Abstract
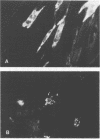
The use of fluorescein-conjugated Helix pomatia lectin was shown to be as effective as fluorescein-conjugated monoclonal antibody reagents for the detection and differentiation of herpes simplex virus type 1 and type 2 (HSV-1 and HSV-2) in MRC-5 cell culture. Cells infected with HSV-1 generally displayed a pattern of nongranular or diffuse fluorescence, while cells infected with HSV-2 were identified by the production of fluorescent grains and flecks. This unique nonimmunological reagent, when used in combination with low-speed centrifugation, provides a remarkably specific, sensitive, rapid, and cost-effective means to detect HSV-infected MRC-5 or BHK-21 cells as early as 20 h postinoculation. In contrast to the immunofluorescence method, the serotypes of HSV can be differentiated with only one fluorescein-H. pomatia reagent in MRC-5 cell cultures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arens M. Q., Swierkosz E. M. Simplified method for typing herpes simplex virus by restriction endonuclease analysis. J Clin Microbiol. 1983 Mar;17(3):548–551. doi: 10.1128/jcm.17.3.548-551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R., Keller K. Lectins in diagnostic microbiology. Eur J Clin Microbiol. 1984 Feb;3(1):4–9. doi: 10.1007/BF02032806. [DOI] [PubMed] [Google Scholar]
- Gleaves C. A., Wilson D. J., Wold A. D., Smith T. F. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J Clin Microbiol. 1985 Jan;21(1):29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., Corey L., McDougall J. K., Tolentino E., Nowinski R. C. Monoclonal antibodies to herpes simplex viruses: use in antigenic typing and rapid diagnosis. J Infect Dis. 1983 May;147(5):829–837. doi: 10.1093/infdis/147.5.829. [DOI] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Becht H., Rott R. Reaction of viruses and virus-infected cells with heterophile agglutinins. Ann N Y Acad Sci. 1974;234(0):355–368. doi: 10.1111/j.1749-6632.1974.tb53048.x. [DOI] [PubMed] [Google Scholar]
- Köhler W., Prokop O., Kühnemund O. Routine identification of group-C streptococci by means of an agglutinin (protectin) from the albumen gland of the edible snail, Helix pomatia. J Med Microbiol. 1973 Feb;6(1):127–130. doi: 10.1099/00222615-6-1-127. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Chatterjee S., Whitley R. J., Roizman B. Identification of a herpes simplex virus 1 glycoprotein gene within a gene cluster dispensable for growth in cell culture. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4303–4307. doi: 10.1073/pnas.84.12.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström M., Olofsson S., Jeansson S., Lycke E., Datema R., Månsson J. E. Host cell-induced differences in O-glycosylation of herpes simplex virus gC-1. I. Structures of nonsialylated HPA- and PNA-binding carbohydrates. Virology. 1987 Dec;161(2):385–394. doi: 10.1016/0042-6822(87)90131-0. [DOI] [PubMed] [Google Scholar]
- Malkinson M., Orgad U., Becker Y. Use of lectins to detect and differentiate subtypes of Marek's disease virus and turkey herpesvirus glycoproteins in tissue culture. J Virol Methods. 1986 May;13(2):129–133. doi: 10.1016/0166-0934(86)90080-7. [DOI] [PubMed] [Google Scholar]
- Michalski F. J., Shaikh M., Sahraie F., Desai S., Verano L., Vallabhaneni J. Enzyme-linked immunosorbent assay spin amplification technique for herpes simplex virus antigen detection. J Clin Microbiol. 1986 Aug;24(2):310–311. doi: 10.1128/jcm.24.2.310-311.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B. Immunochemistry of herpes simplex virus glycoproteins. Curr Top Microbiol Immunol. 1980;90:67–106. doi: 10.1007/978-3-642-67717-5_4. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Cromeans T. L., Nahmias A. J. Participation of three major glycoprotein antigens of herpes simplex virus type 1 early in the infectious cycle as determined by antibody-dependent cell-mediated cytotoxicity. Infect Immun. 1980 Apr;28(1):38–44. doi: 10.1128/iai.28.1.38-44.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Jeansson S., Lycke E. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J Virol. 1981 May;38(2):564–570. doi: 10.1128/jvi.38.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Lundström M., Jeansson S., Lycke E. Different populations of herpes simplex virus glycoprotein C discriminated by the carbohydrate-binding characteristics of N-acetylgalactosamine specific lectins (soybean and Helix pomatia). Brief report. Arch Virol. 1985;86(1-2):121–128. doi: 10.1007/BF01314118. [DOI] [PubMed] [Google Scholar]
- Olofsson S., Lundström M., Marsden H., Jeansson S., Vahlne A. Characterization of a herpes simplex virus type 2-specified glycoprotein with affinity for N-acetylgalactosamine-specific lectins and its identification as g92K or gG. J Gen Virol. 1986 Apr;67(Pt 4):737–744. doi: 10.1099/0022-1317-67-4-737. [DOI] [PubMed] [Google Scholar]
- Olofsson S., Norrild B., Andersen A. B., Pereira L., Jeansson S., Lycke E. Populations of herpes simplex virus glycoprotein gC with and without affinity for the N-acetyl-galactosamine specific lectin of Helix pomatia. Arch Virol. 1983;76(1):25–38. doi: 10.1007/BF01315701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Sjöblom I., Lundström M., Jeansson S., Lycke E. Glycoprotein C of herpes simplex virus type 1: characterization of O-linked oligosaccharides. J Gen Virol. 1983 Dec;64(Pt 12):2735–2747. doi: 10.1099/0022-1317-64-12-2735. [DOI] [PubMed] [Google Scholar]
- PITAL A., JANOWITZ S. L. ENHANCEMENT OF STAINING INTENSITY IN THE FLUORESCENT-ANTIBODY REACTION. J Bacteriol. 1963 Oct;86:888–889. doi: 10.1128/jb.86.4.888-889.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucina J., Bystrická M., Matis J., Lesso J. Synthesis of glycoproteins of syncytial and non-syncytial strains of herpes simplex virus type 1 and their transport to the plasma membrane. Acta Virol. 1985 May;29(3):177–184. [PubMed] [Google Scholar]
- Respess R. A., Pancake B. A., Edson C. M., Schaffer P. A. A rapid procedure for the enrichment of undenaturated, antigenically active herpes simplex virus glycoproteins. J Virol Methods. 1984 Feb;8(1-2):27–45. doi: 10.1016/0166-0934(84)90038-7. [DOI] [PubMed] [Google Scholar]
- Roizman B., Norrild B., Chan C., Pereira L. Identification and preliminary mapping with monoclonal antibodies of a herpes simplex virus 2 glycoprotein lacking a known type 1 counterpart. Virology. 1984 Feb;133(1):242–247. doi: 10.1016/0042-6822(84)90447-1. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon V. C., Turner R. B., Speranza M. J., Overall J. C., Jr Rapid detection of herpes simplex virus in clinical specimens by centrifugation and immunoperoxidase staining. J Clin Microbiol. 1986 Apr;23(4):683–686. doi: 10.1128/jcm.23.4.683-686.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Slifkin M., Cumbie R. Identification of group B streptococcal antigen with lectin-bound polystyrene particles. J Clin Microbiol. 1987 Jul;25(7):1172–1175. doi: 10.1128/jcm.25.7.1172-1175.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifkin M., Gil G. M. Identification of group C streptococcal antigen extracts with lectin-bound polystyrene particles. J Clin Microbiol. 1984 Jan;19(1):83–84. doi: 10.1128/jcm.19.1.83-84.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifkin M., Gil G. M. Rapid biochemical tests for the identification of groups A, B, C, F, and G streptococci from throat cultures. J Clin Microbiol. 1983 Jul;18(1):29–32. doi: 10.1128/jcm.18.1.29-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm B., Olofsson S., Jeansson S., Vahlne A., Lycke E. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with Helix pomatia lectin-purified antigens. J Clin Microbiol. 1984 Feb;19(2):235–239. doi: 10.1128/jcm.19.2.235-239.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vretblad P., Hjorth R., Lås T. The isolectins of Helix pomatia. Separation by isoelectric focusing and preliminary characterization. Biochim Biophys Acta. 1979 Jul 25;579(1):52–61. doi: 10.1016/0005-2795(79)90086-2. [DOI] [PubMed] [Google Scholar]
- Wenske E. A., Courtney R. J. Glycosylation of herpes simplex virus type 1 gC in the presence of tunicamycin. J Virol. 1983 Apr;46(1):297–301. doi: 10.1128/jvi.46.1.297-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z. M., Mayo D. R., Hsiung G. D. Comparison of biological, biochemical, immunological, and immunochemical techniques for typing herpes simplex virus isolates. J Clin Microbiol. 1983 Mar;17(3):396–399. doi: 10.1128/jcm.17.3.396-399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler T., Waris M., Rautiainen M., Arstila P. Herpes simplex virus detection by macroscopic reading after overnight incubation and immunoperoxidase staining. J Clin Microbiol. 1988 Oct;26(10):2013–2017. doi: 10.1128/jcm.26.10.2013-2017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]