Abstract
Objective:
To determine if serum concentrations of testosterone precursors would correlate with intratesticular testosterone (ITT) concentration measured directly by testicular aspiration and allow for a less invasive means of inferring ITT.
Design:
Controlled clinical study.
Setting:
Healthy volunteers in an academic research environment.
Patients:
Twenty-nine normal men.
Intervention:
We determined ITT concentration by testicular aspiration before and after treatment in men receiving exogenous testosterone to block endogenous gonadotropin production and randomly assigned to one of four doses of human chorionic gonadotropin (hCG) (0, 125 IU, 250 IU, 500 IU every other day) for 3 weeks.
Outcome measures:
The association between serum 17-hydroxyprogesterone, androstenedione and dihydroepiandrosterone (DHEA) and ITT.
Results:
With testosterone administration alone, serum 17-hydroxyprogesterone decreased significantly and increased significantly when 500 IU hCG was administered. End-of-treatment ITT strongly correlated with serum 17-hydroxyprogesterone. Moreover, serum 17-hydroxyprogesterone, but not androstenedione or DHEA, was independently associated with end-of-treatment ITT by multivariate linear regression.
Conclusion:
Serum 17-hydroxyprogesterone is highly correlated with ITT in gonadotropin suppressed normal men receiving testosterone and stimulated with hCG. Serum 17-hydroxyprogesterone is a surrogate biomarker of ITT and may be useful in research and in men receiving gonadotropin therapy for infertility.
Keywords: intratesticular testosterone, 17-hydroxyprogesterone, male infertility, male contraception
Introduction
Testosterone is essential for the initiation and maintainence of spermatogenesis within the testes (1-4). Testosterone is synthesized and secreted from the Leydig cells of the testicular interstitium when stimulated by luteinizing hormone (LH) (5). Intratesticular testosterone (ITT) mediates its effects by binding to the androgen receptor, which is found in Leydig cells, Sertoli cells, and peritubular cells but is not present in developing germ cells (6). ITT concentrations in normal men are approximately 100 times those in serum (7-11). Experimental suppression of LH secretion from the pituitary by the administration of exogenous testosterone leads to pronounced decreases in ITT and dramatic reductions in sperm production (7, 9, 10, 12). In contrast, the administration of the LH receptor agonist, human chorionic gonadotropin (hCG) increases ITT and stimulates spermatogenesis, both in men with hypogonadotropic hypogonadism from pituitary disease (13) and in men with experimentally induced gonadotropin deficiency (14).
Despite the central role of ITT in male reproduction, the quantitative relationship between LH signal, ITT and spermatogenesis is unknown. This deficit in knowledge stems largely from the difficulty in evaluating the intratesticular milieu over time within an individual during hormonal manipulation. Current options for measuring ITT include repeat surgical testicular biopsies, which can lead to testicular injury (15), and testicular aspirations, which can be painful and have the potential to cause bleeding or other complications. Therefore, the identification of a serum surrogate or biomarker of ITT would be of great value, since it might allow for serial monitoring of ITT during hormonal manipulation, without the need for more invasive procedures. Such a surrogate marker could be a useful clinically in conjunction with measurements of serum testosterone to determine the optimal dosage of hCG for gonadotropin-deficient men being treated for infertility. In addition, a serum marker of ITT could be useful in monitoring men on treatments aiming to suppress endogenous testosterone biosynthesis, such as therapy for hormone-responsive prostate cancer or experimental hormonal male contraceptives.
Previous work investigating the response of the testis to hCG has demonstrated that serum concentrations of testosterone precursors such as 17-hydroxyprogesterone, androstenedione and dihydroepiandrosterone (DHEA) are increased by hCG administration (16). Therefore, we hypothesized that serum concentrations of these testosterone precursors might correlate with ITT and potentially provide a serum biomarker of ITT and Leydig cell function.
Therefore, we measured serum 17-hydroxyprogesterone, androstenedione and dihydroepiandrosterone (DHEA) in a group of normal men receiving testosterone enanthate to suppress endogenous gonadotropins and various doses of human chorionic gonadotropin (hCG). We correlated ITT with serum measurements of these testosterone precursor hormones to ascertain the relationship between them and ITT during various degrees of Leydig cell stimulation with hCG.
Methods
Subjects
The Institutional Review Board at the University of Washington approved all procedures involving human subjects. All subjects were participants in a previously published study to determine the dose-response of ITT to hCG stimulation conducted at the University of Washington (10). Complete information on study design and results can be found in the original publication. In brief, 29 normal healthy men between 18 and 45 years of age (mean 24 ± 6.5) were recruited for the study. Subjects were healthy as determined by medical history and physical examination, and had normal clinical laboratory tests, normal testicular volume, normal serum T, LH, FSH, and sperm concentration of greater than 20 million/ml after 48 hours of ejaculatory abstinence. Exclusion criteria included chronic medical or mental illness, previous or current ethanol abuse, anabolic steroid use, abnormal screening labs, single testicle, abnormal reproductive physiology, infertility or a family history of congenital adrenal hyperplasia.
Study Procedures
All subjects were treated with testosterone enanthate (TE) 200 mg IM on day 0, 7 and 14 to suppress endogenous gonadotropin secretion from the pituitary. After enrollment, the hospital pharmacist, randomly assigned subjects to one of four hCG treatment groups: hCG 0 (saline placebo), 125, 250 or 500 IU, which was administered subcutaneously every other day for 3 weeks (11 total doses). The first dose of hCG was administered immediately after the first percutaneous fine needle aspirate of testicular fluid on Day 0 while the last dose of hCG was administered on Day 20 of the study. Study personnel administered all injections. On day 21 a second percutaneous testicular fine needle aspiration was performed.
Blood was drawn immediately prior to the aspirations at baseline and on day 21. Serum was separated by centrifugation and stored at −70°C until assayed for hormone concentrations after the completion of the study.
Testicular Fluid Aspiration
Testicular fluid was sampled by fine needle aspiration at baseline and after 3 weeks of treatment with TE plus placebo or hCG (10). With the subject lying on an examination table in the supine position, the skin over the spermatic cord at the external ring was cleansed with alcohol. Then, a bilateral spermatic cord block was performed with 10 cc of 1% buffered lidocaine. The skin over the anterior-superior aspect of the testes was then cleansed with alcohol, and a 19-gauge butterfly needle inserted into the testicular parenchyma. Negative pressure was generated with an attached syringe until testicular fluid was obtained. The testicular fluid sample was immediately placed on ice, and centrifuged at 300g. The aspirate supernatant was stored at −70°C. Fluid from the right and left testis was pooled for the measurement of testosterone.
Measurements
Serum 17-hydroxyprogesterone was measured by immunofluorometric assay (Diagnostic Products Corp, Los Angeles, CA, USA). The sensitivity of the assay was 0.6 nmol/L. The intra and interassay coefficients of variation were 6.7% and 11% for low pools, and 3.5% and 8.5 % for high pools. The normal range in men age 20-59 is 1.8-10.3 nmol/L. Serum androstenedione was measured by radioimmunoassay (Diagnostic Products Corp, Los Angeles, CA, USA). The sensitivity of the assay was 0.45 nmol/L. The intra and interassay coefficients of variation were 5.6% and 9.8% for low pools, and 2.8% and 9.0 % for high pools. The normal range in men age 20-59 is 1.0-9.2 nmol/L. Serum DHEA was measured by radioimmunoassay (Diagnostic Products Corp, Los Angeles, CA, USA). The sensitivity of the assay was 0.7 nmol/L. The intra and interassay coefficients of variation were 5.6% and 10.2% for low pools, and 7.3% and 7.0 % for high pools. The normal range in men age 20-59 is 4.9-43.3 nmol/L. The cross-reactivities of these assays with related hormonal compounds is less than 1%. The measurement of testosterone in testicular aspirates has been described in detail previously (9-12). The sensitivity of the intratesticular fluid testosterone RIA was 10 pg/tube with inter- and intra-assay coefficients of variation of 11.2% and 9.6%, respectively.
Statistical analyses
Due to non-normal distributions within groups, hormone concentrations were expressed as medians, with 25th and 75th percentiles included in parentheses. Change in serum hormone concentrations within a group between baseline and day 21 was compared with a Wilcoxon sign-rank test. Serum hormone concentrations between dose groups of hCG were compared by Kruskal-Wallis ANOVA with a Wilcoxon rank-sum post-hoc test and a Bonferroni correction for multiple comparisons. Correlations between ITT and hormone concentrations were performed using Spearman's technique. The association between ITT and hormone concentrations, age and weight was analyzed by univariate and multivariate linear regression using robust standard errors on log-transformed data and back-transformed for presentation. For all comparisons, a two-sided alpha of <0.05 was considered significant. Statistical analyses were performed using STATA version 8.0 (College Park, TX).
Results
Subjects
All 29 participants completed the study. All testosterone injections were administered on schedule, and 316 of 319 hCG or placebo injections were administered per protocol. Of the three missed injections, one was in a placebo subject and one occurred in two different subjects in the 250 IU hCG group, both on study day 5. The aspiration procedure was well tolerated by all participants without complications or significant discomfort. Unfortunately, in one subject in the 125 IU hCG group and one in the 250 IU hCG group, insufficient testicular fluid was obtained during the end-of-treatment aspiration procedure for measurement of ITT. Therefore, analyses were performed on data from the 27 subjects in whom both baseline and end-of-treatment ITT measurements were available. After three weeks of treatment with exogenous testosterone enanthate, the mean serum LH was suppressed from 4.11 ± 0.37 to 0.21 ± 0.03 IU/L (5% of baseline) on day 21, and the mean serum FSH was suppressed from 2.83 ± 0.37 to 0.09 ± 0.01 IU/L on day 21 (both p <0.0001).
Serum Testosterone Precursors
At end-of-treatment, serum 17-hydroxyprogesterone was significantly reduced in the 0 IU hCG group [median (25%, 75%): 5.1 (3.8, 6.2) nmol/L at baseline vs. 2.1 (1.5, 2.5) nmol/L at end-of-treatment; p = 0.02). In contrast, serum 17-hydroxyprogesterone was significantly increased in the 500 IU hCG group [median (25%, 75%): 4.6 (4.1, 5.4) nmol/L at baseline vs. 7.8 (5.5, 9.4) nmol/L at end-of-treatment; (p = 0.02)]. In addition, serum androstenedione was significantly increased in the 250 IU and 500 IU hCG groups (p = 0.03 and p = 0.02, respectively), but not in the 0 IU or 125 IU hCG groups compared with baseline.
At baseline between hCG dose groups, there were no significant differences in the serum concentrations of 17-hydroxyprogesterone, androstenedione or DHEA (Table 1). At the end-of-treatment, however, serum 17-hydroxyprogesterone was significantly elevated in 250 IU and 500 IU hCG groups compared with the 0 IU and 125 IU groups (p <0.05 for all comparisons) (Table 1). The dose-dependency between hCG dose groups and serum 17-hydroxyprogesterone was highly statistically significant (p < 0.001). In contrast, neither serum androstenedione nor serum DHEA differed significantly between dose groups of hCG at the end-of-treatment.
Table 1.
Serum and intratesticular hormone concentrations at baseline and after three weeks of treatment with one of four doses of human chorionic gonadotropin (hCG). All values are medians (interquartile range).
| hCG treatment group | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| 0 IU (n = 7) | 125 IU (n = 7) | 250 IU (n = 6) | 500 IU (n = 7 ) | p-value (trend) | ||
| Baseline | ||||||
| Intratesticular testosterone (nmol/L) | 1111 (982, 1630) | 1063 (848, 1188) | 1239 (1105, 1535) | 1227 (918, 1338) | 0.48 | |
| 17-hydroxyprogesterone (nmol/L) | 5.1 (3.8, 6.2) | 5.3 (3.5, 6.3) | 4.9 (3.8, 6.8) | 4.6 (4.1, 5.4) | 0.98 | |
| Androstenedione (nmol/L) | 6.6 (5.6, 7.3) | 6.6 (6.1, 9.3) | 6.4 (4.8, 6.8) | 6.5 (6.0, 8.2) | 0.73 | |
| DHEA (nmol/L) | 35 (24, 48) | 30 (22, 53) | 32 (24, 44) | 34 (22, 39) | 0.99 | |
| End of Treatment | ||||||
| Intratesticular testosterone (nmol/L) | 58 (57, 110)a | 780 (409, 1146)b | 1037 (913, 1291)b | 1470 (738, 1967)b | 0.001 | |
| 17-hydroxyprogesterone (nmol/L) | 2.1 (1.5, 2.5)a | 2.1 (1.9, 3.7) | 6.3 (3.2, 8.8)b,c | 7.8 (5.5, 9.4)a,d | <0.001 | |
| Androstenedione (nmol/L) | 6.8 (6.2, 7.6) | 5.6 (5.2, 9.2) | 8.4 (7, 12)a | 9.5 (8.3, 11)a | 0.10 | |
| DHEA (nmol/L) | 28 (23, 31) | 23 (15, 30) | 24 (20, 31) | 27 (24, 41) | 0.44 | |
p<0.05 compared with baseline
p<0.01 compared with group 1
p<0.05 compared with group 2
p<0.01 compared with groups 1 and 2
Intratesticular Testosterone and Testosterone Precursors
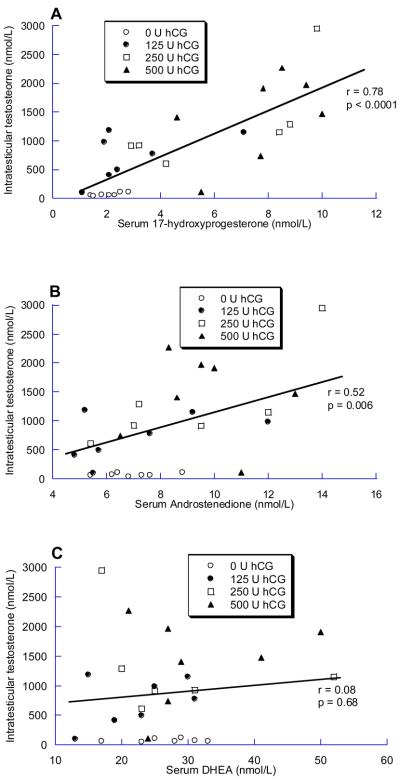
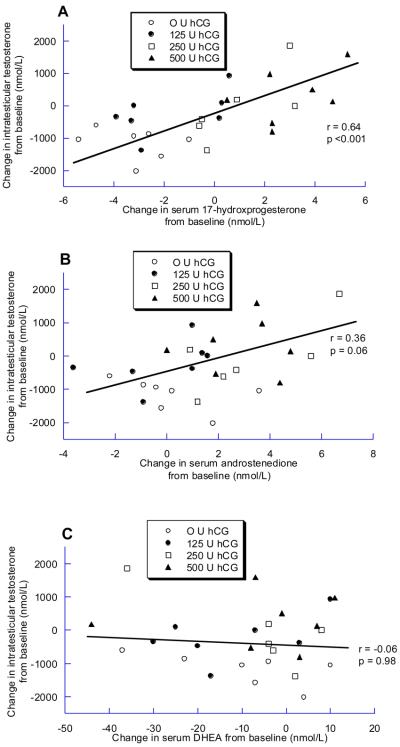
At baseline, ITT did not correlate with serum 17-hydroxyprogesterone or DHEA, and was inversely correlated with androstenedione (r = −0.47; p = 0.01). In contrast, during treatment with hCG, ITT strongly correlated with 17-hydroxyprogesterone (r = 0.78, p<0.0001) (figure 1A), correlated less strongly with androstenedione (r = 0.52, p = 0.006) (Figure 1B), and did not correlate with DHEA (r = 0.08, p = 0.68). Similarly, change in ITT from baseline to end-of-treatment was significantly correlated with change in serum 17-hydroxyprogesterone (r = 0.64, p = <0.001) (Figure 2A), but not with change in serum androstenedione or DHEA (Figure 2B and 2C).
Figure 1.
Association between the end-of-treatment intratesticular testosterone and A) 17-hydroxyprogesterone, B) Androstenedione and C) DHEA (n = 27). Note the differences in the X-axes for each plot.
Figure 2.
Association between the change in intratesticular testosterone and the change in A) 17-hydroxyprogesterone, B) Androstenedione and C) DHEA between baseline and end-of-treatment (n =27). Note the differences in the X-axes for each plot.
Modeling of factors associated with ITT with univariate linear regression revealed a statistically significant association between ITT and end-of-treatment 17-hydroxyprogesterone (β = 199, 95% CI (127, 271); p <0.001). This association remained significant with adjustment for end-of-treatment values of the other testosterone precursors, serum testosterone, age and weight (β = 168, 95% CI (102, 235); p <0.001). End-of-treatment androstenedione was significantly associated with ITT in univariate regression (β = 177, 95% CI (67, 286); p = 0.003); however, with adjustment for confounders this association was no longer significant (p = 0.79).
Discussion
In this work, we demonstrate a significant association between serum concentrations of 17-hydroxyprogesterone and ITT in men receiving TE and various dosages of hCG. Serum 17-hydroxyprogesterone decreased by roughly 60% in men receiving placebo, while it increased by 70% in men at the highest dose of hCG (500 IU). While 17-hydroxyprogesterone did not correlate with ITT at baseline, it was very strongly correlated with ITT on treatment, both when absolute concentration and change from baseline were taken into account. Moreover, end-of-treatment concentrations of serum 17-hydroxyprogesterone were significantly associated with ITT after correction for other variables using linear regression. While the overall correlation between ITT and serum 17-hydroxyprogesterone was strong, in the lowest dose hCG group, the ITT increased much more than the serum 17-hydroxyprogesterone. This implies that serum 17-hydroxyprogesterone might not be as sensitive to changes in ITT mediated by lower levels of hCG stimulation as it is to the larger increases in ITT stimulated by higher doses of hCG.
The finding that 17-hydroxyprogesterone correlates with ITT is the first description of a serum biomarker of ITT during treatment with testosterone plus hCG, and may be of great benefit in clinical research studies aimed at understanding the quantitative relationship between LH-signal, ITT and spermatogenesis. In addition, this observation might have utility in determining the optimal treatment dose of recombinant LH or hCG in men with infertility due to hypogonadotropic hypogonadism, although how much information it could add to measurement of serum testosterone remains to be determined. Moreover, it might provide a useful marker of testicular function as part of an “hCG stimulation test” for men already being treated with replacement doses of testosterone for hypogonadism. For example, it has recently been shown that measurement of serum estradiol can be useful as a marker of testicular reserve since serum estradiol concentrations are more quickly and dramatically elevated after a single dose of hCG than serum testosterone (17). Lastly, measurement of serum 17-hydroxyprogesterone in men enrolled in trials of experimental male hormonal contraceptives might provide insight into the relationship between ITT, endogenous testosterone production and suppression of spermatogenesis. Obviously, since men in these studies are already receiving exogenous testosterone as part of the contraceptive regimen, serum testosterone cannot be used for this purpose (18).
Most of the circulating 17-hydroxyprogesterone in men is likely of testicular and not adrenal origin. Orchiectomy reduces circulating levels of 17-hydroxyprogesterone by approximately 70 % (19,20), a reduction very similar to that seen in our group of subjects receiving exogenous testosterone and placebo hCG (and therefore having little circulating LH activity). Thus, roughly 30% of circulating 17-hydroxyprogesterone is likely of non-testicular, presumably adrenal origin. The testicular secretion of 17-hydroxyprogesterone is known to be second only to that of testosterone, with testosterone accounting for 70% of steroid output and 17-hydroxyprogesterone accounting for 20% (21).
No differences in serum 17-hydroxyprogesterone have been observed in the baseline state in large, cross-sectional studies of men with infertility (22). However, two studies of men with idiopathic infertility have revealed an increased ratio of 17-hydroxyprogesterone to testosterone after stimulation with hCG in infertile men as compared to normal controls (23). This effect is especially pronounced in men with baseline elevations in serum follicle-stimulating hormone (24), and may imply a subtle impairment in the activity of the 17, 20 lyase or 17-beta-hydroxysteroid dehydrogenase in these patients. An increased ratio of 17-hydroxyprogesterone to testosterone has also been observed in a population of men with varicocele-related infertility; however, it is uncertain if the elevated 17-hydroxyprogesterone seen in this series was testicular or adrenal in origin as subjects also had significantly elevated serum concentrations of 11-beta-hydroxyandrostenedione, an exclusively adrenal androgen (25).
This study is limited in that it involves only normal men recruited and treated at one site. As a result, ethnic, nutritional and geographic differences in the ability to respond to stimulation with hCG were not assessed. In addition, there is a fairly large variance in the measurement of ITT that could bias the conclusions. Moreover, a single measurement after 21 days may not accurately reflect changes in ITT over longer periods, perhaps due to atrophy of Leydig cells, which could be of relevance to the treatment of male infertility. Clearly, larger studies of the relationship between ITT and serum 17-hydroxyprogesterone in fertile and infertile men with and without hCG stimulation should be performed to corroborate and extend these findings. In addition, longer-term studies of hCG stimulation will need to be conducted to better understand the relationship between ITT, 17-hydroxyprogesterone and spermatogenesis.
In conclusion, we demonstrate here an association between serum 17-hydroxyprogesterone and ITT in the setting of stimulation of the testes in gonadotropin-suppressed normal men with hCG. Since ITT is central to spermatogenesis, this finding may aid future research designed to understand the relationship between ITT and spermatogenesis as well as optimizing the treatment of men requiring injections of hCG or recombinant LH for the treatment of male infertility.
Table 2.
Simple univariate and multivariate linear regression of end-of-treatment intratesticular testosterone with end-of-treatment serum concentrations of testosterone precursors. Regression was performed using robust standard errors. The multivariate model controlled for age, weight and the other testosterone precursor concentrations.
| Testosterone precursor | Univariate model | Multivariate modela | |||||
|---|---|---|---|---|---|---|---|
| β | 95% CI | p-value | β | 95% CI | p-value | ||
| 17-hydroxyprogesterone | 199 | (127, 271) | <0.001 | 168 | (102, 235) | < 0.001 | |
| Androstenedione | 177 | (67, 286) | 0.003 | 22 | (−147, 191) | 0.79 | |
| DHEA | 12 | (−20, 44) | 0.45 | −19 | (−48, 10) | 0.19 | |
Controlled for age, weight, serum testosterone and serum concentrations of other two testosterone precursors
Acknowledgements
We thank Ms. Kathy Winter, Ms. Marilyn Busher, Ms. Janet Gilchrest, and Ms. Kymberly Anable for assistance with the clinical aspects of the studies. In addition, we thank: Drs. Jonathan J. Jarow, William W. Wright, Terry R. Brown, Xiaohua Yan and Barry R. Zirkin for the measurement of intratesticular testosterone, Dorothy McGuinness for performance of hormone assays, and Dr. David W. Amory Sr. for critical review of the manuscript.
This work was supported by the National Institute of Child Health and Human Development (NICHD) through cooperative agreements U54-HD-12629 and U54 HD-42454 as part of the specialized Cooperative Centers Program in Reproductive Research and the Cooperative Contraceptive Research Centers Program. Dr. Amory is supported, in part, by the National Institute of Child Health and Human Development, a division of the National Institutes of Health by grant # K23 HD45386. A portion of this work was conducted through the Clinical Research Center facility at the University of Washington and supported by the NIH grant M01-RR-00037.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: A.D.C, S.T. P., and B.D.A.,. have nothing to disclose. W.J.B. and A.M.M. has consulted for GlaxoSmithKline and Quatrx. A.M.M. has consulted for Solvay, Amgen, Threshold, GTx and Mattern Pharmaceuticals and received grant support from GlaxoSmithKline, Ascend Therapeutics, and Solvay Pharmaceutical. JKA has received grant support from GlaxoSmithKline, Schering AG and Merrion Pharmaceuticals
References
- 1.Steinberger E, Duckett GE. Hormonal control of spermatogenesis. J Reprod Fertil. 1967;(2):75–81. [Google Scholar]
- 2.Conn PM, Crowley JR. Gonadotropin-releasing hormone and its analogs. N Engl J Med. 1991;(324):93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe RM. Testosterone and spermatogenesis. J Endocrinol. 1987;(113):1–2. doi: 10.1677/joe.0.1130001. [DOI] [PubMed] [Google Scholar]
- 4.Jarow JP, Zirkin BR. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann NY Acad Sci. 2005;(1061):208–220. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- 5.Hall PF. On the stimulation of testicular steroidogenesis in the rabbit by interstitial cell-stimulating hormone. Endocrinology. 1966;(78):690–698. doi: 10.1210/endo-78-4-690. [DOI] [PubMed] [Google Scholar]
- 6.Bremner WJ, Millar MR, Sharpe RM, Sauders PT. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage dependent expression and regulation of androgens. Endocrinology. 1994;(135):1227–1234. doi: 10.1210/endo.135.3.8070367. [DOI] [PubMed] [Google Scholar]
- 7.Morse HC, Horike N, Rowley MJ, Heller CG. Testosterone concentrations in testes of normal men: effects of testosterone propionate administration. J Clin Endocrinol Metab. 1973;(37):882–6. doi: 10.1210/jcem-37-6-882. [DOI] [PubMed] [Google Scholar]
- 8.Jarow JP, Chen H, Rosner TW, Trentacoste S, Zirkin BR. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intratesticular fluid. J Androl. 2001;(22):640–5. [PubMed] [Google Scholar]
- 9.Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, Anawalt BD, et al. Intratesticular testosterone concentrations levels comparable to serum levels are not sufficient to maintain normal sperm production spermatogenesis in men receiving a hormonal contraceptive regimen. J Androl. 2004;(25):931–938. doi: 10.1002/j.1939-4640.2004.tb03164.x. [DOI] [PubMed] [Google Scholar]
- 10.Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, Anawalt BD, et al. Low dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone induced gonadotropin suppression. J Clin Endocrinol Metab. 2005;(90):2595–2602. doi: 10.1210/jc.2004-0802. [DOI] [PubMed] [Google Scholar]
- 11.Jarow JP, Wright WW, Brown TR, Yan X, Zirkin BR. Bioactivity of androgens within the testes and serum of normal men. J Androl. 2005;(26):343–348. doi: 10.2164/jandrol.04100. [DOI] [PubMed] [Google Scholar]
- 12.Matthiesson KL, Stanton PG, O'Donnell L, Meachem S, Amory JK, Berger R, et al. Effects of testosterone and levonorgestrel combined with a 5alpha reductase inhibitor or GnRH antagonist on spermatogenesis and intratesticular steroid levels in normal men. J Clin Endocrinol Metab. 2005;(90):5647–5655. doi: 10.1210/jc.2005-0639. [DOI] [PubMed] [Google Scholar]
- 13.Finkel DM, Phillips BA, Snyder PJ. Stimulation of spermatogenesis by gonadotropins in men with hypogonadotropic hypogonadism. N Eng J Med. 1985;(313):651–655. doi: 10.1056/NEJM198509123131102. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto AM, Bremner WJ. Stimulation of sperm production by human chorionic gonadotropin after prolonged gonadotropin suppression in normal men. J Androl. 1985;(6):137–143. doi: 10.1002/j.1939-4640.1985.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 15.Chan PT, Schlegel PN. Diagnostic and therapeutic testis biopsy. Curr Urol Res. 2000;(1):266–272. doi: 10.1007/s11934-000-0006-4. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto AM, Paulsen CA, Hopper BR, Rebar RW, Bremner WJ. Human chorionic gonadotropin and testicular function: Stimulation of testosterone, testosterone precursors, and sperm production despite high estradiol levels. J Clin Endocrinol Metab. 1983;(56):720–728. doi: 10.1210/jcem-56-4-720. [DOI] [PubMed] [Google Scholar]
- 17.Meier C, Christ-Crain M, Christoffel-Courtin C, Staub JJ, Muller B. Serum estradiol after single dose hCG administration correlates with Leydig cell reserve in hypogonadal men: reassessment of the hCG stimulation test. Clin Lab. 2005;(51):509–15. [PubMed] [Google Scholar]
- 18.Amory JK, Page ST, Bremner WJ. Recent advances in male hormonal contraception. Nat Clin Prac Endocrinol Metab. 2006;(2):32–41. doi: 10.1038/ncpendmet0069. [DOI] [PubMed] [Google Scholar]
- 19.Stege R, Eriksson A, Henriksson P, Carolstrom K. Orchidectomy or estrogen treatment in prostatic cancer: effects on serum levels of adrenal androgens and related steroids. Int J Androl. 1987;(10):581–7. doi: 10.1111/j.1365-2605.1987.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 20.Carlstrom K, Stege R. Adrenocortical function in prostatic cancer patients: effects of orchidectomy or different modes of estrogen treatment on basal steroid levels and on the response to exogenous adrenocorticotropic hormone. Urol Int. 1990;(45):160–163. doi: 10.1159/000281699. [DOI] [PubMed] [Google Scholar]
- 21.Winters SJ, Takahaski J, Troen P. Secretion of testosterone and its delta4 precursor steroids into spermatic vein blood in men with varicocele-associated infertility. J Clin Endocrinol Metab. 1999;(84):997–1001. doi: 10.1210/jcem.84.3.5548. [DOI] [PubMed] [Google Scholar]
- 22.Abbaticchio G, Nacucchi O, Giagulli VA, Brescia F, Giorgio R. Exploration of the testis in infertile men. Relationships among serum levels of FSH, LH, 17-alpha-OH-progesterone and testosterone. Andrologia. 1990;(22):231–237. doi: 10.1111/j.1439-0272.1990.tb01971.x. [DOI] [PubMed] [Google Scholar]
- 23.Abbaticchio G, Nardelli GM, DeFini M, Nacucchi O, Brescia F, Cospite R, et al. 17-a-progesterone to testosterone plasma ratios and their modification after hCG in normal men and in patients with idiopathic infertility. Andrologia. 1988;(20):441–446. [PubMed] [Google Scholar]
- 24.Anapliotou ML, Liparake M, Americanos N, Goulandris N, Papaioannou D. Increased 17-OH-progesterone levels following hCG stimulation in men with idiopathic oligozoospermia and raised FSH levels. Int J Androl. 1994;(17):192–198. doi: 10.1111/j.1365-2605.1994.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 25.Hampl R, Lachman M, Novak Z, Sulcova J, Starka L. Serum levels of steroid hormones in men with varicocele and oligospermia as compared to normozoospermic men. Exp Clin Endocrinol. 1992;(100):117–119. doi: 10.1055/s-0029-1211189. [DOI] [PubMed] [Google Scholar]