. M. Greer, B. A. Coburn, E. Grant, J. E. Basford, M. H. Tschop, and D. Y. Hui. 2008. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes. 57 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J., H. Katagiri, Y. Ishigaki, T. Yamada, T. Ogihara, J. Imai, K. Uno, Y. Hasegawa, M. Kanzaki, T. T. Yamamoto, et al. 2007. Involvement of apolipoprotein E in excess fat accumulation and insulin resistance. Diabetes. 56 24–33. [DOI] [PubMed] [Google Scholar]
- 33.Karagiannides I., R. Abdou, A. Tzortzopoulou, P. J. Voshol, and K. E. Kypreos. 2008. Apolipoprotein E predisposes to obesity and related metabolic dysfunctions in mice. FEBS J. 275 4796–4809. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi S., J. Suzuki, M. Kohno, K. Oida, T. Tamai, S. Miyabo, T. Yamamoto, and T. Nakai. 1995. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J. Biol. Chem. 270 15747–15754. [DOI] [PubMed] [Google Scholar]
- 35.Goudriaan J. R., S. M. Espirito Santo, P. J. Voshol, B. Teusink, K. W. van Dijk, B. J. van Vlijmen, J. A. Romijn, L. M. Havekes, and P. C. Rensen. 2004. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J. Lipid Res. 45 1475–1481. [DOI] [PubMed] [Google Scholar]
- 36.Huang Z. H., C. A. Reardon, and T. Mazzone. 2006. Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 55 3394–3402. [DOI] [PubMed] [Google Scholar]
- 37.Pouliot M. C., J. P. Despres, S. Moorjani, P. J. Lupien, A. Tremblay, and C. Bouchard. 1990. Apolipoprotein E polymorphism alters the association between body fatness and plasma lipoproteins in women. J. Lipid Res. 31 1023–1029. [PubMed] [Google Scholar]
- 38.Feitosa M. F., T. Rice, K. E. North, A. Kraja, T. Rankinen, A. S. Leon, J. S. Skinner, J. Blangero, C. Bouchard, and D. C. Rao. 2006. Pleiotropic QTL on chromosome 19q13 for triglycerides and adiposity: the HERITAGE Family Study. Atherosclerosis. 185 426–432. [DOI] [PubMed] [Google Scholar]
- 39.Volcik K. A., R. A. Barkley, R. G. Hutchinson, T. H. Mosley, G. Heiss, A. R. Sharrett, C. M. Ballantyne, and E. Boerwinkle. 2006. Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC study participants. Am. J. Epidemiol. 164 342–348. [DOI] [PubMed] [Google Scholar]
- 40.Arbones-Mainar J. M., L. A. Johnson, M. K. Altenburg, and N. Maeda. 2008. Differential modulation of diet-induced obesity and adipocyte functionality by human apolipoprotein E3 and E4 in mice. Int. J. Obes. (Lond.). 32 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu K. K., and Y. Huan. 2007. Diabetic atherosclerosis mouse models. Atherosclerosis. 191 241–249. [DOI] [PubMed] [Google Scholar]
- 42.Park L., K. G. Raman, K. J. Lee, Y. Lu, L. J. Ferran, Jr., W. S. Chow, D. Stern, and A. M. Schmidt. 1998. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 4 1025–1031. [DOI] [PubMed] [Google Scholar]
- 43.Calkin A. C., J. M. Forbes, C. M. Smith, M. Lassila, M. E. Cooper, K. A. Jandeleit-Dahm, and T. J. Allen. 2005. Rosiglitazone attenuates atherosclerosis in a model of insulin insufficiency independent of its metabolic effects. Arterioscler. Thromb. Vasc. Biol. 25 1903–1909. [DOI] [PubMed] [Google Scholar]
- 44.Yi X., and N. Maeda. 2006. alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 55 2238–2244. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Navarro H., M. Vila-Caballer, M. F. Pastor, A. Vinue, M. F. White, D. Burks, and V. Andres. 2007. Plasma insulin levels predict the development of atherosclerosis when IRS2 deficiency is combined with severe hypercholesterolemia in apolipoprotein E-null mice. Front. Biosci. 12 2291–2298. [DOI] [PubMed] [Google Scholar]
- 46.Lyngdorf L. G., S. Gregersen, A. Daugherty, and E. Falk. 2003. Paradoxical reduction of atherosclerosis in apoE-deficient mice with obesity-related type 2 diabetes. Cardiovasc. Res. 59 854–862. [DOI] [PubMed] [Google Scholar]
- 47.Wu K. K., T. J. Wu, J. Chin, L. J. Mitnaul, M. Hernandez, T. Q. Cai, N. Ren, M. G. Waters, S. D. Wright, and K. Cheng. 2005. Increased hypercholesterolemia and atherosclerosis in mice lacking both ApoE and leptin receptor. Atherosclerosis. 181 251–259. [DOI] [PubMed] [Google Scholar]
- 48.Taleb S., O. Herbin, H. Ait-Oufella, W. Verreth, P. Gourdy, V. Barateau, R. Merval, B. Esposito, K. Clement, P. Holvoet, et al. 2007. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27 2691–2698. [DOI] [PubMed] [Google Scholar]
- 49.Gruen M. L., V. Saraswathi, A. M. Nuotio-Antar, M. R. Plummer, K. R. Coenen, and A. H. Hasty. 2006. Plasma insulin levels predict atherosclerotic lesion burden in obese hyperlipidemic mice. Atherosclerosis. 186 54–64. [DOI] [PubMed] [Google Scholar]
- 50.Chiba T., S. Shinozaki, T. Nakazawa, A. Kawakami, M. Ai, E. Kaneko, M. Kitagawa, K. Kondo, A. Chait, and K. Shimokado. 2008. Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis. 196 68–75. [DOI] [PubMed] [Google Scholar]