Abstract
The self-assembly of irregular metallo-supramolecular hexagons and parallelograms has been achieved in a self-selective manner upon mixing 120° unsymmetrical dipyridyl ligands with 60° or 120° organoplatinum acceptors in a 1:1 ratio. The polygons have been characterized using 31P and 1H multinuclear NMR spectroscopy and electrospray ionization mass spectrometry (ESI-MS), as well as X-ray crystallography. Geometric features of the molecular subunits direct the self-selection process, which is supported by molecular force field computations.
Coordination-driven, transition metal-mediated self-assembly has become a well-established methodology in supramolecular chemistry for constructing ensembles exhibiting wide structural diversity1 and functionality2. The rational design of rigid complementary molecular subunits - electron poor metal acceptors and electron rich ligands - that takes advantage of the directional bonding approach has enabled the development of coordination-driven self-assembly, as witnessed by the myriad metallo-supramolecular polygons and polyhedra synthesized to date.3 Generally, complementary subunits utilized in this approach exhibit high symmetry and contain equivalent binding sites. Much more complicated systems and situations arise when unsymmetrical building blocks bearing different binding sites are employed. In accord with the 2nd Law of Thermodynamics, a self-assembly involving unsymmetrical subunits will likely produce a statistical mixture of various supramolecular isomers provided that no driving bias exists in the system.4
The selective self-assembly of one discrete structure from within a complex mixture that has the potential of producing multiple isomeric supramolecules can be achieved via a self-selection process provided that there exists some form(s) of molecular information encoded within complementary subunits that biases the formation of one isomer over the other(s).5 We have previously shown that self-selection can occur during the self-assembly of unsymmetrical ambidentate pyridy/carboxylate ligands with ditopic organoplatinum acceptors, wherein the driving force for self-selection rests in the preference for maximum charge separation in the supramolecular rhomboidal isomers.5a Recently, a thorough study of the self-selection of supramolecular squares clearly indicates that steric features encoded within unsymmetrical subunits can also control the fidelity of self-selection.5d
Controlling the geometric features of molecular subunits is an alternative approach for influencing self-selection during self-assembly. In accordance with the directional bonding approach to coordination-driven self-assembly, small changes in the geometry of individual molecular subunits can be used to drive self-organization phenomena.6 In recent reports, we have demonstrated that manipulation of the geometric factors (e.g. size, directionality) of rigid symmetric molecular subunits allows for the selective self-assembly of multiple discrete supramolecular polygons and polyhedra from within multicomponent supramolecular systems,6c,e,f and this approach has been generalized to include many Pt-N coordination-driven self-assembling systems.6g Herein, we present an investigation of the use of unsymmetrical bipyridyl ligands to direct the self-selection of two irregular polygons.
Rigid unsymmetrical bidentate ligands 1a,b contain two geometrically different pyridyl binding sites (meta and para) as shown in Scheme 1. According to the directional bonding model,3b unsymmetrical 120° donor ligands 1a,b can undergo a [2 + 2] and [3 + 3] self-assembly with 60° and 120° organoplatinum acceptors 2 and 3, respectively. The dissymmetry of 1, however, allows for the possibility of two isomeric assemblies to be generated for each of the self-assembly as shown in Scheme 1. The self-assembly of unsymmetrical ligands 1a,b with rigid organoplatinum acceptors 2 or 3 was carried out by mixing the donors and acceptors in a 1:1 ratio and heating at 60-65 °C for 6 h in an aqueous acetone solution (v/v 3:2), followed by KPF6 anion exchange. The resulting self-selection systems have been characterized using 31P and 1H multinuclear NMR spectroscopy, ESI mass spectrometry, and in the case of 4a X-ray crystallography (Figure 1a). The experimental results of each self-assembly indicate only one isomeric species - supramolecular parallelogram 4 or irregular hexagon 6 - is selectively generated, rather than a statistical mixture with 5 or 7.
Scheme 1.
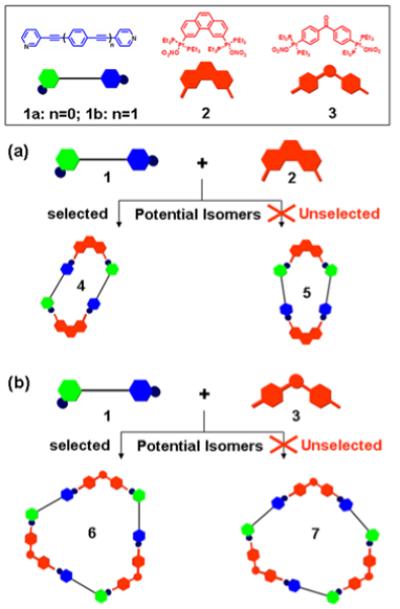
Graphical representation of the self-selection of (a) supramolecular parallelograms 4a,b and (b) irregular hexagons 6a,b from the self-assembly of unsymmetrical ligands 1a,b with 60° and 120° organoplatinum acceptors 2 or 3.
Figure 1.
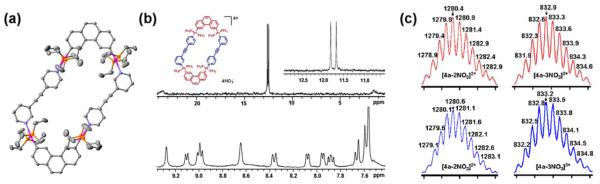
(a) ORTEP drawing (30% probability ellipsoids) of 4a; (b) 31P{1H} (top) and 1H NMR (bottom) spectra recorded for 4a; (c) Calculated (red, upper) and experimental (blue, bottom) ESI mass spectra recorded for 4a.
The 31P{1H} NMR spectra (Figure 1b, Figure 2a, and Figure S4-5 in Supporting Information) recorded for each mixture shows two singlet signals of approximately equal intensity with concomitant 195Pt satellites (δ = 12.69 ppm and 12.56 ppm for 4a; δ = 15.18 ppm and 15.35 ppm for 4b; δ = 13.81 ppm and 13.90 ppm for 6a; δ = 14.72 ppm and 14.77 ppm for 6b). The 31P NMR spectra clearly indicate that only one single isomeric structure is formed in each self-assembly and that the Pt metal centers equally coordinate the two different pyridyl binding sites of 1, as evidenced by the two different 31P NMR signals of equal intensity. Similarly, sharp identifiable proton signals attributable to the formation of highly ordered supramolecular structures are found in the 1H NMR spectra of each mixture (Figure 1b, Figure 2b, and Figure S4-5). Identification of irregular hexagons 6a,b as the selected supramolecular isomers can be further confirmed based on the symmetry of the 31P{1H} NMR spectra because four different phosphorus peaks would be expected given the varying connectivity between 1 and 3 in isomer 7. For self-assembly between 1 and 2, however, NMR spectral results are insufficient to distinguish between isomers 4 and 5.
Figure 2.
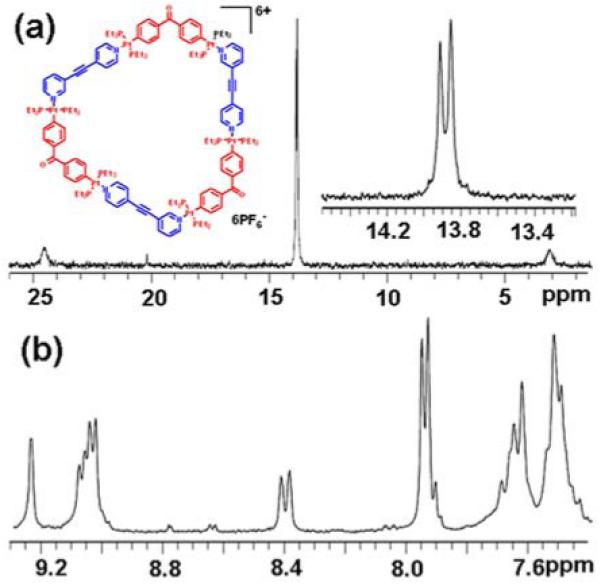
(a) 31P{1H} and (b) 1H NMR spectra recorded for 6a.
An X-ray crystallographic study has been carried out for further identification of 4a. Diffraction-quality single crystals of 4a were grown by slow vapor diffusion of pentane into a dichloromethane solution of the irregular polygon. Crystallographic data and refinement parameters are listed in Table S1 (see Supporting Information). The unambiguously established structure of isomer 4a (Figure 1a) shows that the unsymmetrical donor ligands coordinate with the 60° acceptors in their head-to-tail orientation, as opposed to the head-to-head orientation in isomer 5a (Scheme 2).
Scheme 2.
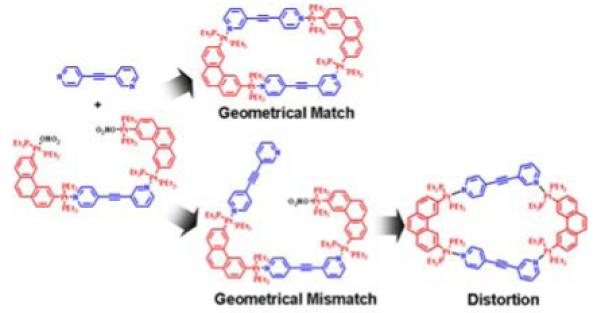
Graphical representation of geometrical match and mismatch during self-assembly of organoplatinum acceptor 2 with unsymmetrical ligand 1a in head-to-tail (top, 4a) and head-to-head (bottom, 5a) orientations.
Formation of [2 + 2] supramolecular parallelograms 4 and [3 + 3] hexagons 6 is further supported by ESI mass spectrometry as shown in Figure 1c and Figure S6 in the Supporting Information. The ESI mass peaks corresponding to the consecutive loss of nitrate anions from the supramolecular parallelograms 4a: m/z = 1280.6 [M - 2NO3]2+ and m/z = 833.2 [M - 3NO3]3+, and 4b: m/z = 1380.6 [M - 2NO3]2+ and m/z = 899.8 [M - 3NO3]3+ are observed, as are those corresponding to the formation of the irregular hexagons: 6a at m/z = 1598.3 [M - 2NO3]2+ and m/z = 948.1 [M - 4NO3]4+, and 6b at m/z = 2108.1 [M - 2NO3]2+ and m/z = 1023.1 [M - 4NO3]4+. All of these peaks are isotopically resolved and agree with their theoretical distribution.
In the systems described above, the geometric information encoded within unsymmetrical ligands 1a,b and organoplatinum acceptors 2 and 3 represent the major factor directing the selective self-assembly. For example, upon forming directional Pt-N coordination bonding interactions between subunits 1 and 2, [2 + 2] self-assembly of 4 in a head-to-tail orientation represents a geometric match, according to the directionality and rigidity of the subunits. However, the same molecular subunits have to undergo energetically unfavorable structural distortions in order to self-assemble in the head-to-head orientation of structure 5 because of the geometrical mismatch between the subunits (Scheme 2). Structural distortions result in an enthalpy increase for the formation of 5 as compared with 4. Thus, a thermodynamic bias between 4 and 5 is established that relies primarily on the geometric features of the molecular subunits. This same analysis holds for supramolecules 6 and 7 as well. The thermodynamic bias between these isomers is able to induce self-selection during self-assembly under thermodynamic control.7
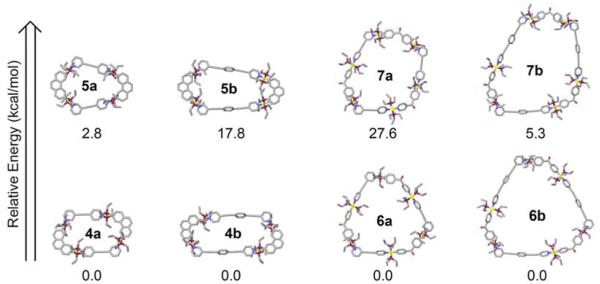
A computational study has also been carried out to investigate this postulation via direct determination of the thermodynamic energy difference between these isomers. Each individual isomer of 4-7 was built8 within the input mode of the program Maestro v9.51.09 and subjected to a 1 ns molecular dynamics simulation (MMFF force field, gas phase, 300 K) in order to equilibrate the structures. The output of each simulation was then minimized to full convergence. The MMFF computational results are given in Figure 3 and show that in each case the relative energies of the unselected species 5 and 7 are significantly greater than those of selected isomers 4 and 6 (E5a-4a = 2.8 kcal/mol; E5b-4b = 17.8 kcal/mol; E7a-6a = 27.6 kcal/mol; E7b-6b = 5.3 kcal/mol). While these calculations are performed at the MMFF molecular force field level, on account of the size of 4-7, the computational results are strongly supportive in favor of the selective self-assembly of 4 rather than 5 and 6 rather than 7.
Figure 3.
Structures and relative energies of supramolecules 4-7 as obtained from MMFF force field modeling. The lowest energy supramolecule is taken as 0.0 kcal/mol for each pair of related supramolecular isomers.
In conclusion, the self-selection of irregular supramolecular parallelograms and hexagons has been achieved during the self-assembly of unsymmetrical ligands 1a,b and organoplatinum acceptors 2 and 3. Self-selection is strongly supported by characterization from 31P and 1H multinuclear NMR spectroscopy, ESI mass spectrometry and, in one case, X-ray crystallography. The selective self-assembly of these supramolecular polygons is directed by a thermodynamic preference for closed, discrete structures that minimize distortion of the Pt-N coordination bonds in the supramolecular systems. The thermodynamic preference between these isomers has been further investigated using molecular modeling based on the MMFF force field, and the computational results support the observed experimental selectivity between each set of polygons: irregular polygons 4 and 6 are thermodynamically favored over 5 and 7.
Experimental Section
Methods and Materials
The rigid and directional organoplatinum acceptor 29 and 310 were prepared as the reported procedure. Deuterated solvents were purchased from Cambridge Isotope Laboratory (Andover, MA). NMR spectra were recorded on a Varian Unity 300 spectrometer. The 1H NMR chemical shifts are reported relative to residual solvent signals, and 31P NMR resonances are referenced to an external unlocked sample of 85% H3PO4 (δ 0.0). Mass spectra for 4a,4b, 6a, and 6b were recorded on a Micromass Quattro II triple-quadrupole mass spectrometer using electrospray ionization with a MassLynx operating system.
General Procedure for the Self-Assembly
Unsymmetrical ligand 1 (1 equiv) and organoplatinum acceptor 2 or 3 (1 equiv) were added to one glass vial. To the vial containing donors and acceptors was added 1 ml acetone aqueous solution (v/v 3:2) and the resulting suspension was stirred at 60-65 °C for 6 h, after which the clear solution had formed. The clear solution was characterized by NMR spectroscopy and ESI mass spectrometry. The NO3- counterions were exchanged for PF6- using a H2O solution of KPF6. The product was washed several times with excess H2O and the resulting solid collected for elemental analysis.
Self-Assembly of Supramolecular Parallelogram 4a
Reaction scale
Unsymmetrical ligand 1a (1.50 mg, 8.32 μmol) and 60° organoplatinum acceptor 2 (9.68 mg, 8.32 μmol). 1H NMR (Acetone-d6 / D2O 1:1, 300MHz) δ 9.28 (s, 2H, H2-3-Pyr), 9.09 (d, 2H, H6-3-Pyr), 8.97 (t, 4H, J=6.3 Hz, Hα-4-Pyr), 8.64 (s, 4H, H4,5), 8.35 (d, 2H, J=7.8 Hz, H4-3-Pyr), 7.94 (dd, 4H, J=38.1, 5.4 Hz, Hβ-4-Pyr), 7.86 (t, 2H, J=7.8 Hz, H5-3-Pyr), 7.65 (d, 4H, J=7.1 Hz, H2,7), 7.56 (m, 8H, H1,8,9,10), 1.33 (m, 48H, PCH2CH3), 1.08 (m, 72H, PCH2CH3). 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) δ 12.69 (195Pt satellites, 1JPt-P= 2709 Hz), 12.56 (195Pt satellites, 1JPt-P= 2700 Hz). MS (ESI) calcd for [M - 2NO3]2+m/z 1280.4, found 1280.6; calcd for [M - 3NO3]3+m/z 832.9, found 833.2. For 4a·4PF6 Anal Calcd C100H152F24N4P12Pt4: C, 39.79; H, 5.08; N, 1.86. Found: C, 40.14; H, 5.07; N, 1.94.
Self-Assembly of Supramolecular Parallelogram 4b
Reaction scale
Unsymmetrical ligand 1b (1.92 mg, 6.85 μmol) and 60° organoplatinum acceptor 2 (7.97 mg, 6.85 μmol). 1H NMR (Acetone-d6 / D2O 1:1, 300MHz) δ 8.94 (m, 8H, H2,6-3-Pyr, Hα-4-Pyr), 8.56 (s, 4H, H4,5), 8.22 (d, 2H, J=7.1 Hz, H4-3-Pyr), 7.80 (m, 6H, Hβ-4-Pyr, H5-3-Pyr), 7.70 (d, 8H, J=3.6 Hz, Hphenylene), 7.65 (d, 4H, J=7.1 Hz, H2,7), 7.55 (m, 8H, H1,8,9,10), 1.31 (m, 48H, PCH2CH3), 1.06 (m, 72H, PCH2CH3). 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) δ 15.35 (195Pt satellites, 1JPt-P= 2690 Hz), 15.18 (195Pt satellites, 1JPt-P= 2705 Hz). MS (ESI) calcd for [M - 2NO3]2+m/z 1380.4, found 1380.6; calcd for [M - 3NO3]3+m/z 899.6, found 899.8. For 4b·4PF6 Anal Calcd C116H160F24N4P12Pt4: C, 43.29; H, 5.01; N, 1.74. Found: C, 43.58; H, 5.02; N, 1.82.
Self-Assembly of Supramolecular Irregular Hexagon 6a
Reaction scale
Unsymmetrical ligand 1a (1.43 mg, 7.94 μmol) and 120° organoplatinum acceptor 3 (9.26 mg, 7.94 μmol). MS (ESI) calcd for [M - 2NO3]2+m/z 1958.1, found 1958.3; calcd for [M - 4NO3]4+m/z 948.1, found 948.1. For 6a·6PF6 H NMR (Acetone-d6 / D2O 8:1, 300MHz) δ 9.24 (s, 3H, H2-3-Pyr), 9.02 (m, 9H, H6-3-Pyr, Hα-4-Pyr), 8.39 (d, 3H, J=7.8 Hz, H4-3-Pyr), 7.92 (m, 9H, H5-3-Pyr, Hβ-4-Pyr), 7.64 (m, 12H, PhH), 7.50 (d, 12H, J=7.2 Hz, PhH), 1.42 (m, 72H, PCH2CH3), 1.10 (m, 108H, PCH2CH3). 31P{1H} NMR (Acetone-d6/D2O: 8/1, 121.4 MHz) δ 14.77 (195Pt satellites, 1JPt-P= 2668 Hz), 14.72 (195Pt satellites, 1JPt-P= 2650 Hz). Anal Calcd C147H228F36N6O3P18Pt6: C, 38.89; H, 5.06; N, 1.85. Found: C, 39.63; H, 5.09; N, 1.94.
Self-Assembly of Supramolecular Irregular Hexagon 6b
Reaction scale
Unsymmetrical ligand 1b (1.95 mg, 6.96 μmol) and 120° organoplatinum acceptor 3 (8.15 mg, 6.98 μmol). MS (ESI) calcd for [M - 2NO3]2+m/z 2108.2, found 2108.1; calcd for [M - 4NO3]4+m/z 1023.1, found 1023.1. For 6b·6PF6 H NMR (Acetone-d6 / D2O 8:1, 300MHz) δ 9.23 (s, 3H, H2-3-Pyr), 9.07 (d, 9H, J=5.7 Hz, H6-3-Pyr, Hα-4-Pyr), 8.36 (d, 3H,J=7.8 Hz, H4-3-Pyr), 7.93 (d, 9H, J=5.7 Hz H5-3-Pyr, Hβ-4-Pyr), 7.77 (d, 12H, J=6.0 Hz, Hphenylene), 7.69 (m, 12H, PhH), 7.57 (d, 12H, J=7.2 Hz, PhH), 1.51 (m, 72H, PCH2CH3), 1.18 (m, 108H, PCH2CH3). 31P{1H} NMR (Acetone-d6 /D2O: 8/1, 121.4 MHz) δ 14.77 (195Pt satellites, 1JPt-P= 2659 Hz), 14.72 (195Pt satellites, 1JPt-P= 2659 Hz). Anal Calcd C171H240F36N6O3P18Pt6: C, 42.44; H, 5.00; N, 1.74. Found: C, 43.12; H, 5.04; N, 1.81.
Supplementary Material
ACKNOWLEDGMENT
P.J.S. thanks the NIH (GM-057052) for financial support. B.H.N. thanks the NIH (GM-080820) for financial support. We thank Dr. Atta M. Arif for technical assistance with X-ray crystallographic study.
REFERENCES
- (1).(a) Lehn J-M, Rigault A, Siegel J, Harrowfield J, Chevrier B, Moras D. Proc. Natl. Acad. Sci. U.S.A. 1987;84:2565. doi: 10.1073/pnas.84.9.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Holliday BJ, Mirkin CA. Angew. Chem., Int. Ed. 2001;40:2022. [PubMed] [Google Scholar]; (c) Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem. Commun. 2001:509. [Google Scholar]; (d) Ruben M, Rojo J, Romero-Salguero FJ, Uppadine LH, Lehn J-M. Angew. Chem., Int. Ed. 2004;43:3644. doi: 10.1002/anie.200300636. [DOI] [PubMed] [Google Scholar]; (e) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:351. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (f) Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (g) Lukin O, Voegtle F. Angew. Chem., Int. Ed. 2005;44:1456. doi: 10.1002/anie.200460312. [DOI] [PubMed] [Google Scholar]; (h) Severin K. Chem. Commun. 2006:3859. doi: 10.1039/b606632c. [DOI] [PubMed] [Google Scholar]; (i) Nitschke JR. Acc. Chem. Res. 2007;40:103. doi: 10.1021/ar068185n. [DOI] [PubMed] [Google Scholar]; (j) Pitt MA, Johnson DW. Chem. Soc. Rev. 2007;36:1441. doi: 10.1039/b610405n. [DOI] [PubMed] [Google Scholar]; (k) Schmittel M, Mahata K. Angew. Chem., Int. Ed. 2008;47:5284. doi: 10.1002/anie.200800583. [DOI] [PubMed] [Google Scholar]
- (2).(a) Yoshizawa M, Takeyama Y, Kusukawa T, Fujita M. Angew. Chem., Int. Ed. 2002;41:1347. doi: 10.1002/1521-3773(20020415)41:8<1347::aid-anie1347>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]; (b) Würthner F, You CC, Saha-Möller CR. Chem. Soc. Rev. 2004;33:133. doi: 10.1039/b300512g. [DOI] [PubMed] [Google Scholar]; (c) Murase T, Sato S, Fujita M. Angew. Chem., Int. Ed. 2007;46:1083. doi: 10.1002/anie.200603561. [DOI] [PubMed] [Google Scholar]; (d) Balzani V, Bergamini G, Campagna S, Puntoriero F. Top. Curr. Chem. 2007;280:1. [Google Scholar]; (e) Northrop BH, Yang H-B, Stang PJ. Chem. Commun. 2008:5896. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502. [Google Scholar]; (b) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (c) Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (d) Northrop BH, Chercka D, Stang PJ. Tetrahedron. 2008;64:11495. doi: 10.1016/j.tet.2008.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Barboiu M, Petit E, van der Lee A, Vaughan G. Inorg. Chem. 2006;45:484. doi: 10.1021/ic0517220. [DOI] [PubMed] [Google Scholar]; (b) Nitschke JR. Acc. Chem. Res. 2007;40:103. doi: 10.1021/ar068185n. [DOI] [PubMed] [Google Scholar]; (c) Legrand Y-M, van der Lee A, Barboiu M. Inorg. Chem. 2007;46:9540. doi: 10.1021/ic701122a. [DOI] [PubMed] [Google Scholar]; (d) Hutin M, Cramer CJ, Gagliardi L, Shahi ARM, Bernardinelli G, Cerny R, Nitschke JR. J. Am. Chem. Soc. 2007;129:8774. doi: 10.1021/ja070320j. [DOI] [PubMed] [Google Scholar]; (e) Langner A, Tait SL, Lin N, Rajadurai C, Ruben M, Kern K. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17927. doi: 10.1073/pnas.0704882104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Rang A, Engeser M, Maier NM, Nieger M, Lindner W, Schalley CA. Chem. Eur. J. 2008;14:3855. doi: 10.1002/chem.200800113. [DOI] [PubMed] [Google Scholar]
- (5).(a) Chi K-W, Addicott C, Arif AM, Stang PJ. J. Am. Chem. Soc. 2004;126:16569. doi: 10.1021/ja045542l. [DOI] [PubMed] [Google Scholar]; (b) Chi K-W, Addicott C, Moon M-E, Lee HJ, Yoon SC, Stang PJ. J. Org. Chem. 2006;71:6662. doi: 10.1021/jo060971i. [DOI] [PubMed] [Google Scholar]; (c) Ghosh S, Turner DR, Batten SR, Mukherjee PS. Dalton Trans. 2007:1869. doi: 10.1039/b702353g. [DOI] [PubMed] [Google Scholar]; (d) Zhao L, Northrop BH, Zheng Y-R, Yang H-B, Lee HJ, Lee YM, Park JY, Chi K-W, Stang PJ. J. Org. Chem. 2008;73:6580. doi: 10.1021/jo800957r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Krämer R, Lehn J-M, Marquis-Rigault A. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5394. doi: 10.1073/pnas.90.12.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Caulder DL, Raymond KN. Angew. Chem., Int. Ed. Engl. 1997;36:1440. [Google Scholar]; (c) Addicott C, Das N, Stang PJ. Inorg. Chem. 2004;43:5335. doi: 10.1021/ic049326p. [DOI] [PubMed] [Google Scholar]; (d) Kamada T, Aratani N, Ikeda T, Shibata N, Higuchi Y, Wakamiya A, Yamaguchi S, Kim KS, Yoon ZS, Kim D, Osuka A. J. Am. Chem. Soc. 2006;128:7670. doi: 10.1021/ja0611137. [DOI] [PubMed] [Google Scholar]; (e) Yang H-B, Ghosh K, Northrop BH, Stang PJ. Org. Lett. 2007;9:1561. doi: 10.1021/ol070371l. [DOI] [PubMed] [Google Scholar]; (f) Zheng Y-R, Yang H-B, Northrop BH, Ghosh K, Stang PJ. Inorg. Chem. 2008;47:4706. doi: 10.1021/ic800038j. [DOI] [PubMed] [Google Scholar]; (g) Zheng Y-R, Yang H-B, Ghosh K, Zhao L, Stang PJ. Chem. Eur. J. Submitted. [Google Scholar]
- (7).(a) Hiraoka S, Fujita M. J. Am. Chem. Soc. 1999;121:10239. [Google Scholar]; (b) Hiraoka S, Kubota Y, Fujita M. Chem. Commun. 2000;1509 [Google Scholar]; (c) Lehn J-M. Science. 2002;295:2400. doi: 10.1126/science.1071063. [DOI] [PubMed] [Google Scholar]; (d) Lehn J-M. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4763. doi: 10.1073/pnas.072065599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yamamoto T, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:12309. doi: 10.1021/ja0302984. [DOI] [PubMed] [Google Scholar]; (f) Zheng Y-R, Stang PJ. J. Am. Chem. Soc. doi: 10.1021/ja809788x. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).The MMFF force field employed lacks quantitative parameters for square planar platinum structures. Therefore, a generic “dummy atom” was used in place of platinum and all bond lengths and angles involving the platinum dummy were constrained to fit X-ray data of analogous platinum-based supramolecular polyhedra.
- (9).Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:5193. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]
- (10).Yang H-B, Das N, Huang F, Hawkridge AM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2006;128:10014. doi: 10.1021/ja063377z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.