Abstract
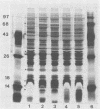
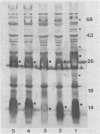
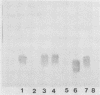
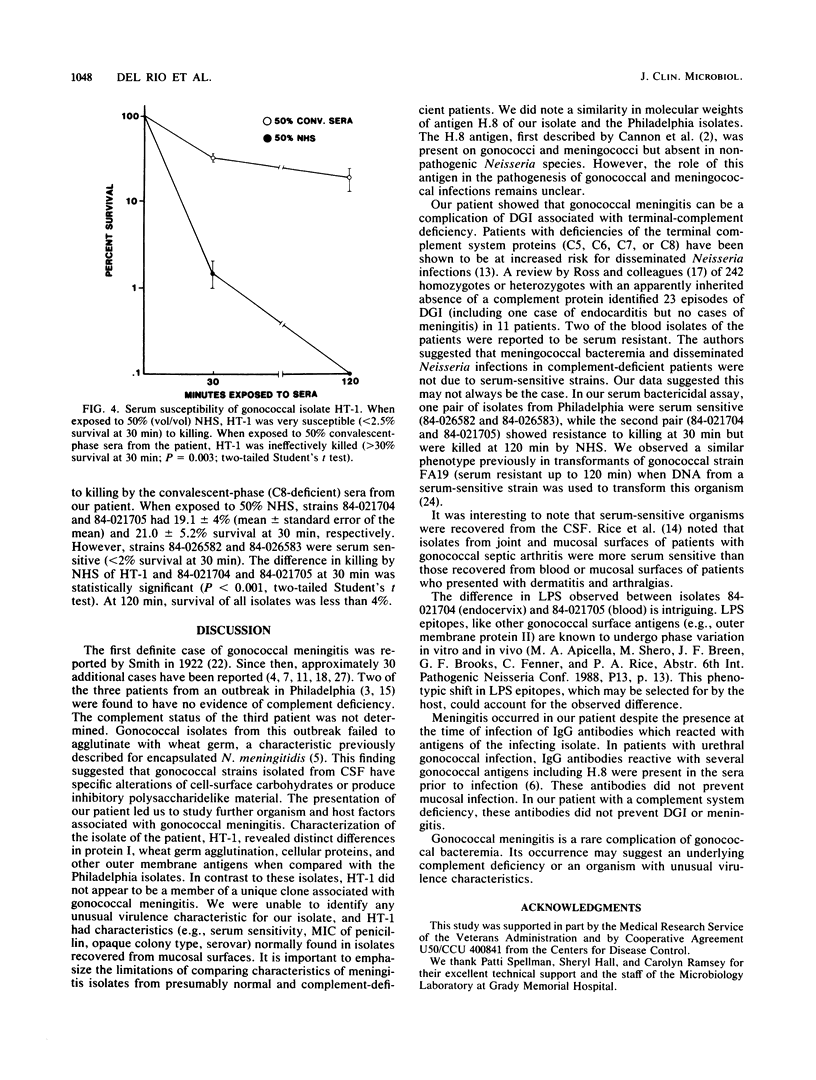
We studied a previously healthy 20-year-old woman who presented with gonococcal meningitis. The gonococcal isolate, HT-1, was prototrophic by auxotyping, was protein I serovar IB-1, and agglutinated with wheat germ lectin. This isolate differed from the proline-requiring, serovar IA-1 and IB-4, wheat germ-agglutination-negative gonococcal isolates recovered from three patients during a recent outbreak of gonococcal meningitis in Philadelphia. HT-1 was killed by normal pooled human sera (greater than or equal to 98% at 30 min) but not effectively killed by the convalescent-phase sera of the patient (greater than 30% survival at 30 min). Similar results were obtained when mucosal and cerebrospinal fluid isolates from a Philadelphia patient were exposed to these sera, but mucosal and blood isolates from another Philadelphia case showed increased resistance to killing by normal pooled human sera. Further characterization revealed multiple differences in outer membrane and cellular proteins and lipopolysaccharide between case isolates. Absence of the L8 lipopolysaccharide epitope was noted for all isolates. Sera of our patient were found to have low total hemolytic complement (CH100 = 21 U/ml; normal = 55 to 100 U/ml) due to deficiency of C8 (C8 less than 1,000 CH50 U/ml; normal = greater than or equal to 16,000 CH50 U/ml). This is the first reported case of gonococcal meningitis occurring in a patient with a terminal-complement deficiency. Gonococcal meningitis is a rare complication of gonococcal bacteremia. Both defects in host defenses (e.g., terminal-complement deficiency) and organisms with unusual virulence appear to contribute to the pathogenesis of this complication of gonococcal bacteremia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Black W. J., Nachamkin I., Stewart P. W. Monoclonal antibody that recognizes an outer membrane antigen common to the pathogenic Neisseria species but not to most nonpathogenic Neisseria species. Infect Immun. 1984 Mar;43(3):994–999. doi: 10.1128/iai.43.3.994-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H. A. Meningococcus and gonococcus: never the Twain--well, hardly ever. N Engl J Med. 1971 Aug 26;285(9):518–520. doi: 10.1056/NEJM197108262850914. [DOI] [PubMed] [Google Scholar]
- Frasch C. E. Role of lipopolysaccharide in wheat germ agglutinin-mediated agglutination of Neisseria meningitidis and Neisseria gonorrhoeae. J Clin Microbiol. 1980 Oct;12(4):498–501. doi: 10.1128/jcm.12.4.498-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C. B., Boslego J. W., Brandt B. Evidence of serum antibodies to Neisseria gonorrhoeae before gonococcal infection. J Infect Dis. 1987 Jun;155(6):1276–1281. doi: 10.1093/infdis/155.6.1276. [DOI] [PubMed] [Google Scholar]
- Holmes K. K., Counts G. W., Beaty H. N. Disseminated gonococcal infection. Ann Intern Med. 1971 Jun;74(6):979–993. doi: 10.7326/0003-4819-74-6-979. [DOI] [PubMed] [Google Scholar]
- Knapp J. S. Laboratory methods for the detection and phenotypic characterization of Neisseria gonorrhoeae strains resistant to antimicrobial agents. Sex Transm Dis. 1988 Oct-Dec;15(4):225–233. doi: 10.1097/00007435-198810000-00009. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Masi A. T., Eisenstein B. I. Disseminated gonococcal infection (DGI) and gonococcal arthritis (GCA): II. Clinical manifestations, diagnosis, complications, treatment, and prevention. Semin Arthritis Rheum. 1981 Feb;10(3):173–197. doi: 10.1016/s0049-0172(81)80002-9. [DOI] [PubMed] [Google Scholar]
- Noble R. C., Cooper R. M. Gonococcal meningitis and ventriculitis in the presence of a ventriculoperitoneal shunt. Sex Transm Dis. 1977 Jan-Mar;4(1):9–11. doi: 10.1097/00007435-197701000-00003. [DOI] [PubMed] [Google Scholar]
- O'Brien J. P., Goldenberg D. L., Rice P. A. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine (Baltimore) 1983 Nov;62(6):395–406. [PubMed] [Google Scholar]
- Petersen B. H., Lee T. J., Snyderman R., Brooks G. F. Neisseria meningitidis and Neisseria gonorrhoeae bacteremia associated with C6, C7, or C8 deficiency. Ann Intern Med. 1979 Jun;90(6):917–920. doi: 10.7326/0003-4819-90-6-917. [DOI] [PubMed] [Google Scholar]
- Rice P. A., McCormack W. M., Kasper D. L. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J Immunol. 1980 May;124(5):2105–2109. [PubMed] [Google Scholar]
- Rice R. J., Schalla W. O., Whittington W. L., JeanLouis Y., Biddle J. W., Goldberg M., DeWitt W., Pasquariello C. A., Abrutyn E., Swenson R. Phenotypic characterization of Neisseria gonorrhoeae isolated from three cases of meningitis. J Infect Dis. 1986 Feb;153(2):362–365. doi: 10.1093/infdis/153.2.362. [DOI] [PubMed] [Google Scholar]
- Ross S. C., Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984 Sep;63(5):243–273. [PubMed] [Google Scholar]
- Sayeed Z. A., Bhaduri U., Howell E., Meyers H. L., Jr Gonococcal meningitis. A review. JAMA. 1972 Mar 27;219(13):1730–1731. [PubMed] [Google Scholar]
- Schalla W. O., Whittington W. L., Rice R. J., Larsen S. A. Epidemiological characterization of Neisseria gonorrhoeae by lectins. J Clin Microbiol. 1985 Sep;22(3):379–382. doi: 10.1128/jcm.22.3.379-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Mandrell R. E., Jarvis G. A. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect Immun. 1985 Dec;50(3):672–677. doi: 10.1128/iai.50.3.672-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short H. B., Ploscowe V. B., Weiss J. A., Young F. E. Rapid method for auxotyping multiple strains of Neisseria gonorrhoeae. J Clin Microbiol. 1977 Sep;6(3):244–248. doi: 10.1128/jcm.6.3.244-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Association of virulence of Neisseria meningitidis with transparent colony type and low-molecular-weight outer membrane proteins. J Infect Dis. 1983 Feb;147(2):282–292. doi: 10.1093/infdis/147.2.282. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., Shafer W. M. Evidence that the serum resistance genetic locus sac-3 of Neisseria gonorrhoeae is involved in lipopolysaccharide structure. J Gen Microbiol. 1987 Sep;133(9):2671–2678. doi: 10.1099/00221287-133-9-2671. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., Whitney A. M., Rothbard J., Schoolnik G. K. Pili of Neisseria meningitidis. Analysis of structure and investigation of structural and antigenic relationships to gonococcal pili. J Exp Med. 1985 Jun 1;161(6):1539–1553. doi: 10.1084/jem.161.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubin H. L., Landsberg L. Gonococcal meningitis. N Engl J Med. 1971 Aug 26;285(9):504–505. doi: 10.1056/NEJM197108262850909. [DOI] [PubMed] [Google Scholar]