Abstract
Background
In vitro and clinical observations in HIV-infected patients receiving interferon α therapy have shown a reduction in HIV loads. Limited investigations have explored the innate or adaptive immune responses of IFN-α on SIV replication in vivo.
Methods
Seven chronically infected rhesus macaques were given pegylated IFN-α2a (n = four) or saline (n = three) injections once weekly for 14 weeks. Weekly periphera blood samples were taken for safety parameters, viral load determinations, and measurement of innate and adaptive immune responses.
Results
Pharmacokinetic measurements demonstrated therapeutic peg-IFN-α levels for the initial period of therapy and IFN-α inducible antiviral molecules were increased sporadically in the PBMC mRNA of the treatment group. Despite the demonstrable effect of the IFN-α injections, the treatment had no effect on plasma viral RNA levels.
Conclusions
This work demonstrates that while short term IFN-α therapy induces innate antiviral immunity, it does not dramatically enhance or suppress viral replication. However, studies in the SIV model to determine therapeutic potential of chronic IFN-α therapy for the treatment of HIV will require macaque specific cytokines.
Keywords: innate immune responses, interferon inducible molecules, macaque, pegylated interferon-α2a, simian immunodeficiency virus (SIV), Type I interferons
Background
Interferon (IFN)-α is produced by many cell types, but redominantly by plasmacytoid dendritic cells, after exposure to viruses, bacteria, and/or foreign eukaryotic, tumor, or virus-infected cells [5]. Precise molecular mechanisms for the antiviral, antiproliferative, and immunomodulatory effects of IFN are not completely understood. The mechanisms by which IFNs exert their antiviral effects have been studied most thoroughly [15]. Studies of both RNA and DNA viruses indicate that the inhibition of translation and virion assembly appear to be the principal modes of the antiviral effects of interferons. Some of the earliest cytokines induced in viral infections are α/β interferons. Their antiviral effect function is mediated by several IFN-inducible genes that interfere with viral RNA (vRNA) and protein synthesis [17].
Interferons have potent and diverse immunoregulatory effects, which include induction of other cytokines, activation of macrophages and dendritic cells, augmentation of natural killer (NK) cytotoxicity, antibody-dependent cellular cytotoxicity, or T-cell cytotoxicity, and alterations of cell trafficking [3, 4, 7, 13, 14, 17]. Limited data are available regarding monitoring immune responses following administration of therapeutic IFN-α to humans. New insights into IFN-α effects on NK-cell regulation and dendritic-cell maturation suggest intermediary cellular interactions as well as inducing a TH1-type cytokine response locally [5, 16]. Indeed, studies in rhesus macaques demonstrate that IFN may protect the animals against vaccinia challenge even when the virus is resistant to IFN in vitro, suggesting that immune modulation may predominate in controlling viral infections even when its antiviral mechanisms are not effective [19].
Extensive in vitro experiments examining the effect of IFN-α on HIV replication have been performed [12]. IFN-α inhibits both early HIV replication and integration as well as late-stage assembly and packaging of viral particles [8]. However, in vivo experiments examining the immunomodulatory pathways triggered by therapeutic IFN-α administration are limited and have not been performed in the setting of HIV/SIV infection.
Pegasys® [pegylated (40 kDa) branched IFN-α2a; Roche Pharmaceuticals, Nutley, NJ, USA] offers an opportunity to revisit the safety, tolerability, and efficacy of IFN-α for the treatment of HIV infection because of its pharmacokinetic and pharmacodynamic properties. These pilot experiments were designed to explore the virologic effects of IFN-α in the setting of chronic SIV infection.
Methods
Animals
California National Primate Research Center colonybred juvenile male rhesus macaques (Macaca mulatta), seronegative for simian-type D retroviruses, simian T-cell lymphotropic virus, and SIV, were housed in accordance with the American Association for Accreditation of Laboratory Animal Care Standards. The investigators adhered to the guidelines of the Committee on Care and Use of Laboratory Animals, National Resources Council. All experiments were approved by the institution animal use committee prior to implementation.
Seven juvenile macaques (none were MamuA01 +) chronically infected by the intravenous route with SIVmac251 (100 TCID50) approximately 20 weeks prior to enrollment were randomized to receive either normal saline (n = 3) or pegylated IFN-α2a (Pegasys®) at 50 µg (n = 4) once per week by the intramuscular injection. At the time of randomization, none had evidence of opportunistic infections.
Blood samples were collected weekly for analysis of pharmacologic, biochemical, virologic, and immunologic parameters.
Virus load measurement
Plasma samples were analyzed for vRNA by a quantitative branched DNA assay [6, 21]. Virus load in plasma samples is reported as log10 vRNA copy numbers per ml of plasma. The detection limit of this assay is 125 vRNA copies.
Pegylated IFN-α2a levels
Serum trough drug levels were measured on samples drawn just prior to the next dose by Prevalere (Albany, NY, USA).
Cytokine mRNA analysis
Peripheral blood mononuclear cell (PBMC) cytokine mRNA levels were determined by real-time RT-PCR as described previously [1, 2].
Results
The animals selected for these experiments were chronically infected with SIV and all had plasma vRNA levels > 104 copies/ml and relatively low CD4+ T-cell counts, when IFN therapy was initiated. While hematological and biochemical parameters were similar between treatment groups (data not shown), two animals randomized to the pegylated IFN-α2a developed SAIDS-related enterocolitis that was not responsive to standard therapy, prompting the animals to be killed at week 6 and 10. Thus, only five animals completed the entire 14-week period of observation (experimental group = 2; control group = 3).
Virologic and immunologic results of IFN-α therapy
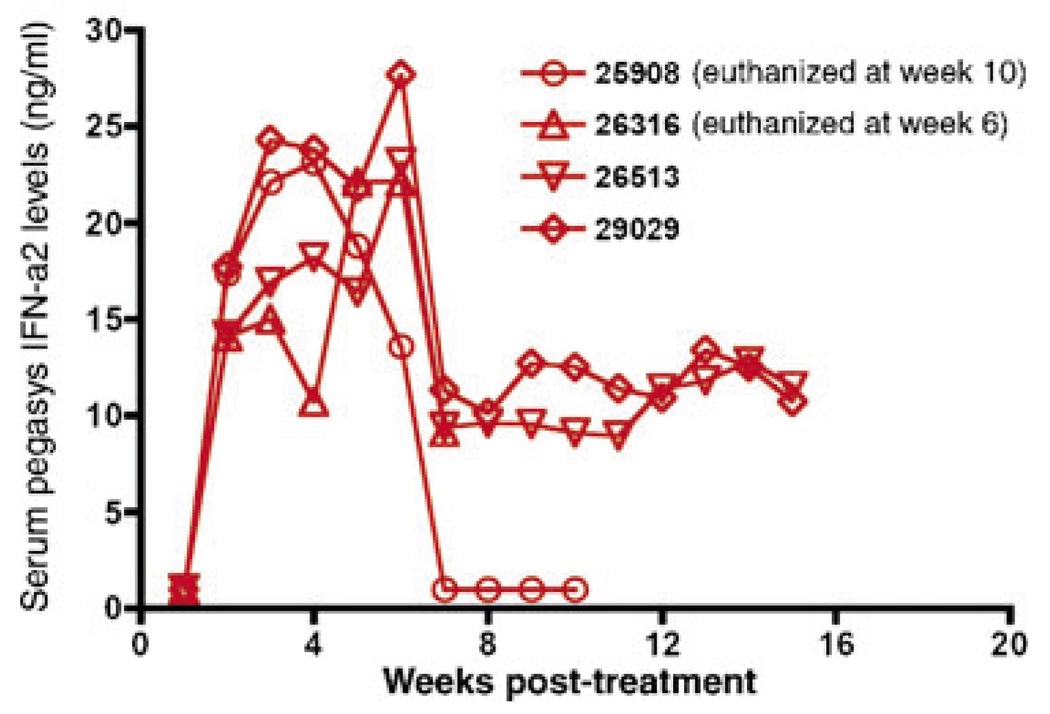
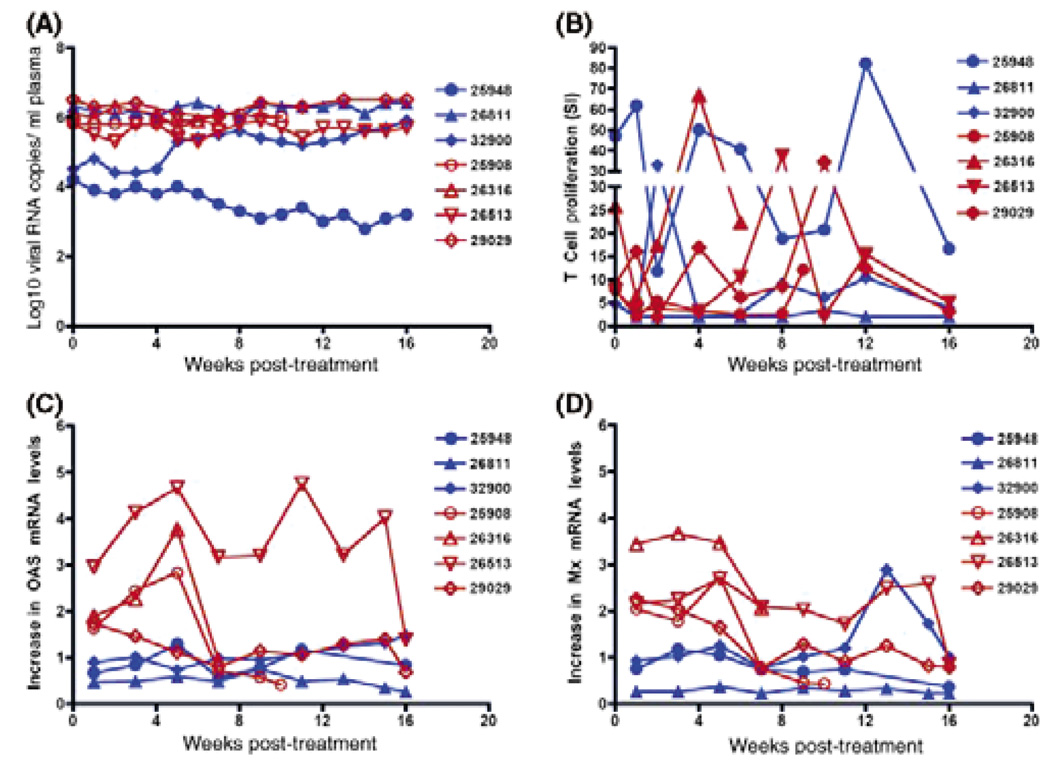
Figure 1 depicts the serum pegylated IFN-α2a levels. It should be noted that serum IFN-α levels were not sustained throughout the treatment period. After 5 weeks of therapy, apparent gastrointestinal side effects of the drug treatment necessitated a 50% dose reduction. This decreased dosage produced a sharp drop in serum IFN-α levels. The first 5 weeks of high-dose IFN-α treatment stimulated considerable innate antiviral immunity in the animals as PMBC mRNA levels of the IFN-α-inducible antiviral effector molecules 2′–5′ oligoadenylate synthetase (OAS) and Mx were increased in PBMC through 5 weeks PI (Fig. 2, Panels C and D). However, the OAS and Mx PBMC mRNA levels in most animals decreased to near baseline after the IFN-α dose was reduced. Despite unequivocal stimulation of innate immunity by the IFN-α therapy, there was no effect on plasma SIV RNA levels (Fig. 2, Panel A). Further, the treatment seemed to have little effect on anti-SIV T-cell proliferative responses (Fig. 2, Panel B).
Fig. 1.
Trough IFN-α2a levels in Pegasys-treated monkeys. Serum IFN-α levels are reported in ng/ml for various time points after the initiation of Pegasys treatment.
Fig. 2.
Effect of Pegasys treatment in chronic SIV-infected macaques. Panel A: Plasma viral RNA levels for Pegasys-treated (Brown symbols) and untreated (Blue symbols) monkeys are reported as log10 viral RNA copies per ml of plasma. Panel B: T-cell proliferative responses are shown for various time points after Pegasys treatment initiation. The y-axis shows the Stimulation Index (SI) as calculated by cpm of SIV-stimulated PBMC/cpm of media control cultures. Panels C and D show the increase in 2′–5′ oligoadenylate synthetase (OAS) and Mx PBMC mRNA levels, respectively, compared with OAS and Mx PBMC mRNA levels in the same animal prior to treatment.
Conclusions
Interferon-α exerts antiviral and immunomodulatory affects through multiple pathways. In the pilot project reported here, chronically SIVmac251-infected macaques responded to therapeutic IFN-α2a therapy with increased expression of key antiviral innate immune effector molecules but there was no evidence of that the IFN-α therapy altered SIV replication. In contrast, human trials employing both pegylated and unpegylated IFN-α therapy either in the setting of HIV infection alone or coinfection with hepatitis C virus infection have demonstrated a significant, dose-dependent decrease on HIV plasma vRNA levels [11, 20, 22].
There are many potential explanations for the inability of our study to detect effects of IFN-α treatment on SIV replication in vivo. First, the advanced stage of immune suppression in the chronic SIV-infected macaques selected may have precluded an optimal adaptive antiviral response to immunomodulatory therapy. Second, although the plasma IFN-α levels were adequate in all animals during the first 5 weeks of therapy, we did not measure antibodies to IFN-α to determine if neutralizing antibodies were produced. In fact, the homology between IFN-α2a and simian IFN-α is only 94%, and antibody formation has been found in nonhuman primates administered other human IFN-α molecules [18]. Thus, while the pharmacokinetic assays employed for this study distinguished pegylated and endogenous IFN, the presence of antibodies to the human IFN-α cannot be excluded. Third, the number of animals needed to be able to detect a significant effect in SIV replication may need to be much larger than the five animals included in this study. In fact, chronically SIV-infected macaques with low CD4+ T cells were selected for these experiments, with the hope that anti-IFN-α neutralizing antibody responses would be delayed.
It is not possible to discern whether IFN-α therapy contributed to the development of clinical signs of SAIDS in the two monkeys in the treatment arm or if this clinical progression was just part of the normal course of SIV infection. Hematologic and chemistry profiles in the animals terminated early were not consistent with the specific toxicities associated with IFN-α therapy in humans (data not shown).
These experiments represent the first trial of therapeutic IFN-α therapy in the non-human primate model for AIDS and the results indicate that the levels of drug achieved in the first 5 weeks of therapy were comparable to those in humans receiving pegylated IFN-α2a therapy for the treatment of hepatitis C. As in the human trials, immunologic evidence of a therapeutic effect was observed as demonstrated by the increase in PBMC mRNA levels of IFN-inducible antiviral molecules. A unique feature of these experiments was the simultaneous, although limited, measurement of innate and adaptive immune response subsequent to therapeutic IFN-α administration. A rise in IFN-inducible antiviral effector molecules was observed, as a predicted effect of administration of exogenous IFN-α on the innate immune system. However, an enhancement of SIV- specific T-cell proliferative responses or the increased control of viral replication that should be associated with enhanced effective adaptive anti-SIV immunity was not observed.
In summary, these results support further study into the value of therapeutic immunomodulators in chronic viral infection in rhesus monkeys. Future studies of IFN-α therapy in SIV-infected macaques will focus on potential applications of this strategy in humans, including its potential role as an additional element within a highly active antiretroviral therapy regimen, as a means to consolidate immune reconstitution, or as strategy for delaying the need to initiate antiretroviral therapy. Simian IFN will likely be required for these experiments to circumvent interfering antibody formation, however.
Acknowledgments
This work was supported by Public Health Services grants U51 RR00169, from the National Center for Research Resources, by the North-Central California Center for AIDS Research, #P30-AI49366-01, R01-AI51999 (DMA), PHS-AI49366 (MDG), 3U01AI38858-09S1 and 204IC005 (DMA and RBP) from the National Institute of Health, and by a restricted grant from Roche Laboratories, Inc. (Nutley, NJ, USA). The authors thank the Immunology Core Laboratory and Primate Services Unit at the CNPRC and Ding Lu and Linda Fitts for excellent technical assistance.
Footnotes
This study was presented in part at the Nonhuman Primate Model for AIDS Conference in San Antonio TX, November, 2004.
References
- 1.Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol. 2002;76:8433–8445. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel K, Alegria-Hartman MJ, Zanotto K, McChesney MB, Marthas ML, Miller CJ. Anatomic site and immune function correlate with relative cytokine mRNA expression levels in lymphoid tissues of normal rhesus macaques. Cytokine. 2001;16:191–204. doi: 10.1006/cyto.2001.0961. [DOI] [PubMed] [Google Scholar]
- 3.Akbar AN, Lord JM, Salmon M. IFN-alpha and IFN-beta: a link between immune memory and chronic inflammation. Immunol Today. 2000;21:337–342. doi: 10.1016/s0167-5699(00)01652-2. [DOI] [PubMed] [Google Scholar]
- 4.Biron CA. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 5.Biron CA. Interferons alpha and beta as immune regulators – a new look. Immunity. 2001;14:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 6.Dailey PJ, Zamroud M, Kelso R, Kolberg J, Urdea M. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay. Abstracts of the 13th Annual Symposium on Nonhuman Primate Models of AIDS; Monterey, CA: 1995. Sep, p. 180. [Google Scholar]
- 7.Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dianzani F, Castilletti C, Gentile M, Gelderblom HR, Frezza F, Capobianchi MR. Effects of IFNα on late stages of HIV-1 replication cycle. Biochemie. 1998;80:745–754. doi: 10.1016/s0300-9084(99)80028-5. [DOI] [PubMed] [Google Scholar]
- 9.Emilié D, Burgard M, Lascoux-Combe C, Laughlin M, Krzysiek R, Pignon C, Rudent A, Molina JM, Livrozet JM, Souala F, Chene G, Grangeot-Keros L, Galanaud P, Sereni D Rouzioux C and the Primoferon A Study Group. Early control of HIV replication in primary HIV-1 infection treated with antiretroviral drugs and pegylated IFNα: results from the Primoferon A (ANRS 086) Study. AIDS. 2001;15:1345–1347. doi: 10.1097/00002030-200107270-00014. [DOI] [PubMed] [Google Scholar]
- 10.Frissen PH, de Wolf F, Reiss P, Bakker PJ, Veenhof CH, Danner SA, Goudsmit J, Lange JM. High-dose interferon-alpha2a exerts potent activity against human immunodeficiency virus type 1 not associated with antitumor activity in subjects with Kaposi’s sarcoma. J Infect Dis. 1997;176:811–814. doi: 10.1086/517309. [DOI] [PubMed] [Google Scholar]
- 11.Haas DW, LaVelle J, Nadler JP, Greenberg SB, Frame P, Mustafa N, St Clair M, McKinnis R, Dix L, Elkins M, Rooney J. A randomized trial of interferon α therapy for HIV type 1 infection. AIDS Res Hum Retroviruses. 2000;16:183–190. doi: 10.1089/088922200309278. [DOI] [PubMed] [Google Scholar]
- 12.Ho DD, Hartshorn KL, Rota TR, Andrews CA, Kaplan JC, Schooley RT, Hirsch MS. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet. 1985;1:602–604. doi: 10.1016/s0140-6736(85)92144-0. [DOI] [PubMed] [Google Scholar]
- 13.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 14.Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- 15.Nokta MA, Reichman RC, Pollard RB. Pathogenesis of viral infections. In: Galasso, Whitley, Merigan, editors. Antiviral Agents and Viral Diseases of Man. 3rd edn. New York: Raven Press; 1990. pp. 49–85. [Google Scholar]
- 16.O’Shea JJ, Visconti R. Type I IFNs and regulation of TH1 responses: enigmas both resolved and emerge. Nat Immunol. 2000;1:17–19. doi: 10.1038/76872. [DOI] [PubMed] [Google Scholar]
- 17.Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 18.Schellekens H, Niphuis H, Buijs L, Douw vdK, Hochkeppel HK, Heeney JL. The effect of recombinant human interferon alpha B/D compared to interferon alpha 2b on SIV infection in rhesus macaques. Antiviral Res. 1996;32:1–8. doi: 10.1016/0166-3542(95)00955-8. [DOI] [PubMed] [Google Scholar]
- 19.Schellekens H, Weimar W, Cantell K, Stitz L. Antiviral effect of interferon in vivo may be mediated by the host. Nature. 1979;278:742. doi: 10.1038/278742a0. [DOI] [PubMed] [Google Scholar]
- 20.Schugt I, Dreuter A, Schlottmann R. Pegylated interferon alfa-2b: a new therapeutic option in the treatment of early-stage HIV infection [Abstract 59]. 10th Conference of Retroviruses & Opportunistic Infections; Boston, MA: 2003. Feb 10–14, p. 78. [Google Scholar]
- 21.Wingfield C, Booth J, Sheridan P, Detmer J, Turczyn J. SIV 4.0, performance characteristics of an exceptionally sensitive, quantitative assay for SIV RNA using branched DNA technology. Abstracts of the 20th Annual Symposium on Nonhuman Primate Models of AIDS; Monterey, CA: 2002. Sep, p. 200. [Google Scholar]
- 22.Wright SE, Hutcheson DP, Cummins JM. Low dose oral interferon alpha 2a in HIV-1 seropositive patients: a double-blind, placebo-controlled trial. Biotherapy. 1998;11:229–234. doi: 10.1023/a:1008050000064. [DOI] [PubMed] [Google Scholar]