Abstract
A growing body of evidence indicates a role for D3 receptors in L-DOPA-induced dyskinesias. This involvement could be amenable to non-invasive in vivo analysis using functional neuroimaging. With this goal, we examined the hemodynamic response to the dopamine D3-preferring agonist 7-hydroxy-N,N-di-n-propyl-2 aminotetralin (7-OHDPAT) in naïve, parkinsonian and L-DOPA-treated, dyskinetic rodents and primates using pharmacological MRI (phMRI) and relative cerebral blood volume (rCBV) mapping. Administration of 7-OHDPAT induced minor negative changes of rCBV in the basal ganglia in naïve and parkinsonian animals. Remarkably, the hemodynamic response was reversed (increased rCBV) in the striatum of parkinsonian animals rendered dyskinetic by repeated L-DOPA treatment. Such increase in rCBV is consistent with D1 receptor-like signaling occurring in response to D3 stimulation, demonstrates a dysregulation of dopamine receptor function in dyskinesia and provides a potentially novel means for the characterization and treatment of L-DOPA-induced dyskinesia in patients.
Keywords: Dyskinesia, Parkinson’s disease, Dopamine, Dopamine receptor, D3, Striatum, phMRI, Primate
Long-term dopamine (DA) replacement therapy in Parkinson’s disease (PD) often leads to development of abnormal motor response and dyskinesia (Olanow et al., 2004) through poorly understood mechanisms. DA modulates the basal ganglia output through opposite effects on the postsynaptic DA receptors: D1 facilitation and D2-like (D2 and D3) inhibition (Beaulieu et al., 2005). Anatomically, D1 and D2-like receptors are partially segre- gated into the striatonigral (“direct”) and striatopallidal (“indirect”) projections or pathways (Gerfen et al., 1990). However, there is evidence of substantial co-localization of functional D1- and D2-like receptors on striatal medium spiny projection neurons (Aizman et al., 2000; Pollack, 2004) implying that cross-talk may occur both at circuitry and intracellular levels. DA receptors are 7 transmembrane G-protein-coupled receptors: D1 receptors are coupled to G αs/olf, increase cAMP levels and phosphorylation of DARPP-32 and proteins downstream (Bonci and Hopf, 2005). D2 are linked to Gαi, inhibit adenyl cyclases and activate G-protein-coupled inward-rectifying potassium channels (Girk) and phosphatases (Bonci and Hopf, 2005). D3 have no net effect on cAMP levels and may couple to both Gs and i proteins (Ilani et al., 2002). Notably, D1 and D2/D3 agonists induce opposite hemodynamic changes in the striatum measured by functional pharmacologic (ph)MRI; D1 agonists increase relative cerebral blood volume (rCBV) while D2 and D3 agonists decrease it (Chen et al., 2005; Choi et al., 2006). Pramipexole, a D3-preferring agonist, has been shown to reduce cerebral blood flow in cingulate and orbitofrontal areas in monkeys in a PET study (Black et al., 2002). These opposite effects correlate well with the D1-mediated facilitation and D2 gating roles on glutamate transmission in the striatum.
While D3 receptors are not highly expressed in the motor regions of the striatum (Murray et al., 1994; Sokoloff et al., 1990), there is compelling evidence from postmortem studies, of L-DOPA induction of ectopic D3 receptor expression in D1-expressing medium spiny neurons in the striatum of parkinsonian rats (Bordet et al., 1997; Bordet et al., 2000) and macaques (Bezard et al., 2003; Quik et al., 2000). Furthermore, both the presence of L-DOPA-induced dyskinesias in primates (Bezard et al., 2003) and sensitization to L-DOPA in rats (Bordet et al., 1997; Bordet et al., 2000; Guillin et al., 2003) have been correlated with changes in D3 receptor expression in postmortem analyses. In this study we examined in vivo hemodynamic changes in response to D3 activation using ph MRI and (7-hydroxy-N,N-di-n-propyl-2 aminotetralin) 7-OHDPAT, in naïve, parkinsonian and L-DOPA-treated rats and primates. 7-OHDPAT has a 10-fold higher affinity for the D3 (Missale et al., 1998; Sokoloff et al., 1990) compared to the D2 receptor.
Methods
Rodent studies
All animal studies were performed following NIH guidelines and were approved by the IACUC at McLean Hospital and Harvard Medical Area. Naïve and 6-OHDA-lesioned adult female Sprague-Dawley rats (200–250 g) were purchased from Charles River Laboratories and Taconic. The animals were unilaterally lesioned by 6-OHDA injection (4 μl; 2 μg/μl) into the medial forebrain bundle. An appropriate level of denervation was evaluated by the rotational response to apomorphine (0.05 mg/kg) and amphetamine (4 mg/kg) at least 6 weeks after the lesion as described (Ferrari et al., 2006). A group of 10 animals showing good rotational response to apomorphine, 9±1.4 contralateral turns/min for 30 min, and to amphetamine, 11.6±1.5 ipsilateral turns/min for 90 min, was selected. In this group 6 animals were treated twice a day with i.m. injections of L-DOPA methylester (12 mg/kg) and benserazide (a peripheral inhibitor of L-aromatic amino acid decarboxylase, 2.5 mg/kg) for 2 weeks and 5 of them developed abnormal involuntary movements (AIMs). The response to L-DOPA was scored in a standard acute challenge session 5–7 days after the induction phase was completed, according to Cenci et al. (1998); the average AIMs score was 33.9±1.2.
Pharmacological magnetic resonance imaging
Studies were performed in naïve (n=7) and 6-OHDA-lesioned rats (n=10) as described (Chen et al., 2005) in a 4.7-T magnet (Bruker, Billerica, MA). Briefly, the rats were anesthetized with a 1% halothane in 1:1 mixture of O2 and NO2 and positioned in a stereotaxic frame. Eight 1.5-mm-thick coronal slices were acquired using a gradient echo sequence (600/20). After baseline acquisition, animals received an i.v. injection of a superparamagnetic agent (MION, 10 mg/kg, synthesized in-house (Mandeville et al., 2001)) through the tail vein to sensitize images to cerebral blood volume (CBV) changes (Chen et al., 2001). After collecting images for 20 min, the animals received an i.v. injection of 7-OHDPAT; 1.3–3 mg/kg) and image acquisition continued for 60 to 90 min. Analyses of the changes was performed as described (Chen et al., 2001) by generating CBV maps and analyzing the changes over time (0–40 min) in the regions of interest by curve fitting. Analysis of the effect of L-DOPA on regional CBV changes across groups was performed using nonparametric analysis of variance, because the signal change did not follow a normal distribution. Imaging was performed at least 3 days after the last L-DOPA administration.
Primate studies
Twelve adult male macaques (Macaca fascicularis) were included in the study. Animals were single-housed at the New England Regional Primate Research Center. Parkinsonism was induced by systemic administration of MPTP (Jenkins et al., 2004) (n=8). Three months after the last dose of MPTP animals showed a stable parkinsonian syndrome (Jenkins et al., 2004) and corresponding decrease in locomotor activity and loss of dopamine transporter binding sites [measured by PET and 11C (2β-carbomethoxy-3β-(4-fluorophenyl) tropane) (CFT) binding potential] in the posterior putamen as described (Jenkins et al., 2004) (Table 1). Four parkinsonian animals were dosed chronically with oral L-DOPA/carbidopa (Sinemet® 100/10) for 12–15 weeks and three of them developed dyskinesias in response to L-DOPA. Dyskinesias were rated after a single i.m. administration of L-DOPA (methylester, 45 mg/kg) and benserazide (10 mg/kg) at least 1 week after the last dose. The abnormal movements were classified as chorea (rapid, random flicking movements), athetosis (sinuous, writhing distal limb movements) dystonia (sustained twisting movements resulting in abnormal posturing), myoclonus (jerky) or stereotypy (repetitive purposeless behavior) and the severity was rated from 1 to 4, based on frequency and interference with normal behavior (Pearce et al., 1995) as described for macaques by Bezard et al. (2003) (1: mild, fleeting and rare dyskinetic movements and postures, >5 in 10 min; 2: moderate, more prominent dyskinesias but not interfering with normal behavior, 5–20 in 10 min; 3: marked, frequent dyskinesias intruding on normal behavior, 21–50 in 10 min, and 4: severe, virtually continuous dyskinesias, disabling the animal and replacing normal behavior). The severity was scored independently by two observers at 30, 45, 60 and 90 min post injection, for each region (orofacial, right and left arm, right and left leg, axial) and composite scores (sum of scores) were obtained at 45 min (Table 2). Disability (interference with normal behavior) was calculated by dividing the composite score by the number of affected regions. Imaging was performed at least 3 days after the last L-DOPA administration.
Table 1.
Primate descriptive statistics
| Group | Naïve, n=4 | MPTP-PD, n=5 | MPTP-Dysk, n=3 |
|---|---|---|---|
| Age (years) | 5±0.5 | 7±0.5 | 7±0.3 |
| Weight (kg) | 6.3±0.3 | 8.6±0.8 | 6.2±0.5 |
| MPTP (total mg) | NA | 22±5 | 13±5 |
| MPTP (doses) | NA | 17±3.4 | 11±4 |
| Parkinson rating scale | 0 | 13.6±3.4 | 16±2.5 |
| Locomotor activity | 42±7.5 | 18±3 *(57%) | 23±3.8 * (45%) |
| 11C CFT BP (% loss) | 3.14±0.1 | 1.31±0.05 * (58%) | 1.42±0.1 * (55%) |
BP: binding potential. All results are mean±SEM, (%): average decrease from naive baseline.
MPTP-lesioned animals showed a significant reduction of locomotor activity compared to naive animals (ANOVA, F2,9=6.2, p<0.05, with no difference between dyskinetic and non-dyskinetic groups) and a significant loss of dopamine terminals in the posterior putamen measured by PET and the dopamine transporter ligand 11C-CFT (ANOVA, F2,9=98, p<0.0001, with no difference between dyskinetic and non-dyskinetic groups).
Table 2.
L-DOPA-induced dyskinesias in primates
| Primate | Mf1 | Mf2 | Mf3 | Mf4 |
|---|---|---|---|---|
| Daily L-DOPA (mg) | 424±16 | 482±12 | 462±12 | 437±14 |
| Time on L-DOPA (days) | 85 | 90 | 77 | 85 |
| Dyskinesia: severity | 0 | 1.85 | 3.6 | 3.5 |
| Dyskinesia: type | Stereotypic | Dyst/chorea | Dyst/chorea | Dyst/chorea |
| Dyskinesia: peak score | 2 | 9 | 18 | 14 |
| Putamen rCBV | −4.66 | 15.23 | 6.08 | −0.43 |
| Putamen corrected rCBV | 0.65 | 7.89 | 14.98 | 8.59 |
Dyskinesia was induced using high doses of L-DOPA in order to shorten the length of the induction phase (Parkinson’s disease patients usually developed dyskinesias only after several years of daily treatment). Dyskinesia was evaluated 45 min after the i.m. administration of a standard dose of L-DOPA methylester with benserazide, at least 1 week after completion of the induction phase. rCBV: relative cerebral blood volume; corrected rCBV was obtained by subtracting whole brain CBV changes to eliminate the effect of CO2 changes. Corrected rCBV values in the putamen were correlated with dyskinesia peak scores (R2=0.93, p<0.05).
Pharmacological magnetic resonance imaging
Studies were performed on a Siemens’ 3-T Trio system using an in-house built transmitter/receiver 3-in. surface coil under halothane anesthesia as described (Jenkins et al., 2004) with collection of serial gradient echo images (TR/TE 500/10 ms; FOV=120 mm, 1 mm transaxial slices, 128 × 128 matrix). After collection of 6–10 baseline images, 10–15 more images were acquired after i.v. administration of 10 mg/kg of MION (Jenkins et al., 2004). 7-OHDPAT (0.25 mg/kg) was administered i.v. and imaging continued for 40–60 min. Continuous monitoring of end tidal (Et) CO2 was made through tracheal intubation with a gas monitor (Puritan-Bennett Model 254, Pleasanton, CA) and heart rate was monitored using EKG leads (Siemens). Data analysis was performed using region of interest (ROI)-based analyses of regional changes in rCBV as described (Jenkins et al., 2004). We used the same ROIs as outlined in our previous study describing amphetamine stimulation (Jenkins et al., 2004), as those are most likely responsive to changes in DA signals. Data are presented as mean±SEM. One-way ANOVA and repeated measures ANOVA were used to examine the effect of DA lesion and L-DOPA treatment as well as physiological changes. Comparison of CBV changes between groups was performed using a nonparametric test (Kruskal–Wallis). All statistical analyses were performed using Statview software (SAS Institute Inc., Carny, NC).
Results
Effects of DA denervation and chronic L-DOPA administration on the rCBV response to 7-OHDPAT in 6-OHDA-lesioned hemiparkinsonian rats
In naïve anesthetized rats 7-OHDPAT administration induced a negative change in rCBV in the nucleus accumbens (Figs. 1a–b) (Choi et al., 2006) corresponding to the anatomical distribution of the D3 receptor (Murray et al., 1994), which is highly expressed in the ventromedial shell of the nucleus accumbens, olfactory tubercle and islands of Calleja and low in the motor striatum (Sokoloff et al., 1990). The hemodynamic changes induced by 7-OHDPAT in the nucleus accumbens were larger than the insignificant response elicited in the caudate–putamen (<5%, Fig. 1b) and returned to baseline ~60 min after the injection.
Fig. 1.
(a–b) In naive rats, 7-OHDPAT administration induced mild negative changes in regional relative cerebral blood volume (rCBV), more pronounced in limbic than in motor regions: (a) representative rCBV map in a naive rat showing the anatomical distribution of signal changes on coronal brain slices (all maps are color-coded by significance level according to the color scale bar shown on the right); (b) the hemodynamic response in the nucleus accumbens was larger than in the caudate–putamen (n=7), as predicted by the low expression of D3 receptors in the motor regions of the striatum. (c) Effect of 6-OHDA lesion on the rCBV response to 7-OHDPAT:rCBV map of a representative 6-OHDA-lesioned rat showing a small enhancement of the response, at the level of the nucleus accumbens ipsilateral to the lesion (IL). (d) Animals treated with L-DOPA showed different distribution and opposite sign of the hemodynamic response to 7-OHDPAT:rCBV map in a representative lesioned animal treated with L-DOPA. (e) Quantification of the average % signal change from 0 to 40 min in the nucleus accumbens (Acc), caudate–putamen (CP), cingulate cortex (Cin) and thalamus (Th) of hemiparkinsonian animals with and without L-DOPA chronic administration; ipsilateral rCBV changes were significantly affected by L-DOPA in the caudate–putamen (Mann–Whitney U test, P ≤ 0.01) and also in the cingulate cortex and thalamus (P ≤ 0.05); in the nucleus accumbens, two dyskinetic animals showed a negative response and the average change was not significant. (f) Time course of rCBV signal change in the caudate–putamen of 6-OHDA-lesioned animals with and without L-DOPA treatment revealed a significant effect of L-DOPA treatment, more marked on the side ipsilateral to the 6-OHDA lesion. Time 0 represents the injection time of 7-OHDPAT, approximately 20 min after the administration of an iron superparamagnetic agent (MION, 10 mg/kg) to sensitize the images to vascular changes. Acc: nucleus accumbens; CP: caudate–putamen; Cin: cingulate cortex; Th: thalamus; IL: ipsilateral to lesion (6-OHDA injection in the midbrain forebrain bundle); CL: contralateral to lesion.
Unilateral DA denervation by 6-OHDA did not induce major changes in the response to 7-OHDPAT (Fig. 1c). Ipsilateral to the side of the lesion, hemiparkinsonian animals showed a small enhancement in the magnitude of the rCBV response in the nucleus accumbens with no significant differences between sides (Fig. 1e). In contrast, in the 6-OHDA-lesioned animals chronically treated with L-DOPA, there was a rapid positive activation in response to 7-OHDPAT both in the caudate–putamen and accumbens nuclei, ipsilateral to the side of the 6-OHDA lesion (Figs. 1d–f). Analysis of the rCBV changes over time by curve fitting to a gamma-variate model demonstrated a significant effect of L-DOPA treatment (p≤0.001) on the hemodynamic response to 7-OHDPAT in the caudate–putamen (Fig. 1f). A non-parametric analysis of variance revealed a significant effect of L-DOPA in the rCBV response (0–40 min) (U test, z=−4.37, p≤0.001). Regional analysis showed significant changes in the ipsilateral caudate–putamen (p≤0.01), thalamus (p≤0.05) and cingulate cortex (p≤0.05) (Fig. 1e). These results show that the hemodynamic response to D3 DA agonist administration was altered (sensitized) by chronic L-DOPA administration in hemiparkinsonian rodents.
Effects of DA denervation and L-DOPA administration on the rCBV response to 7-OHDPAT in MPTP-lesioned parkinsonian primates
We next studied the effect of 7-OHDPAT, in naïve (n=4) and MPTP-lesioned primates (n=8) showing a stable, moderate parkinsonism, defined by a parkinsonian score of 14.5±1.9 (PRS: 0–24) and a 57% loss of dopamine transporter binding in the putamen measured by PET and the dopamine transporter tracer 11C CFT (Table 1). For induction of dyskinesias, 4 parkinsonian animals were treated repeatedly with L-DOPA (Mf1–4, Table 2) and 3 of them developed typical L-DOPA-induced dyskinesias. The dyskinesias consisted on choreiform and dystonic movements predominantly affecting limbs, tail, and orolingual muscles, peaked ~45 min after L-DOPA injection and were moderate to severe, according to interference with normal behavior (Bezard et al., 2003). There were no significant differences between the L-DOPA-treated and not treated MPTP-lesioned PD primates with respect to MPTP dose or to the severity of parkinsonism, as determined by parkinsonian score, decrease in locomotor activity and 11C CFT binding potential in the posterior putamen (ANOVA, p>0.05, Table 1).
In the anesthetized primates, 7-OHDPAT had a potent systemic physiological effect, inducing a significant decrease of EtCO2 immediately after injection, from 38±0.9 to 32.7±2 (repeated-measures ANOVA, p≤0.05) and returned to 38.8±2.2 at the end of the imaging session. There was also a significant drop in heart rate (from 121±4 to 98±6, p≤0.001) which remained slow until the end of the study (99±5; p≤0.05). These measures did not differ between groups.
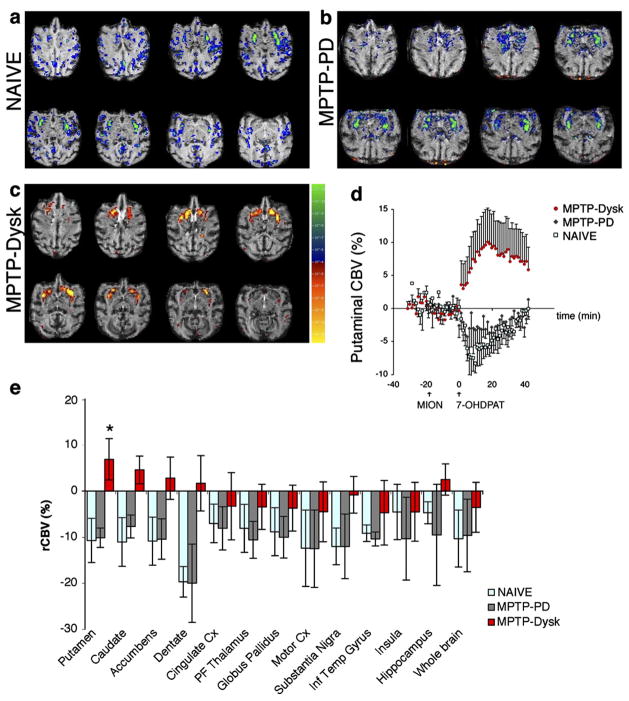
In the brain, naïve animals showed minor changes in rCBV in striatal motor regions in response to 7-OHDPAT administration and mild (0–10%) global negative hemodynamic responses that were correlated with the decrease in EtCO2 (R =0.76; p ≤ 0.01). As a group, MPTP-lesioned animals (MPTP-PD) did not differ from naïve animals. After removing the effect of CO2 by fitting the rCBV values to a general linear model using EtCO2 as a regressor (Fig. 2), both naïve and MPTP-PD showed a small decrease in rCBV in the basal ganglia (Figs. 2a–b). In contrast, the parkinsonian animals that developed dyskinesias, MPTP-Dysk, showed a selective, significant increase in rCBV in the putamen (Figs. 2c–d). This increase occurred in spite of the decrease in whole brain rCBV and the decrease in EtCO2; without using the CO2 as a regressor, the rCBV change in putamen was still positive for the dyskinetic group. ROI group analysis demonstrated significant changes between groups only in the putamen (Kruskal–Wallis, H=6.23, p ≤ 0.05) (Fig. 2e). The putaminal changes in rCBV were not correlated with whole brain CBV changes whereas changes in other brain regions examined showed a strong correlation with whole brain rCBV changes and EtCO2. After removing the contributions from whole brain CBV changes, there were significant correlations only between putamen, caudate, accumbens and dentate nucleus of the cerebellum, using Pearson product moment correlations. Putaminal changes in rCBV corrected for the effect of EtCO2 by subtracting the whole brain CBV changes (Table 2) were correlated with the severity of dyskinesia (R2=0.93, F=26.7, p≤0.05).
Fig. 2.
(a–c) Representative maps of significant hemodynamic changes (p values) in response to 7-OHDPAT are shown overlayed on axial echo gradient brain images. These maps were derived by fitting the cerebral blood volume (CBV) p values to a general linear model using the EtCO2 as a regressor to remove the effect of CO2. In the basal ganglia, a small reduction in CBV was observed in both naive (a) and MPTP-PD animals (b), without differences in signal intensity or anatomical distribution between these groups. MPTP-dyskinetic primates (c) showed a significant increase in CBV in the putamen. Color scale bar for a–c: significance (p) values for t test comparisons of CBV before and after 7-OHDPAT (increase from baseline is coded red–yellow and decrease is blue–green). (d) Time course of rCBV change in the putamen in the three groups of primates. Time 0 marks the injection time of 7-OHDPAT, approximately 10–15 min after the administration of an iron superparamagnetic agent (MION, 10 mg/kg) to sensitize the images to vascular changes and baseline collection. (e) Regional analysis of rCBV changes in response to 7-OHDPAT showed a significant increase in the putamen of L-DOPA-treated primates (Kruskal–Wallis H=6.23, P ≤ 0.05) with respect to MPTP-treated only and naive animals. Regression analysis of rCBV changes showed a lack of significant correlation between putaminal and whole brain signal change (r=0.37, n.s.), in contrast with other brain regions such as cingulate cortex (r=0.8, p ≤ 0.01), globus pallidus (r=0.73, p ≤ 0.01) and insular cortex (r=0.65, p ≤ 0.05).
Discussion
In this study we examined in vivo dynamic changes in response to 7-OHDPAT using phMRI in naïve, parkinsonian and L-DOPA- treated, dyskinetic animals. We used 2 complementary models: the unilateral acute 6-OHDA rodent model that, although not without limitations as a model for dyskinesia, is quite informative and allow us to compare this study with our previous studies mapping normal distribution of DA receptors in the rodent brain, and a bilateral primate model that closely resembles PD and the L-DOPA-related motor complications that most Parkinson patients develop during the course of the disease. Both naïve rodents and primates had a negative hemodynamic response to D3 consistent with previous studies (Black et al., 2002; Choi et al., 2006) that was only marginally affected by DA denervation. In contrast, 7-OHDPAT induced a significant increase in rCBV, i.e. a D1-like response (Choi et al., 2006; Jenkins et al., 2004), in the L-DOPA-treated, dyskinetic rodents and primates. This reversed, sensitized hemodynamic response was mainly restricted to the putamen and was independent of CO2 effects.
The D3 selectivity of 7-OHDPAT in vivo is not well characterized (Pritchard et al., 2003) and in the present study we used a relatively high dose in order to induce a measurable hemodynamic response in naïve anesthetized animals. However, we have carefully examined the selectivity of D3 and D2 preferring agonists in our recently published studies (Chen et al., 2005; Choi et al., 2006). The D2 preferring agonists quinpirole and norpropylapomorphine elicit negative rCBV changes over the entire striatum (motor and limbic); thus, using a ratio of the induced rCBV change in caudate–putamen over nucleus accumbens, the D2 preferring agonists have a ratio close to 1 while the ratio for 7-OHDPAT is 0.2–0.7 (depending upon the dose). These data clearly indicate a D3 preference of 7-OHDPAT in vivo. Moreover, in another recent study (Delfino et al., 2007), quinpirole (a D2 preferring agonist) did not increase BOLD signal, or induce sensitization – strongly arguing against a sensitization of D2 receptors in dyskinetic rats. Lastly, we have some preliminary evidence using the selective D3 antagonist SB277011 supporting D3 involvement in the rCBV change observed in the striatum of dyskinetic animals (Sanchez-Pernaute and Jenkins, unpublished observation) as the aberrant activation reported here was suppressed on the ipsilateral side by the D3 antagonist, while 6-OHDA-lesioned rats showed bilateral medial activation similar to what we have shown previously with eticlopride, a D3/D2 antagonist (Chen et al., 2005).
Sensitization of the inhibitory response to DA involving D3 receptors has been proposed to enhance D1 receptor signaling (Bordet et al., 1997; Bordet et al., 2000) and thus facilitate the activation of the striatonigral (direct) pathway. Several recent studies (Aubert et al., 2005; Picconi et al., 2003; Tong et al., 2004) have indicated a preferential (or unbalanced) activation of the direct (D1) pathways in dyskinesia in patients and animal models, as shown by an increase in adenyl cyclase activity in the striatum of parkinsonian patients (Tong et al., 2004), increased D1 sensitivity and phosphorylation of proteins downstream the D1 receptor in MPTP-treated dyskinetic primates (Aubert et al., 2005). D3 and D1 can have opposite or synergistic effects (Schwartz et al., 1998). It has been proposed that tolerance at the D3 receptor underlies motor sensitization to indirect DA agonists, including transporter blockers (Richtand et al., 2003). G-protein-coupled receptors are typically regulated at multiple levels (receptor affinity, coupling, phosphorylation, regulators of G-protein signaling proteins, receptor internalization and degradation, clustering of adaptor and scaffolding proteins) (Pierce et al., 2002). Because DA has a higher affinity for D3 than for D2 receptor, adaptive changes may occur faster at the D3 DA receptor (Richtand et al., 2003). In our study, dyskinetic animals showed a D1-like pattern (Choi et al., 2006; Jenkins et al., 2004) of phMRI activation in response to a D3-preferring agonist, suggesting that either directly, by abnormal G-coupling, or indirectly (i.e. by release of inhibition) sensitized D3 signaling involves downstream activation of D1 transduction pathways.
The observation of a complete reversal of the CBV changes after administration of 7-OHDPAT to the dyskinetic animals compared to the non-dyskinetic parkinsonian animals raises a number of interesting questions with regards to the mechanism underlying CBV changes. We previously showed that D1 agonists produce large positive CBV changes in the striatum, while D2/D3 agonists (norpropylapomorphine, quinpirole and 7-OHDPAT) produce negative CBV changes in rats (Chen et al., 2005; Choi et al., 2006). Using BOLD a recent study in dyskinetic rodents has reported similar positive changes with D1 (or non-selective) agonists (Delfino et al., 2007) while D2 did not produce positive BOLD changes. Thus, since the CBV change observed in the dyskinetic animals is positive it is unlikely due to lack of specificity of the 7-OHDPAT for D3 over D2 receptors as the D2 receptor activation should produce a negative CBV change. In contrast, if the post-synaptic D3 signaling in the putamen occurs through activation of the D1 pathway as discussed above, an increase in rCBV, as observed, may result. The fact that this increase occurred in spite of the decrease in EtCO2 means that the increase in CBV was quite strong, as we have observed for D1 agonists (Choi et al., 2006). These results are consistent with a number of studies showing that regulation of CBV and CBF consequent to neuronal activity (neurovascular coupling) is associated with post-synaptic signaling, not energy deficits (Attwell and Iadecola, 2002). Recent studies point to a key role of astrocytes in the regulation of the microvasculature, via release of vasoactive substances, such as prostaglandins (Takano et al., 2006), triggered by calcium waves after purinergic or glutamatergic stimuli (Mulligan and MacVicar, 2004). We cannot rule out a direct effect of 7-OHDPAT on astrocytes, as we recently showed that astrocytes express D3 receptors (Choi et al., 2006), a fact also demonstrated by Miyazaki et al. (2004), and could therefore contribute to the hemodynamic response. However, while this possibility highlights how complicated is the interpretation of hemodynamic events related to neuronal activity, it does not lessen the relevance of D3 sensitization in dyskinesia.
In this study, one of the monkeys did not develop dyskinesia after L-DOPA and did not show an increase in putaminal CBV changes further suggesting that the observed hemodynamic changes are linked to dyskinesia not to L-DOPA treatment itself. In naïve rhesus monkeys, acute administration of L-DOPA methylester (300 mg, i.m.) failed to induce fMRI changes in the forebrain (Chen et al., 1999). Although our imaging technique and experimental paradigm are different, a wealth of experimental and clinical data support the notion that in normal individuals with intact buffering capacity (uptake and storage) for exogenous L-DOPA, administration of L-DOPA does not alter the levels of DA or availability of receptors, thus being highly unlikely to modify the response to 7-OHDPAT or other DA agonists. For example, experimental studies using microdialysis have shown that the extracellular DA concentration in the intact striatum is not greatly (and only briefly) augmented by L-DOPA administration (Abercrombie et al., 1990; Miller and Abercrombie, 1999; Sanchez-Pernaute et al., 2001) in the rodent. In addition, chronic administration of L-DOPA does not affect DA receptor density or affinity in the normal striatum (Schneider et al., 1984) (Rouillard et al., 1987). On the other hand, in Parkinson patients, DA increase after L-DOPA administration measured by raclopride displacement and PET is larger in patients with more severe disease (Pavese et al., 2006) – probably related to failure to transport and store the neurotransmitter – and dyskinesias are correlated with larger increases in DA. Finally, Bezard et al. (2003) showed that postmortem changes in D3 receptor expression in putamen and internal segment of the globus pallidus (GPi) were associated with dyskinesia, and not with L-DOPA per se.
Our results emphasize that repeated L-DOPA administration in a system lacking proper uptake and storage presynaptic capacity can lead to dysregulation of synaptic DA (Olanow et al., 2004) (de la Fuente-Fernandez et al., 2004) and persistent postsynaptic receptor changes, and support a role of D3 receptor sensitization in the pathophysiology of dyskinesias. Further, and more importantly, this study provides a methodology for mapping the L-DOPA-induced dyskinesias that is readily translatable to human studies and may lead to novel or improved therapeutic approaches.
Acknowledgments
We are grateful to Jack McDowell and Jennifer Pagel for excellent technical help and to Dr Anna Liisa Brownell and staff in her lab for the PET studies. This work was supported by the NINDS P50 NS-39793 and N.E.R.P.R.C. Center Grant P51RR00168.
References
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-DOPA on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525 (1):36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3 (3):226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25 (12):621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Aubert I, Guigoni C, Hakansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE, Fisone G, Bloch B, Bezard E. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57 (1):17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122 (2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med. 2003;9 (6):762–767. doi: 10.1038/nm875. [DOI] [PubMed] [Google Scholar]
- Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, Perlmutter JS. A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci USA. 2002;99 (26):17113–17118. doi: 10.1073/pnas.012260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Hopf FW. The dopamine D2 receptor: new surprises from an old friend. Neuron. 2005;47 (3):335–338. doi: 10.1016/j.neuron.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci USA. 1997;94 (7):3363–3367. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Schwartz JC, Sokoloff P. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 2000;12 (6):2117–2123. doi: 10.1046/j.1460-9568.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10 (8):2694–2706. [PubMed] [Google Scholar]
- Chen Q, Andersen AH, Zhang Z, Ovadia A, Cass WA, Gash DM, Avison MJ. Functional MRI of basal ganglia responsiveness to levodopa in parkinsonian rhesus monkeys. Exp Neurol. 1999;158 (1):63–75. doi: 10.1006/exnr.1999.7089. [DOI] [PubMed] [Google Scholar]
- Chen YC, Mandeville JB, Nguyen TV, Talele A, Cavagna F, Jenkins BG. Improved mapping of pharmacologically induced neuronal activation using the IRON technique with superparamagnetic blood pool agents. J Magn Reson Imaging. 2001;14 (5):517–524. doi: 10.1002/jmri.1215. [DOI] [PubMed] [Google Scholar]
- Chen YC, Choi JK, Andersen SL, Rosen BR, Jenkins BG. Mapping dopamine D2/D3 receptor function using pharmacological magnetic resonance imaging. Psychopharmacology (Berl) 2005;180 (4):705–715. doi: 10.1007/s00213-004-2034-0. [DOI] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuro-Image. 2006;30 (3):700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Sossi V, Huang Z, Furtado S, Lu JQ, Calne DB, Ruth TJ, Stoessl AJ. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain. 2004;127 (Pt 12):2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- Delfino M, Kalisch R, Czisch M, Larramendy C, Ricatti J, Taravini IR, Trenkwalder C, Murer MG, Auer DP, Gershanik OS. Mapping the effects of three dopamine agonists with different dyskinetogenic potential and receptor selectivity using pharmacological functional magnetic resonance imaging. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301329. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Sanchez-Pernaute R, Lee H, Studer L, Isacson O. Transplanted dopamine neurons derived from primate ES cells preferentially innervate DARPP-32 striatal progenitors within the graft. Eur J Neurosci. 2006;24 (7):1885–1896. doi: 10.1111/j.1460-9568.2006.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250 (4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Guillin O, Griffon N, Bezard E, Leriche L, Diaz J, Gross C, Sokoloff P. Brain-derived neurotrophic factor controls dopamine D3 receptor expression: therapeutic implications in Parkinson’s disease. Eur J Pharmacol. 2003;480 (1–3):89–95. doi: 10.1016/j.ejphar.2003.08.096. [DOI] [PubMed] [Google Scholar]
- Ilani T, Fishburn CS, Levavi-Sivan B, Carmon S, Raveh L, Fuchs S. Coupling of dopamine receptors to G proteins: studies with chimeric D2/D3 dopamine receptors. Cell Mol Neurobiol. 2002;22 (1):47–56. doi: 10.1023/a:1015341712166. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Sanchez-Pernaute R, Brownell AL, Chen YC, Isacson O. Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. J Neurosci. 2004;24 (43):9553–9560. doi: 10.1523/JNEUROSCI.1558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, Jenkins BG, Kosofsky BE, Moskowitz MA, Rosen BR, Marota JJ. Regional sensitivity and coupling of BOLD and CBV changes during stimulation of rat brain. Magn Reson Med. 2001;45 (3):443–447. doi: 10.1002/1522-2594(200103)45:3<443::aid-mrm1058>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Miller DW, Abercrombie ED. Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous L-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J Neurochem. 1999;72 (4):1516–1522. doi: 10.1046/j.1471-4159.1999.721516.x. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78 (1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res. 2004;1029 (1):120–123. doi: 10.1016/j.brainres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte end-feet cause cerebrovascular constrictions. Nature. 2004;431 (7005):195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA. 1994;91 (23):11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonuccelli U, Damier P, De Yebenes J, Gershanik O, Guttman M, Grandas F, Hallett M, Hornykiewicz O, Jenner P, Katzenschlager R, Langston WJ, LeWitt P, Melamed E, Mena MA, Michel PP, Mytilineou C, Obeso JA, Poewe W, Quinn N, Raisman-Vozari R, Rajput AH, Rascol O, Sampaio C, Stocchi F. Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord. 2004;19 (9):997–1005. doi: 10.1002/mds.20243. [DOI] [PubMed] [Google Scholar]
- Pavese N, Evans AH, Tai YF, Hotton G, Brooks DJ, Lees AJ, Piccini P. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology. 2006;67 (9):1612–1617. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- Pearce RK, Jackson M, Smith L, Jenner P, Marsden CD. Chronic L-DOPA administration induces dyskinesias in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated common marmoset (Callithrix jacchus) Mov Disord. 1995;10 (6):731–740. doi: 10.1002/mds.870100606. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6 (5):501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev, Mol Cell Biol. 2002;3 (9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Pollack A. Coactivation of D1 and D2 dopamine receptors: in marriage, a case of his, hers, and theirs. Sci STKE. 2004;255:pe50. doi: 10.1126/stke.2552004pe50. [DOI] [PubMed] [Google Scholar]
- Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J, Berger SP, Richtand NM. 7-OH-DPAT and PD 128907 selectively activate the D3 dopamine receptor in a novel environment. Neuropsychopharmacology. 2003;28 (1):100–107. doi: 10.1038/sj.npp.1300018. [DOI] [PubMed] [Google Scholar]
- Quik M, Police S, He L, Di Monte DA, Langston JW. Expression of D(3) receptor messenger RNA and binding sites in monkey striatum and substantia nigra after nigrostriatal degeneration: effect of levodopa treatment. Neuroscience. 2000;98 (2):263–273. doi: 10.1016/s0306-4522(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Welge JA, Levant B, Logue AD, Hayes S, Pritchard LM, Geracioti TD, Coolen LM, Berger SP. Altered behavioral response to dopamine D3 receptor agonists 7-OH-DPAT and PD 128907 following repetitive amphetamine administration. Neuropsychopharmacology. 2003;28 (8):1422–1432. doi: 10.1038/sj.npp.1300182. [DOI] [PubMed] [Google Scholar]
- Rouillard C, Bedard PJ, Falardeau P, Dipaolo T. Behavioral and biochemical evidence for a different effect of repeated administration of L-DOPA and bromocriptine on denervated versus non-denervated striatal dopamine receptors. Neuropharmacology. 1987;26 (11):1601–1606. doi: 10.1016/0028-3908(87)90008-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Harvey-White J, Cunningham J, Bankiewicz KS. Functional effect of adeno-associated virus mediated gene transfer of aromatic L-amino acid decarboxylase into the striatum of 6-OHDA-lesioned rats. Mol Ther. 2001;4 (4):324–330. doi: 10.1006/mthe.2001.0466. [DOI] [PubMed] [Google Scholar]
- Schneider MB, Murrin LC, Pfeiffer RF, Deupree JD. Dopamine receptors: effects of chronic L-DOPA and bromocriptine treatment in an animal model of Parkinson’s disease. Clin Neuropharmacol. 1984;7 (3):247–257. [PubMed] [Google Scholar]
- Schwartz JC, Ridray S, Bordet R, Diaz J, Sokoloff P. D1/D3 receptor relationships in brain coexpression, coactivation, and coregulation. Adv Pharmacol. 1998;42:408–411. doi: 10.1016/s1054-3589(08)60775-9. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347 (6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9 (2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tong J, Fitzmaurice PS, Ang LC, Furukawa Y, Guttman M, Kish SJ. Brain dopamine-stimulated adenylyl cyclase activity in Parkinson’s disease, multiple system atrophy, and progressive supra-nuclear palsy. Ann Neurol. 2004;55 (1):125–129. doi: 10.1002/ana.10814. [DOI] [PubMed] [Google Scholar]