Abstract
In Drosophila, the female-specific SEX-LETHAL (SXL) protein is required for oogenesis, but how Sxl interfaces with the genetic circuitry controlling oogenesis remains unknown. Here we use an allele of sans fille (snf) that specifically eliminates SXL protein in germ cells to carry out a detailed genetic and cell biological analysis of the resulting ovarian tumor phenotype. We find that tumor growth requires both Cyclin B and zero population growth, demonstrating that these mutant cells retain at least some of the essential growth-control mechanisms used by wild-type germ cells. Using a series of molecular markers, we establish that while the tumor often contains at least one apparently bona fide germline stem cell, the majority of cells exhibit an intermediate fate between a stem cell and its daughter cell fated to differentiate. In addition, snf tumors misexpress a select group of testis-enriched markers, which, remarkably, are also misexpressed in ovarian tumors that arise from the loss of bag of marbles (bam). Results of genetic epistasis experiments further reveal that bam's differentiation-promoting function depends on Sxl. Together these data demonstrate a novel role for Sxl in the lineage progression from stem cell to committed daughter cell and suggest a model in which Sxl partners with bam to facilitate this transition.
THE continuous supply of gametes throughout adulthood is accomplished by germline stem cells (GSCs) that divide asymmetrically to produce one daughter cell that remains a stem cell and a second daughter cell that differentiates. In the Drosophila gonad this asymmetric cell division is controlled by a combination of cell-autonomous regulatory mechanisms and extrinsic signaling pathways that communicate with and respond to the surrounding somatic gonadal cells (reviewed by Fuller and Spradling 2007; Kirilly and Xie 2007; Lin 2008). Although there are mechanistic differences in how asymmetry is accomplished in males and females, mutations that disrupt asymmetric cell fate choice lead to similar phenotypes; mutations that force both daughter cells to differentiate result in the depletion of the stem cell population and an empty gonad while mutations that interfere with differentiation result in the accumulation of undifferentiated germ cells and a tumor phenotype.
Germ cell tumors also arise from defects in sexual development (reviewed by Oliver 2002; Casper and van Doren 2006). For example, when the sexual identity of the soma and the sexual identity of the germ cells are mismatched, the resulting discordance leads to a failure in differentiation and tumor formation. Although there is little definitive information about the nature or the purpose of sex-specific germline/soma communication, it is clear that the female gonad can signal XY germ cells to initiate a female-specific gene expression program that includes Sex-lethal (Sxl) (Oliver et al. 1993; Waterbury et al. 2000; Janzer and Steinmann-Zwicky 2001). Loss of Sxl function in XX germ cells also leads to germline tumors that inappropriately express some testis-specific markers (Schüpbach 1985; Wei et al. 1994; Staab and Steinmann-Zwicky 1996). While these studies suggest that Sxl expression in the germline is governed by extrinsic factors and is necessary for oocyte differentiation, how Sxl interfaces with the genetic program known to control differentiation is still an enigma.
In contrast to the situation in the germline, Sxl regulation and function in the soma have been extensively studied (reviewed by Cline and Meyer 1996; Penalva and Sanchez 2003). In somatic cells, Sxl functions as the developmental switch gene for both somatic sex determination and X chromosome dosage compensation. Throughout most of a fly's life cycle the female-specific SXL RNA-binding protein controls expression of its downstream target genes via alternative splicing and/or translational repression. Although sex determination defects are not lethal to the organism, upsets in dosage compensation are lethal; by virtue of being at the top of the regulatory cascade leading to X chromosome dosage compensation, loss of Sxl function leads to female-specific embryonic lethality.
Here we evaluate Sxl's role in the germline by analyzing the ovarian tumor phenotype of a female-sterile allele of sans-fille, snf148, which specifically eliminates Sxl in the germline without disrupting Sxl regulation or function in the soma (this study; Nagengast et al. 2003). We find the ovarian tumors in snf148 females arise from a continuously dividing population of Sxl-deficient germ cells that have acquired a fate that is intermediate between a stem cell and its daughter cell. We also find an unexpected functional link to the differentiation-promoting factor bag of marbles (bam), including the observation that a select group of testis-enriched markers are ectopically expressed in both snf and bam ovarian tumors. We therefore propose a novel model in which Sxl and bam act together to facilitate the progression from germline stem cell to committed daughter cell.
MATERIALS AND METHODS
Drosophila strains:
The following mutant alleles and deficiencies were used in this study, snf148 (Nagengast et al. 2003), CycB2 (Jacobs et al. 1998), Df(2R)59AB (Knoblich and Lehner 1993), bamΔ86 (McKearin and Spradling 1990), and zpgz2533 (Tazuke et al. 2002; Gilboa et al. 2003). Transgenic, enhancer trap, and protein trap lines used include P{otu∷SxlcDNA} (Hager and Cline 1997), P{bamP-GFP} (Chen and McKearin 2003a), P{myc-Piwi} (Cox et al. 2000), and P{dad-lacZ} (Tsuneizumi et al. 1997) and P{PTT-GC}CycBCC01846 (Buszczak et al. 2007).
Antibodies, immunofluorescence, and image analysis:
Ovaries isolated from 3- to 7-day-old adult females were fixed and stained by standard methods. The antibodies and dilutions used were mouse anti-SXL (m18, 1:350; Bopp et al. 1991) mouse anti-HTS (1B1, 1:10; Zaccai and Lipshitz 1996), rabbit anti-PH3 (1:1000, Upstate), rabbit anti-GFP (1:2000; Molecular Probes, Eugene, OR), mouse anti-cMyc (1:50, Santa Cruz), rat anti-PUM 1637 (1:200; Macdonald 1992), rabbit anti-VASA (1:4000; Lasko and Ashburner 1990), mouse anti-β-galactosidase (1:1500; Sigma, St. Louis), and rabbit anti-β-galactosidase (1:1500, Cappel). Secondary antibodies coupled to FITC or Cy3 (Jackson ImmunoResearch) were used at 1:800 dilutions, while secondary antibodies coupled to Alexa 488, 555, and 568 (Molecular Probes) were used at 1:1000 dilutions. DNA staining was detected using DAPI or TO-PRO-3 (Molecular Probes) and was applied during the final washes of the immunostaining procedure. Samples were analyzed either on a Zeiss Axiophot microscope or on an inverted Leica DM IRE2 microscope with a Leica TCS SP2 AOBS filter-free UV/spectral confocal laser scanner.
When making comparisons between wild-type and mutant expression patterns, samples were processed together and images for different genotypes were acquired under identical conditions. Relative fluorescence intensity was determined by calculating the pixel intensity using the Stack Profile tool in the Leica Confocal Software program. The pixel intensity was measured within the boundaries of a 7-μm diameter circle and a 2-μm thick Z-section of a single germ cell directly adjacent to a cap cell. Images for the figures were processed with Adobe Photoshop.
Analysis of third instar larval gonads:
To collect gonads of approximately the same age, 0- to 3-hr-old eggs were collected from snf148/FM7c, P{w+,GAL4-Kr.C}DC1, P{w+, UAS-GFP.S65T}DC5 females crossed to snf148 males, allowed to age at 25°, and dissected from midthird instar larvae collected ∼96 hr after egg lay. Genotypes were determined by the presence or absence of GFP expression from the balancer chromosome. Gonads from homozygous mutant animals did not express GFP. Gonads from heterozygous siblings, with GFP expression in the fat bodies, were used as wild-type controls. After immunostaining, the number of germ cells per gonad was determined by counting VASA-positive cells from confocal serial sections.
5-bromo-2-deoxyuridine incorporation:
Ovaries isolated from 3- to 7-day-old flies were incubated in 1 μg/ml BrdU (Sigma) for 1 hr. After washes, ovaries were fixed for 30 min in 4% paraformaldehyde made in PBS and acid treated for 30 min in 2 n HCl at 37°, followed by neutralization in 100 mm borax solution for 2 min. 5-bromo-2-deoxyuridine (BrdU) incorporation was detected by immunostaining with a mouse anti-BrdU antibody (1:20; Roche, Indianapolis).
Western blots:
Tissue lysates were made by homogenizing ovaries isolated from ∼50 females of the appropriate genotype in PBS supplemented with complete miniprotease inhibitor (Roche). Westerns were performed according to standard procedures with the following antibodies: rat anti-BAM-C (1:350; McKearin and Ohlstein 1995) and rabbit anti-U170K-151 (1:6000; Nagengast et al. 2003).
RT–PCR:
Total RNA was isolated from ovaries and testis using TRIzol (Invitrogen, Carlsbad, CA) as directed by the manufacturer. RNA was incubated with DNase I (RQ1 DNase; Promega, Madison, WI), and then 0.5 μg of RNA was subjected to reverse transcription in a 20-μl reaction volume using the Superscript first strand synthesis kit (Invitrogen) primed with random hexamers. The RT reaction was serially diluted and then subjected to PCR using the Expand High Fidelity PCR system (Roche) adjusted to be in the linear range. For all measurements, except for Act5c, 2.5 and 0.5 μl of each cDNA sample were used as a template in a 25-μl PCR reaction. For measurements of Act5C, which is more abundant than the other RNAs analyzed here, only 1 and 0.2 μl of cDNA were used as a template. The following parameters were used for each PCR reaction: (1) 94° for 1 min; (2) 30 cycles at 94° for 1 min, 55° for 1 min, and 72° for 1 min; and (3) an additional 10 min at 72°. To ensure that the PCR signals were from RNA and not from contaminating DNA, primer sequences were designed to span an intron whenever possible, and control reactions without reverse transcriptase were carried out in parallel. The primers used in this study are listed in Table 1.
TABLE 1.
Primers used for RT–PCR analysis
| Transcript | Forward | Reverse |
|---|---|---|
| Act5C | 5′ GTATCCTCACCCTGAAGTAC 3′ | 5′ CATGATGGAGTTGTAGGTGG 3′ |
| aret-RB | 5′ AGTTCGGTCTCGCGGAGGTG 3′ | 5′ GAAATAGCTAGGCAACTCGG 3′ |
| cg15930 | 5′ CAATGAAGATGCATGAACTGC 3′ | 5′ GAAATAGCTAGGCAACTCGG 3′ |
| esg | 5′ CGCCCATGAGATCTGAAATC 3′ | 5′ GGTCTTGTCACAATCCTTGC 3′ |
| gskt | 5′ CATAAAGAAGGTCCTGCAGG 3′ | 5′ CAGCAACTCTGACATCACAC 3′ |
| Wnt2 | 5′ GCGGCACTGCGTGGTCCTTCG 3′ | 5′ GTGAGGCTGCCTACACCTATGCG 3′ |
RESULTS
SXL protein expression in wild-type germaria:
The adult ovary consists of 15–20 tubes, called ovarioles. The GSCs are located at the anterior end of each ovariole, in a structure called the germarium (Figure 1A). When the GSC cell divides, one daughter cell remains in direct contact with the somatic cap cells at the tip of the germarium to retain its GSC identity. The other daughter cell, called a cystoblast (CB), is no longer in contact with the somatic cap cell and differentiates. Each CB cell undergoes four rounds of mitosis with incomplete cytokinesis to yield a 16-cell cyst. One cell per cyst, identified by the accumulation of the ORB protein, is destined to become the oocyte and enter meiosis. The remaining 15 cells become polyploid nurse cells.
Figure 1.—
Sxl and bamP-GFP coexpression in the germarium. (A) Schematic of a wild-type germarium. The cell destined to become the oocyte is blue, and all other germline-derived cells are pink. Spectrosomes and fusomes are red. Cells of somatic origin are in shades of green and gray. The following abbreviations are used: GSC, germline stem cell; CB, cystoblast; fu, fusome; sp, spectrosome. (B, B′, and B″) Wild-type germarial tip from a female carrying the bamP-GFP reporter construct stained for GFP (green), SXL (red), and DNA (blue). Cytoplasmic SXL staining is prominent in two cell types: GSC cells, which do not express bamP-GFP, and CB cells, where bamP-GFP is expressed. As differentiation proceeds, bamP-GFP staining increases and cytoplasmic SXL staining decreases.
The SXL protein accumulates to high levels in the cytoplasm of only a few germ cells located at the tip of the germarium (Bopp et al. 1993; Vied et al. 2003). To definitively determine the identity of the Sxl-expressing cells, we compared expression of SXL with P{bamP-GFP}, a reporter construct typically used to distinguish GSCs from their progeny fated to differentiate. P{bamP-GFP} is a transcriptional reporter in which the bam promoter is fused to the GFP coding sequence (Chen and McKearin 2003b). In agreement with published studies, we find that P{bamP-GFP} is expressed in cells presumed to be CBs and dividing cysts, on the basis of their position within the germarium (Figure 1B). Costaining experiments reveal that SXL is expressed at high levels the GSCs where P{bamP-GFP} is not expressed and in the adjacent two to three cells where P{bamP-GFP} is first detectable (Figure 1B). High levels of cytoplasmic SXL protein, however, are no longer detectable in cells with the highest levels of P{bamP-GFP} expression. This tightly regulated expression pattern suggests that Sxl may have a role in GSCs and/or their immediate daughter cells.
snf148 mutant ovaries contain germline tumors that fail to accumulate SXL protein:
To evaluate Sxl's role in the germline, we chose not to use the extant female-sterile Sxl alleles because the female-sterile phenotype of these alleles is reversible by a variety of factors including temperature, genetic background, or infection with the reproductive parasite Wolbachia (Salz et al. 1987; Bopp et al. 1993; Starr and Cline 2002; Vied et al. 2003). Instead, we chose to use a female-sterile allele of the general splicing factor sans fille, snf148, previously shown to interfere specifically with Sxl splicing regulation in the germline (Nagengast et al. 2003). We find that the germ cells in snf148 mutant germaria fail to accumulate SXL protein even though SXL protein is clearly detectable in the surrounding somatic cells (Figure 2A). Additional evidence that the ovarian tumors in snf148 mutant females arise from Sxl-deficient germ cells is provided by studies showing that the ovarian tumor phenotype is fully rescued by P{otu∷SxlcDNA}, a transgene expressing Sxl under control of the germline-specific otu promoter (this study; Nagengast et al. 2003). The analysis of the snf148 mutant phenotype, therefore, directly informs us of Sxl function in the germline.
Figure 2.—
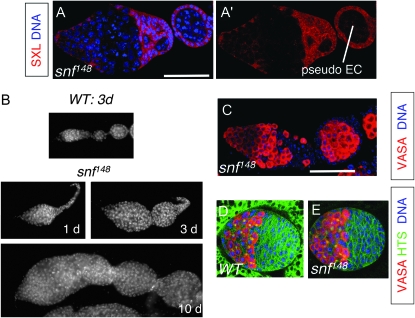
snf148 germline tumors. (A and A′) snf148/snf148 mutant germarium and adjoining pseudo-egg chamber (EC) stained for SXL (red) and DNA (blue). Bar, 50 μm. (B) DAPI-stained ovariole from a 3-day-old wild-type (WT) female (top) and a 1-day-old, a 3-day-old, and a 10-day-old snf148/snf148 mutant female (bottom). (C) snf148/snf148 mutant germarium and adjoining pseudo-egg chamber (EC) stained for VASA (red) and DNA (blue). The image is the same magnification as in A. (D and E) Control (D) and snf148/snf148 (E) gonads from third instar female larvae stained for VASA (red), HTS (green), and DNA (blue). Note that HTS staining labels both spectrosomes and the somatic cell membranes. In control gonads (D) the fat body surrounding the gonad is also green because of expression of a GFP marker, as described in materials and methods. The images in D and E are the same magnification.
During the course of our studies, we noted that the snf148 ovarian tumor, already apparent in newborn flies, increases in size as the fly ages (Figure 2B). As the tumor grows, the somatic gonadal cells surround some of the tumor cells to form a structure that resembles an egg chamber. Formation of this pseudo-egg chamber suggests that the somatic cells identify the tumor cells as germ cells. This conclusion is supported by the majority of cells within the snf ovarian tumor expressing VASA, a germ cell-specific marker (Figure 2C).
Larval snf148 gonads are not tumorous:
Oogenesis begins just after the larval/pupal transition and continues throughout adulthood. To determine whether the snf tumor phenotype is apparent before oogenesis begins, we compared the number of germ cells in wild-type and snf mutant third instar larval ovaries. Interestingly, the larval ovaries do not contain an excess of germ cells. On average a snf148 mutant ovary contains 50 ± 3 VASA staining cells (mean ± SEM, n = 25), as compared to 64 ± 4 VASA staining cells in a control ovary (n = 29; Figure 2, D and E). Similarly, a tumorous phenotype was not apparent in ovaries from animals at the larval/pupal transition (data not shown). These data are consistent with earlier studies showing that Sxl is not essential for embryonic and larval female germline development (Steinmann-Zwicky 1994). We therefore focused our analysis on the adult ovary isolated from 3- to 7-day-old females.
Dividing snf148 tumor cells are not restricted to the anterior end of the adult germarium:
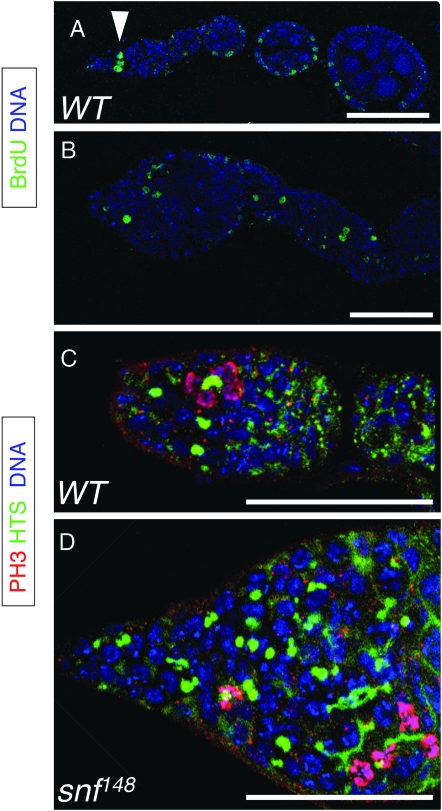
In the adult ovary, mitotically active germ cells are restricted to the anterior end of the germarium. To determine whether the snf tumor cells are similarly restricted, we identified cells in S-phase by pulse labeling with the thymidine analog BrdU. In wild type, germ cells in S-phase were observed as single cells dividing at the tip of the germarium (the GSC and CB cells) or as two-, four-, and eight-cell cysts dividing together (Figure 3A). In contrast, dividing cells in snf tumors were not confined to the anterior half of the germarium and were observed throughout the ovary, even in pseudo-egg chambers (Figure 3B).
Figure 3.—
Proliferating snf148 tumor cells are located throughout the germarium and in pseudo-egg chambers. (A and B) Ovarioles from wild-type (A) and snf148/snf148 females (B) stained for BrdU incorporation (green) to label cells in S-phase and for DNA (blue). In wild type, BrdU positive germ cells are limited to the tip of the germarium (arrowhead). Other cells that are observed to be BrdU positive include the somatic follicle cells that surround the egg chambers. In snf148 mutants, BrdU-positive germ cells are dispersed throughout the germarium and in the pseudo-egg chamber. Bars, 50 μm. (C and D) Germaria from wild-type (C) and snf148/snf148 females (D) stained for HTS (green), DNA (blue), and PH3 (magenta when merged with blue DNA stain). In wild type, interconnected germ cells in mitosis are judged to be in synchrony on the basis of the morphology of the PH3 staining chromosomes. In snf148 mutants, both single cells and groups of interconnected cells are found to be in mitosis. Bars, 50 μm.
To confirm that the dividing cells we located in the snf tumor were in fact germ cells, we double stained ovaries for phosphorylated histone H3 (PH3) and HU LI TAI SHAO (HTS). In wild type, PH3 stains chromosomes starting in late G2 phase and continuing throughout mitosis until the end of telophase. HTS stains the spectrosome, a spherical germ cell-specific organelle found in both GSC and CB cells. During the subsequent divisions, the round spectrosome elongates and branches out to form a fusome that connects the synchronously dividing two-, four-, and eight-cell cysts (Figure 3C). The snf mutant tumors are composed of both single cells with round spectrosomes and cells with abnormal fusome-like structures. Irrespective of their position within the germarium, we observed both single and interconnected germ cells to be in mitosis, as indicated by the intense PH3 staining patterns (Figure 3D).
Proliferation of snf148 tumor cells requires Cyclin B function:
The abnormal distribution of dividing tumor cells raises the question of whether the normal cell-cycle program has been bypassed. Others have reported that Cyclin B (CycB) expression is dependent on Sxl function (Vied et al. 2003). However, and in contrast to the conclusions of their report, when we examined CycB expression in snf tumor cells using an antibody against CYCB, we detected significant staining in snf tumors (data not shown). To confirm this observation with a more robust antibody, we examined expression via a CycB-GFP protein trap line from the Carnegie collection, P{PTT-GC}CycBCC01846 (Buszczak et al. 2007). In wild-type animals, we found that the CYCB-GFP fusion protein expressed from this protein trap allele mimics the endogenous pattern with high levels of expression restricted to the anterior end of the germarium where the dividing germ cells are located (Figure 4A). In snf mutant animals carrying a copy of the protein trap insertion, expression of the CYCB-GFP fusion protein was detectable throughout the tumor (Figure 4B). Occasionally we noted pockets of cells within the tumor that were not stained, suggesting that CYCB undergoes a cell-cycle coordinated pattern of transient accumulation and decay as it does in wild type.
Figure 4.—
CycB is required for snf148 tumor growth. (A and B) Germaria from wild-type (A) and snf148/snf148 mutant (B) females carrying the protein trap allele, P{PTT-GC}CycBCC01846 stained for GFP to detect the CYCB-GFP fusion protein (green) and DNA (blue). In wild type, CYCB-GFP expression is highest at the anterior of the germarium, where the dividing germ cells reside. Staining is also visible in the dividing somatic cells, including the follicle cells that surround the egg chamber. In snf148 mutant ovaries, CYCB-GFP expression is observed throughout the tumor. The images in A and B are the same magnification. (C and D) Ovaries from double-mutant snf148/snf148; CycB2/Df(2R)59AB females (C) or single-mutant snf148/+; CycB2/Df(2R)59AB females (D) stained for VASA (red) and DNA (blue) to show that the removal of CycB suppresses the snf tumor phenotype. While the majority of both double-mutant and control ovaries were found to be devoid of VASA staining cells, the images in C and D are examples of the few ovarioles that contained germ cells. The images in C and D are the same magnification.
CycB is essential for germ cell proliferation (Wang and Lin 2005). To determine whether tumor growth depends on CycB function, we analyzed the phenotype of snf148; CycB2 double mutants. This analysis was carried out by comparing the number of germ cells, stained with VASA, present in homozygous double-mutant animals and control siblings. In sharp contrast to the snf148 tumor phenotype, the double mutant ovaries are not tumorous and contain few or no germ cells. Ninety-five percent of the double-mutant germaria (n = 218) completely lacked VASA stained cells (data not shown). The remaining double-mutant germaria had an average of four germ cells per germarium (Figure 4D). This phenotype is similar to the CycB2 mutant phenotype where 81% (n = 277) of the germaria contained no VASA staining cells (data not shown), and the remaining 19% contained on average eight germ cells (Figure 4C). These results demonstrate that without CycB function, the proliferative capacity of snf tumor cells is sharply curtailed. We conclude that snf tumor cells require CycB for growth. This suggests that the snf tumor cells have not bypassed the normal cell-cycle program utilized by dividing germ cells.
Tumor growth requires zpg-mediated intercellular communication:
Recent studies have suggested that dividing germ cells also require the germ cell-specific gap junction protein encoded by zero population growth (zpg) (Gilboa et al. 2003). To test whether the snf tumor cells require zpg, we compared the number of germ cells, stained with VASA, present in snf148; zpgz2533 double-mutant ovaries with the number present in control siblings. These studies reveal that the double-mutant ovaries are not tumorous and contain an average of only 11 ± 0.4 germline cells per germarium (mean ± SEM, n = 95). This phenotype is similar to the zpg mutant phenotype where the germaria contain on average 6 ± 0.3 germline cells (mean ± SEM, n = 67). Therefore, we conclude that the formation of snf ovarian tumors depends on zpg function.
snf148 tumor cells express both GSC- and CB-specific markers:
Are snf tumor cells arrested at a particular stage of germ cell development? The morphology of the spectrosomes and fusomes is typically used to stage germ cells. As described earlier, when snf tumors are stained with the spectrosome/fusome marker HTS, the mutant ovary is filled with both single and interconnected germ cells (see Figure 3). Within the germarium, ∼40% of the structures are spherical and resemble spectrosomes. Fifty percent are short cylindrical structures that form a bridge between two cells. The remaining 10% are irregular branching structures that connect three or more cells together (see Figure 7E). On the basis of this criterion alone, we found it difficult to stage the snf tumor cells, although no single cell accumulates the oocyte-specific marker ORB, indicating that snf blocks germline development prior to oocyte specification (data not shown). To more accurately stage the snf tumor cells, we followed the expression of three molecular markers known to differ between GSCs and their daughter cells: PIWI, PUMILIO (PUM), and BAM.
Figure 7.—
snf148; bamΔ86 double-mutant analysis. (A–D) Germaria from wild-type (A), snf148/snf148; bamΔ86/+ (B), snf148/+; bamΔ86/bamΔ86 (C), and double-mutant snf148/snf148; bamΔ86/bamΔ86 (D) animals stained for HTS (green) and DNA (blue). In wild type (A) HTS labels the round spectrosomes (arrowhead, sp) and fusomes (arrowhead, fu). Note that HTS also labels somatic cell membranes. In snf mutants (B), round (arrowhead, R), short barbell-shaped (arrowhead, S), and longer branched structures (arrowhead, L) are visible throughout the germarium. In bam mutants (C), the majority of HTS staining material resembles round spectrosomes. In double mutants (D), the morphology of the spectrosomes/fusomes is more similar to the snf mutant phenotype with accumulation of short and elongated fusome-like structures. Bars, 50 μm. (E) Quantification of the spectrosome/fusome morphology observed in double-mutant and control germaria. For each genotype, the number and shape of the spectrosome/fusome-like structures in each germarium were recorded (sample size, 10 germaria per genotype). The structures were classified as (1) round (spherical and not protruding into other cells), (2) short (branches into one adjacent cell), or (3) long (branches into two or more adjacent cells).
The fully functional myc-tagged piwi transgene, P{myc-piwi}, is an effective GSC marker because its nuclear localization pattern within the germline is highest in the GSCs but then rapidly downregulated in CBs and mitotic cysts (Cox et al. 2000). As previously reported, nuclear myc-piwi is highly expressed in germ cells located at the tip of the germarium (Figure 5A). In contrast to wild type, we find the region of myc-piwi expression is dramatically expanded in snf tumors, suggesting a stem cell-like character (Figure 5B).
Figure 5.—
GSC- and CB-specific markers are expressed throughout snf148 mutant ovaries. (A and B) Germaria from wild-type (A) and snf148/snf148 females (B) carrying the nuclear myc-Piwi reporter construct stained for MYC (red or magenta when merged with the blue DNA stain) and DNA (blue). In wild-type germ cells, PIWI is limited to two to three cells at the tip of the germarium. In snf tumors, PIWI-expressing cells are located throughout the tumor. Bar in A, 50 μm. The images in A–F are the same magnification. (C and D) Germaria from wild-type (C) and snf148/snf148 females (D) stained for PUM protein expression (green) and DNA (blue). In wild type, the cytoplasmic PUM protein is highly expressed in two to three cells at the tip of the germarium. In snf148 mutant germaria, PUM-expressing cells are located throughout the tumor. (E and F) Germaria from wild-type (A) and snf148/snf148 females (B) carrying the bamP-GFP reporter construct stained for GFP (green) and DNA (blue). In wild type, only CB cells and dividing cysts express bamP-GFP. Germ cells within the snf148 mutant germaria, however, continue to express bamP-GFP, even in the pseudo-egg chamber. Furthermore, in many cases we observed stained cells located at the tip of the germaria (see text and Figure 6). (G) Western blot of extracts made from ovaries dissected from wild-type and snf148/snf148 mutant females probed with a polyclonal antibody against the Bam-C protein and the U1-70K protein as a loading control.
To confirm that snf tumor cells have retained stem cell-like characteristics, we used the cytoplasmic protein PUM as a second marker. In wild-type germ cells, expression of PUM is independent of piwi function but nevertheless parallels its expression pattern (Figure 5C; Szakmary et al. 2005). In contrast to wild type, PUM-positive cells are distributed throughout the snf mutant germarium (Figure 5, C and D). This observation supports our conclusion that snf tumor cells retain GSC-like characteristics.
To extend this analysis, we examined expression of the P{bamP-GFP} transgene, a transcriptional reporter that is not expressed in GSC cells, but is expressed in the daughter cells fated to differentiate (Figures 1B and 5E). Surprisingly, and in contrast to wild type, the zone of P{bamP-GFP} expression is expanded to include the majority of the cells within the snf tumor (Figure 5F). Two lines of evidence indicate that the expanded P{bamP-GFP} expression domain reflects the accumulation of the endogenous BAM protein. First, whole-mount immunostaining with an antibody against the cytoplasmic BAM protein (BAM-C) reveals that the majority of snf tumor cells express the endogenous BAM protein (data not shown). Second, Western blot analysis of ovarian extracts shows that snf tumors have an increased level of BAM protein compared with wild-type ovaries (Figure 5G).
Together, these studies indicate that the majority of snf tumor cells retain characteristics of both stem cells and their differentiating daughter cells. This atypical molecular signature is due to Sxl dysregulation in the germline because the expression pattern of these markers is restored to wild type in snf mutant animals carrying the P{otu∷SxlcDNA} transgene (data not shown). Thus, germ cells fail to complete the GSC to CB cell fate transition in the absence of SXL protein.
Some snf148 germ cells resemble bona fide GSC cells:
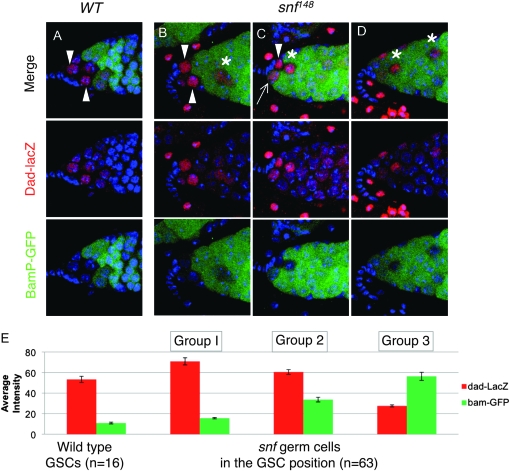
We then focused on the identity of the germ cells found in the GSC position. In the wild-type gonad, the GSCs receive and interpret a short-range somatic TGF-β/BMP signal to silence bam transcription (Chen and McKearin 2003a; Casanueva and Ferguson 2004; Song et al. 2004). Reception of the TGF-β/BMP signal can be monitored by activation of a P{dad-lacZ} enhancer trap line (Tsuneizumi et al. 1997; Kai and Spradling 2003; Casanueva and Ferguson 2004). Therefore, we compared expression patterns in wild-type and snf mutant animals carrying both P{dad-lacZ} and the P{bamP-GFP} transcriptional reporter construct. In agreement with earlier reports, these double-staining studies reveal that in wild type two to three cells adjacent to the cap cells consistently express a high level of dad-lacZ staining and a low level of bamP-GFP staining (arrowheads in Figure 6A). On the other hand, only 64% (n = 88) of the snf mutant germaria had one or more dad-lacZ staining cells in the GSC position (Figure 6, B–D). Interestingly, while some of the dad-lacZ expressing cells resembled wild type with low bamP-GFP staining (arrowheads in Figure 6, B and C), others appeared to be expressing bamP-GFP prematurely (arrow in Figure 6C). We also observed a number of germaria (36%) in which all the cells in the GSC position prematurely expressed bamP-GFP (Figure 6D).
Figure 6.—
TGF-β signaling reception in snf148 mutant germ cells. (A–D) Germaria from wild-type (A) and snf148/snf148 (B–D) females carrying both the dad-lacZ and bamP-GFP reporter constructs stained for β-galactosidase (red), GFP (green), and DNA (blue). Arrows and Arrowheads indicate the presumptive GSCs, identified by their position within the germarium and expression of dad-lacZ. In snf mutant germaria the additional β-galactosidase staining cells located elsewhere in the tumor are marked with an asterisk. (E) Quantification of β-galactosidase and GFP staining intensity in cells adjacent to the somatic cap cells. The average intensity (± SEM) is presented. Wild-type cells were all very similar to each other, with levels of β-galactosidase staining ranging from 40 to 80 and basal levels of GFP. snf148/snf148 mutant cells on the other hand varied and were divided into three groups on the basis of their staining patterns. Groups 1 and 2 all had levels of β-galactosidase staining that fell within the wild-type range, i.e., >40. This group was further subdivided into those that expressed GFP within the wild-type range, i.e., <20 (group 1), and those that had higher levels of GFP staining (group 2). Those cells with levels of β-galactosidase staining of <40 were placed in group 3.
As illustrated in Figure 6E, when we compared the fluorescence intensity of GFP and β-galactosidase staining of individual germ cells located in the GSC position (n = 16), we found that in wild-type germaria the cells were very similar to each other with high levels of dad-lacZ staining (average relative pixel intensity value of 53.4 ± 2.9; mean ± SEM) and basal levels of bamP-GFP (10.9 ± 0.9). When we compared the fluorescence intensity in individual snf mutant germ cells located in the GSC position (n = 63), we found that the cells fell roughly into three groups. The first group (22%) resembled wild-type GSC cells with high levels of dad-lacZ (70.9 ± 3.5) and basal levels of bamP-GFP (15.6 ± 0.4). The second group (38%) also had similar levels of dad-lacZ to wild type (60.6 ± 2.0), but increasing levels of bamP-GFP expression (33.7 ± 2.3). The third group (40%) was markedly different from wild-type GSC cells with basal levels of dad-lacZ staining (27.5 ± 1.0) and high levels of bamP-GFP staining (56.3 ± 4.0).
On the basis of these observations, we conclude that there is a population of snf mutant germ cells that resembles bona fide GSCs: e.g., cells that receive and interpret the TGF-β/BMP signal to repress bam transcription in a position-appropriate manner. However, this population does not appear to be stable, as we find that 36% of the mutant germaria prematurely express bamP-GFP in all cells in the GSC position (Figure 6D). It is worth noting that we also consistently observed one or more dad-lacZ staining cells, with a correspondingly low level of bamP-GFP staining in other areas of the tumor (asterisks in Figure 6, B–D). This observation raises the possibility that some of the tumor cells retain their GSC identity, but have been pushed out of their native location at the tip of the germarium. Other explanations, including tumor cells undergoing dedifferentiation, are also possible.
BAM protein, although present, is not functional in snf tumor cells:
Previous studies have shown that bam expression is sufficient to promote differentiation (Ohlstein and McKearin 1997), yet we have shown that snf mutant ovaries accumulate significant amounts of BAM protein without showing signs of differentiation. Given that snf is a splicing factor, one plausible molecular explanation for the lack of bam function is that the snf148 mutation compromises the production of functional BAM protein by reducing the efficiency and/or accuracy of splicing. However, this is unlikely to be the case because (1) we found no evidence of misspliced bam RNA products in snf148/snf148 mutants and (2) the snf tumor phenotype is not rescued by P{hsp70∷bam}, a heat-shock-inducible transgene expressing the full-length bam cDNA (data not shown).
To elaborate on the genetic relationship of snf and bam, we analyzed the phenotype of snf148; bamΔ86 double mutants. snf and bam mutant phenotypes can be distinguished by fusome morphology (Figure 7). The shapes of the fusome-like structures in snf tumors range from spherical (40%), to short and cylindrical (50%), to branching structures (10%). In contrast, long branching fusome-like structures are not observed in bam tumors. Instead, 75% of the fusomes are spherical and the remaining 25% are short and cylindrical. The double-mutant phenotype is more similar to snf than to bam, with the same proportion and type of structures that range from spherical to branching. This result indicates that bam is not functional in snf mutant ovaries and, together with earlier studies showing that SXL protein is expressed in bam mutant germ cells (Bopp et al. 1993), leads us to suggest that Sxl and bam function in parallel to promote differentiation.
bam and snf tumors misexpress a common set of testis-enriched transcripts:
If the BAM and SXL proteins do in fact function in parallel to control entry into a common differentiation pathway, then snf and bam tumors should have similar molecular signatures. In light of studies with testis-specific enhancer trap lines showing inappropriate expression in female germ cells with reduced Sxl expression (Wei et al. 1994; Staab and Steinmann-Zwicky 1996), we asked whether snf and bam tumors express a common set of testis-enriched transcripts.
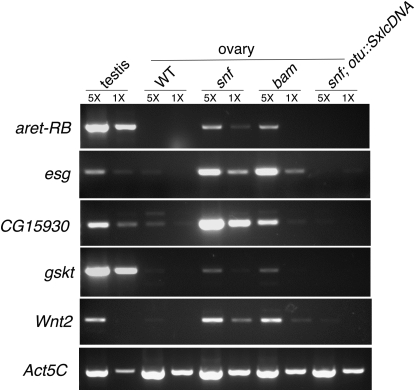
To begin this analysis we searched for a set of testis-enriched transcripts whose expression is markedly increased in snf mutant ovaries relative to wild-type ovaries and ovaries isolated from rescued snf148/snf148; P{otu∷SxlcDNA} females. As illustrated in Figure 8, we identified four transcripts that fit this criterion:
arrest-RB (aret, aka bruno): aret encodes multiple transcripts, one of which, aret-RB, is enriched in testis (Webster et al. 1997). Our attention was drawn to aret because its expression, like that of Sxl, is dictated by the phenotypic sex of the somatic gonad (Waterbury et al. 2000).
escargot (esg): Gonadal expression of esg is male specific and has been used by a number of investigators as a marker for male sexual identity (Staab and Steinmann-Zwicky 1996; Kiger et al. 2000; Streit et al. 2002; Wawersik et al. 2005). esg was chosen for this analysis because previous studies, using an esg enhancer trap line, indicated that esg was inappropriately expressed in female germ cells with reduced Sxl expression (Wei et al. 1994; Staab and Steinmann-Zwicky 1996).
gasket (gskt, aka mojoless): gskt is enriched in male germline cells and was recently reported to be ectopically expressed in female germ cells with reduced Sxl expression (Kalamegham et al. 2007; R. Kalamegham, personal communication).
Wnt oncogene analog 2 (Wnt2): Wnt2 encodes a testis-enriched member of the wingless family of genes and has been used as a marker for male sexual identity (Kozopas et al. 1998; Chintapalli et al. 2007; Defalco et al. 2008).
Figure 8.—
Testis-enriched markers are expressed in both snf and bam mutant ovaries. RT–PCR analysis is shown of aret-RB, esg, CG15930, gskt, Wnt2, and Act5C mRNA levels in testis from wild-type animals and in ovaries from wild-type, snf148/snf148, bamΔ86/bamΔ86, and snf148/snf148; P{otu∷SxlcDNA} females. Act5C is used here as a loading control. To ensure that the mRNA measurements fall within a linear range of amplification, each RT reaction was serially diluted fivefold (1× and 5×). Similar results were obtained in three independent biological replicates.
Remarkably, we discovered that all four of these RNAs are also expressed in bam mutant ovaries, indicating that the snf and bam tumors have a similar molecular signature. To follow up on this observation we asked whether CG15930, a transcript whose expression was noted to be significantly enriched in purified bam mutant germ cells (Kai et al. 2005), was similarly enriched in snf tumors. In agreement with this and other array-based measurements (Terry et al. 2006; Chintapalli et al. 2007), our RT–PCR analysis shows that CG15930 is a testis-enriched transcript whose expression is markedly increased in bam mutant ovaries (Figure 8). Intriguingly, we found that CG15930 was similarly enriched in snf mutant ovaries, but not in ovaries isolated from rescued snf148/snf148; P{otu∷SxlcDNA} females.
Together these data provide additional support for our conclusion, based on genetic epistasis experiments, that bam and Sxl function in parallel. While testis-enriched transcripts are clearly expressed in both bam and snf tumors, we continue to detect a series of ovary-enriched RNAs in both tumor types, indicating that the tumor cells are not sex reversed (e.g., hts-RA, klu-RA, ovo-RC, and stet-RA).
DISCUSSION
The observation that female germ cells lacking Sxl are tumorigenic was first published >20 years ago, yet the place of this female-specific RNA binding protein in the genetic circuitry controlling oogenesis has remained elusive. Here, we investigate Sxl's role in the germline by taking advantage of a snf mutant allele that specifically eliminates Sxl expression in the germline. Our genetic and cell biological analysis established that Sxl is required for the transition from stem cell to committed daughter cell by showing that the majority of Sxl-deficient germ cells have acquired an intermediate fate. These findings are in contrast to the commonly held view, based on fusome morphology alone, that Sxl mutant germ cells arrest development later in the differentiation pathway. This study also offers new insight into the function of bam by demonstrating that its differentiation-promoting function depends on Sxl and, importantly, that Sxl and bam control the same sex-specific expression network.
In current models, maintenance of GSC identity requires contact with the niche to trigger the signal transduction cascade required for transcriptional repression of bam. This in turn provides a permissive environment that allows PUM, which forms a complex with its partner protein Nanos (NOS), to inhibit translation of a yet unidentified set of mRNAs required for differentiation. Differentiation begins when one of the daughter cells is displaced from the niche and can no longer receive the signals that silence bam transcription. BAM then initiates the differentiation program by antagonizing the translation-inhibitory functions of the PUM/NOS complex. This model predicts a strong negative correlation between the expression of bam and the GSC markers, and, while this is true in general, there have been reports of rare single cells that coexpress bam and one or more GSC-specific markers (Szakmary et al. 2005). These and other studies have suggested that cells fated to differentiate first pass through an intermediate stage that transitions, without dividing, to a mature CB (Ohlstein and McKearin 1997; Chen and McKearin 2003a; Gilboa et al. 2003; Kai and Spradling 2003; Casanueva and Ferguson 2004; Szakmary et al. 2005).
We show that Sxl is required to complete the transition from GSC to a mature CB by demonstrating that the majority of germ cells lacking Sxl resemble an immature CB-like cell. Furthermore, genetic epistasis experiments suggest that the failure to progress beyond this intermediate stage is attributable to a lack of bam function. This conclusion is supported by studies showing that the tumors resulting from the lack of Sxl and bam are remarkably similar. Specifically, the loss of Sxl and bam results in germ cell tumors with the same unique molecular signature including expression of stem cell markers (this study; Szakmary et al. 2005) and with the same set of testis-enriched markers (this study). Both types of germ cell tumors also require CycB and zpg for growth (this study; Gilboa et al. 2003; Wang and Lin 2005). This comparison reveals that snf and bam tumors both result from a failure to initiate the differentiation pathway in stem cell progeny. It will be interesting to determine what role the misregulated testis-enriched markers play in this process.
On the basis of these data, we propose that Sxl partners with bam to facilitate the transition between GSCs and the daughter cell that is fated to differentiate. In females, differentiation via control of bam transcription is initiated in response to position-dependent extrinsic cues from the somatic gonad. Extrinsic cues from the somatic gonad also provide essential sex-specific information, via control of Sxl expression. Our findings suggest that the intrinsic Sxl/bam partnership serves to integrate these two different extrinsic signaling pathways. This proposal is particularly compelling because it explains how bam function is substantially different in males and females (McKearin and Ohlstein 1995; Gonczy et al. 1997).
How might Sxl and bam function converge to promote female germ cell differentiation? SXL acts post-transcriptionally to repress splicing and translation. The molecular function of BAM, on the other hand, is unknown but is also thought to act post-transcriptionally (Ohlstein et al. 2000). At a genetic level, one function of bam is to antagonize the differentiation-inhibiting activity of PUM/NOS. The presence of putative high-affinity SXL-binding sites in both the 5′-UTR and the 3′-UTR of the nos mRNA leads us to speculate that SXL functions with BAM to promote differentiation by inhibiting the translation of nos. Although this model is consistent with our finding that SXL and BAM are coexpressed in the appropriate cell type, biochemical studies to address this point have proved to be technically challenging.
In summary, our studies support a model in which the Sxl/bam pathway is required for germ cells to progress from a stem cell fate to a differentiation-competent CB fate (Figure 9). Our studies also suggest that if this pathway is blocked, germ cells will continue to proliferate, forming a tumor. We propose that the block in the developmental progression from stem cell to fully committed daughter cell is the initial tumorigenic event. This model is consistent with the general view that adult stem cells are the source of some, and perhaps all, tumors. Not only do some human germ cell tumors display many of the same characteristics as the Drosophila tumors described here, including expression of stem cell markers, but also they occur frequently in individuals with intersex disorders (reviewed by Clark 2007; Looijenga et al. 2008). While true orthologs of Sxl and bam are not found in vertebrates, the processes that they regulate are likely to be conserved. Future studies aimed at understanding the functional connections between the failure to engage the Sxl/bam genetic programs, misexpression of testis-enriched markers, and tumorigenesis will likely provide mechanistic insight into the pathogenesis of germ cell tumors in humans.
Figure 9.—
A model for ovarian tumor formation. (A) In wild type the GSC cell (pink) divides asymmetrically to produce one daughter cell that will remain a stem cell and a second daughter cell destined to differentiate (pink and green striped) called a pre-CB cell because these cells express both GSC markers and BAM. Before the next division, the cell matures and GSC marker expression is attenuated (green cell). Here we propose that the SXL protein, which is highly expressed in the cytoplasm of both GSC and CB cells, partners with the newly expressed BAM protein, to antagonize the GSC-specific gene expression program. The mature CB cell then undergoes four rounds of mitosis to yield a 16-cell differentiated cyst. (B) In the absence of SXL or BAM protein the pre-CB cells continue to express GSC markers and misexpress a set of testis-specific markers (blue stripes). As a result, these differentiation-defective cells fail to mature and continue to divide symmetrically, forming a germline tumor.
Acknowledgments
We gratefully acknowledge gifts of antibodies and/or fly stocks from M. Buszczak, D. McKearin, P. Lasko, R. Lehmann, H. Lin, P. MacDonald, T. Xie, the Iowa Hybridoma Center, and the Bloomington Stock center. We are grateful to P. Hunt, M. L. Johnson, H. Lou, K. Molyneaux, J. McDonald, and J. Wise for helpful discussions. National Institutes of Health (NIH) grant R01-GM61039 provided support for this work. NIH National Center for Research Resources shared instrumentation grant RR-017980 funded the confocal microscope used in this study and partial salary support for J.C. was provided by NIH training grant T32-HD07104.
References
- Bopp, D., L. R. Bell, T. W. Cline and P. Schedl, 1991. Developmental distribution of female-specific SEX-LETHAL proteins in Drosophila melanogaster. Genes Dev. 5 403–415. [DOI] [PubMed] [Google Scholar]
- Bopp, D., J. I. Horabin, R. A. Lersch, T. W. Cline and P. Schedl, 1993. Expression of the Sex-lethal gene is controlled at multiple levels during Drosophila oogenesis. Development 118 797–812. [DOI] [PubMed] [Google Scholar]
- Buszczak, M., S. Paterno, D. Lighthouse, J. Bachman, J. Planck et al., 2007. The Carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 175 1505–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanueva, M. O., and E. L. Ferguson, 2004. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control DPP signaling. Development 131 1881–1890. [DOI] [PubMed] [Google Scholar]
- Casper, A., and M. Van Doren, 2006. The control of sexual identity in the Drosophila germline. Development 133 2783–2791. [DOI] [PubMed] [Google Scholar]
- Chen, D., and D. M. McKearin, 2003. a DPP signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr. Biol. 13 1786–1791. [DOI] [PubMed] [Google Scholar]
- Chen, D., and D. M. McKearin, 2003. b A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130 1159–1170. [DOI] [PubMed] [Google Scholar]
- Chintapalli, V. R., J. Wang and J. A. Dow, 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39 715–720. [DOI] [PubMed] [Google Scholar]
- Clark, A. T., 2007. The stem cell identity of testicular cancer. Stem Cell Rev. 3 49–59. [DOI] [PubMed] [Google Scholar]
- Cline, T. W., and B. J. Meyer, 1996. Vive la difference: males vs. females in flies vs. worms. Annu. Rev. Genet. 30 637–702. [DOI] [PubMed] [Google Scholar]
- Cox, D. N., A. Chao and H. Lin, 2000. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127 503–514. [DOI] [PubMed] [Google Scholar]
- Defalco, T., N. Camara, S. Le Bras and M. Van Doren, 2008. Nonautonomous sex determination controls sexually dimorphic development of the Drosophila gonad. Dev. Cell 14 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, M. T., and A. C. Spradling, 2007. Male and female Drosophila germline stem cells: two versions of immortality. Science 316 402–404. [DOI] [PubMed] [Google Scholar]
- Gilboa, L., A. Forbes, S. I. Tazuke, M. T. Fuller and R. Lehmann, 2003. Germ line stem cell differentiation in Drosophila requires gap junctions and proceeds via an intermediate state. Development 130 6625–6634. [DOI] [PubMed] [Google Scholar]
- Gonczy, P., E. Matunis and S. Dinardo, 1997. bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development 124 4361–4371. [DOI] [PubMed] [Google Scholar]
- Hager, J. H., and T. W. Cline, 1997. Induction of female Sex-lethal RNA splicing in male germ cells: implications for Drosophila germline sex determination. Development 124 5033–5048. [DOI] [PubMed] [Google Scholar]
- Jacobs, H. W., J. A. Knoblich and C. F. Lehner, 1998. Drosophila CYCLIN B3 is required for female fertility and is dispensable for mitosis like CYCLIN B. Genes Dev. 12 3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer, B., and M. Steinmann-Zwicky, 2001. Cell-autonomous and somatic signals control sex-specific gene expression in XY germ cells of Drosophila. Mech. Dev. 100 3–13. [DOI] [PubMed] [Google Scholar]
- Kai, T., and A. Spradling, 2003. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc. Natl. Acad. Sci. USA 100 4633–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai, T., D. Williams and A. C. Spradling, 2005. The expression profile of purified Drosophila germline stem cells. Dev. Biol. 283 486–502. [DOI] [PubMed] [Google Scholar]
- Kalamegham, R., D. Sturgill, E. Siegfried and B. Oliver, 2007. Drosophila mojoless, a retroposed GSK-3, has functionally diverged to acquire an essential role in male fertility. Mol. Biol. Evol. 24 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger, A. A., H. White-Cooper and M. T. Fuller, 2000. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature 407 750–754. [DOI] [PubMed] [Google Scholar]
- Kirilly, D., and T. Xie, 2007. The Drosophila ovary: an active stem cell community. Cell Res. 17 15–25. [DOI] [PubMed] [Google Scholar]
- Knoblich, J. A., and C. F. Lehner, 1993. Synergistic action of Drosophila CYCLINS A and B during the G2-M transition. EMBO J. 12 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozopas, K. M., C. H. Samos and R. Nusse, 1998. DWnt-2, a Drosophila wnt gene required for the development of the male reproductive tract, specifies a sexually dimorphic cell fate. Genes Dev. 12 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko, P. F., and M. Ashburner, 1990. Posterior localization of VASA protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 4 905–921. [DOI] [PubMed] [Google Scholar]
- Lin, H., 2008. Cell biology of stem cells: an enigma of asymmetry and self-renewal. J. Cell Biol. 180 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looijenga, L. H., A. J. Gillis, H. J. Stoop, R. Hersmus and J. W. Oosterhuis, 2008. Chromosomes and expression in human testicular germ-cell tumors: insight into their cell of origin and pathogenesis. Ann. N Y Acad. Sci. 1120 187–214. [DOI] [PubMed] [Google Scholar]
- Macdonald, P. M., 1992. The Drosophila pumilio gene: an unusually long transcription unit and an unusual protein. Development 114 221–232. [DOI] [PubMed] [Google Scholar]
- McKearin, D., and B. Ohlstein, 1995. A role for the Drosophila BAG-OF-MARBLES protein in the differentiation of cystoblasts from germline stem cells. Development 121 2937–2947. [DOI] [PubMed] [Google Scholar]
- McKearin, D. M., and A. C. Spradling, 1990. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 4 2242–2251. [DOI] [PubMed] [Google Scholar]
- Nagengast, A. A., S. M. Stitzinger, C.-H. Tseng, S. M. Mount and H. K. Salz, 2003. Sex-lethal splicing autoregulation in vivo: interactions between SEX-LETHAL, the U1 snRNP and U2AF underlie male exon skipping. Development 130 463–471. [DOI] [PubMed] [Google Scholar]
- Ohlstein, B., and D. McKearin, 1997. Ectopic expression of the Drosophila BAM protein eliminates oogenic germline stem cells. Development 124 3651–3662. [DOI] [PubMed] [Google Scholar]
- Ohlstein, B., C. A. Lavoie, O. Vef, E. Gateff and D. M. McKearin, 2000. The Drosophila cystoblast differentiation factor, benign gonial cell neoplasm, is related to DExH-box proteins and interacts genetically with bag-of-marbles. Genetics 155 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, B., 2002. Genetic control of germline sexual dimorphism in Drosophila. Int. Rev. Cytol. 219 1–60. [DOI] [PubMed] [Google Scholar]
- Oliver, B., Y.-J. Kim and B. S. Baker, 1993. Sex-lethal, master and slave: a hierarchy of germline sex determination in Drosophila. Development 119 897–908. [DOI] [PubMed] [Google Scholar]
- Penalva, L. O., and L. Sanchez, 2003. RNA binding protein SEX-LETHAL (SXL) and control of Drosophila sex determination and dosage compensation. Microbiol. Mol. Biol. Rev. 67 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz, H. K., T. W. Cline and P. Schedl, 1987. Functional changes associated with structural alterations induced by mobilization of a P element inserted in the Sex-lethal gene of Drosophila. Genetics 117 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach, T., 1985. Normal female germ cell differentiation requires the female X chromosome to autosome ratio and expression of Sex-lethal in Drosophila melanogaster. Genetics 109 529–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X., M. D. Wong, E. Kawase, R. Xi, B. C. Ding et al., 2004. BMP signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131 1353–1364. [DOI] [PubMed] [Google Scholar]
- Staab, S., and M. Steinmann-Zwicky, 1996. Somatic sex-determining signals act on XX germ cells in Drosophila embryos. Development 122 4065–4071. [DOI] [PubMed] [Google Scholar]
- Starr, D. J., and T. W. Cline, 2002. A host parasite interaction rescues Drosophila oogenesis defects. Nature 418 76–79. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky, M., 1994. Sxl in the germline of Drosophila: a target for somatic late induction. Dev. Genet. 15 265–274. [DOI] [PubMed] [Google Scholar]
- Streit, A., L. Bernasconi, P. Sergeev, A. Cruz and M. Steinmann-Zwicky, 2002. mgm 1, the earliest sex-specific germline marker in Drosophila, reflects expression of the gene esg in male stem cells. Int. J. Dev. Biol. 46 159–166. [PubMed] [Google Scholar]
- Szakmary, A., D. N. Cox, Z. Wang and H. Lin, 2005. Regulatory relationship among piwi, pumilio, and bag-of-marbles in Drosophila germline stem cell self-renewal and differentiation. Curr. Biol. 15 171–178. [DOI] [PubMed] [Google Scholar]
- Tazuke, S. I., C. Schulz, L. Gilboa, M. Fogarty, A. P. Mahowald et al., 2002. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development 129 2529–2539. [DOI] [PubMed] [Google Scholar]
- Terry, N. A., N. Tulina, E. Matunis and S. Dinardo, 2006. Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev. Biol. 294 246–257. [DOI] [PubMed] [Google Scholar]
- Tsuneizumi, K., T. Nakayama, Y. Kamoshida, T. B. Kornberg, J. L. Christian et al., 1997. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature 389 627–631. [DOI] [PubMed] [Google Scholar]
- Vied, C., N. Halachmi, A. Salzberg and J. I. Horabin, 2003. Antizyme is a target of Sex-lethal in the Drosophila germline and appears to act downstream of hedgehog to regulate Sex-lethal and cyclin B. Dev. Biol. 253 214–229. [DOI] [PubMed] [Google Scholar]
- Wang, Z., and H. Lin, 2005. The division of Drosophila germline stem cells and their precursors requires a specific cyclin. Curr. Biol. 15 328–333. [DOI] [PubMed] [Google Scholar]
- Waterbury, J. A., J. I. Horabin, D. Bopp and P. Schedl, 2000. Sex determination in the Drosophila germline is dictated by the sexual identity of the surrounding soma. Genetics 155 1741–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawersik, M., A. Milutinovich, A. L. Casper, E. Matunis, B. Williams et al., 2005. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature 436 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, P. J., L. Liang, C. A. Berg, P. Lasko and P. M. Macdonald, 1997. Translational repressor BRUNO plays multiple roles in development and is widely conserved. Genes Dev. 11 2510–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, G., B. Oliver, B. Pauli and A. P. Mahowald, 1994. Evidence for sex transformation of germline cells in ovarian tumor mutants of Drosophila. Dev. Biol. 161 318–320. [DOI] [PubMed] [Google Scholar]
- Zaccai, M., and H. D. Lipshitz, 1996. Role of Adducin-like (hu-li tai shao) mRNA and protein localization in regulating cytoskeletal structure and function during Drosophila oogenesis and early embryogenesis. Dev. Genet. 19 249–257. [DOI] [PubMed] [Google Scholar]