Abstract
Background
Daphniids, commonly known as waterfleas, serve as important model systems for ecology, evolution and the environmental sciences. The sequencing and annotation of the Daphnia pulex genome both open future avenues of research on this model organism. As proteomics is not only essential to our understanding of cell function, and is also a powerful validation tool for predicted genes in genome annotation projects, a first proteomic dataset is presented in this article.
Results
A comprehensive set of 701,274 peptide tandem-mass-spectra, derived from Daphnia pulex, was generated, which lead to the identification of 531 proteins. To measure the impact of the Daphnia pulex filtered models database for mass spectrometry based Daphnia protein identification, this result was compared with results obtained with the Swiss-Prot and the Drosophila melanogaster database. To further validate the utility of the Daphnia pulex database for research on other Daphnia species, additional 407,778 peptide tandem-mass-spectra, obtained from Daphnia longicephala, were generated and evaluated, leading to the identification of 317 proteins.
Conclusion
Peptides identified in our approach provide the first experimental evidence for the translation of a broad variety of predicted coding regions within the Daphnia genome. Furthermore it could be demonstrated that identification of Daphnia longicephala proteins using the Daphnia pulex protein database is feasible but shows a slightly reduced identification rate. Data provided in this article clearly demonstrates that the Daphnia genome database is the key for mass spectrometry based high throughput proteomics in Daphnia.
Background
During the last two decades, genome sequencing efforts are providing us with complete genome sequences from many organisms (for a summary refer to http://www.ncbi.nlm.nih.gov/Genomes/). The generated sequence databases are fundamental tools used by researchers in almost every field of modern biology. In addition they provide the basis for powerful technologies to quantitatively analyze the gene expression profile on the mRNA-level using DNA microarrays [1,2]. However, it has to be considered that mRNA molecules are only intermediate products towards the production of functional proteins and that protein abundance is not necessarily reflected by the amount of the corresponding mRNA transcript [3,4]. The concentration of individual proteins at the cellular level or in biological fluids mainly depends on four completely different processes: (i) protein synthesis, (ii) protein processing, (iii) protein secretion and (iv) protein degradation. As a consequence, systematic quantitative predictions of protein populations are impossible to deduce from genomic or transcriptional data. Moreover, proteins frequently undergo post-translational modifications (PTMs) crucial for their function, activity, and stability and they often play major roles in regulatory networks [5]. Comprehensive datasets addressing the protein level, therefore, are indispensable for a functional and biochemical characterization of both cells and organisms. The field of high-throughput identification and quantification of proteins using systematic approaches is commonly referred to as proteomics. Recent developments in mass spectrometry have revolutionized the field and dramatically increased the sensitivity of protein identification compared to classical techniques like Edman sequencing. As a consequence, large proteome investigations have been established covering, e.g., human plasma [6], human brain [7] and human liver [8] as well as model organisms such as Caenorhabditis elegans [9] and Drosophila melanogaster [10].
This, in turn, has led to the realization that proteomics is not only essential to our understanding of cell function, but in addition is a validation tool for genes predicted in genome annotation projects. Recently published results demonstrate that peptide mass spectrometry complements gene annotation in Drosophila [10] and humans [11,12].
Although a multitude of whole-genome sequencing projects ranging from microbial (e.g. [13]) to vertebrate genomes [14] have been initiated in the last decade, no complete genome sequence is available for crustaceans, a species-rich taxa with additional high economical impact.
Hence, the Daphnia Genomics Consortium (DGC; http://daphnia.cgb.indiana.edu) was founded in 2003 to develop the waterflea Daphnia, a small planktonic crustacean, as a further model system in genomics, but with the added advantage of being able to interpret the results in the context of natural ecological challenges. Even though the ecology and ecotoxicology of Daphnia has been well studied, because they are a major link between limnetic primary production and higher trophic levels, less work has been done on the genetics of this organism. Nevertheless, their clonal reproduction, short generation times, and their transparent body also make them well suited for experimental molecular research.
In this special series of papers published in BMC journals, the first description of the Daphnia pulex draft genome sequence http://wFleaBase.org is described. Besides investigation on the DNA and mRNA level, the availability of the Daphnia genome sequence opens the door to investigate the proteome of this fascinating species. In this article we present the generation of a first data-set consisting of 701,274 peptide tandem-mass-spectra derived from Daphnia pulex. In order to demonstrate the impact of the Daphnia genome sequence on proteomics based studies we compared the number of identified proteins using the Daphnia protein database with the number of identifications obtained by searching against the Swiss-Prot and the Drosophila melanogaster protein database http://flybase.org/. To validate the utility of the Daphnia pulex genome for research on different Daphnia species, additional 407,778 peptide tandem-mass-spectra derived from Daphnia longicephala were generated and evaluated. In addition, the peptides identified in our approach provide the first experimental evidence for the translation of a broad variety of predicted coding regions within the Daphnia genome.
Results
Sample preparation
To generate protein lysates suitable for SDS gel electrophoresis, pools of about 300 waterfleas (Daphnia pulex and Daphnia longicelphala respectively) were homogenated. The protein concentration of the obtained lysates (2 mL) was 2.6 mg/mL for Daphnia pulex and 2.3 mg/mL for Daphnia longicephala corresponding to a total protein yield of 17 μg and 15 μg per Daphniid, respectively.
SDS-gel pre-fractionation of Daphnia proteins
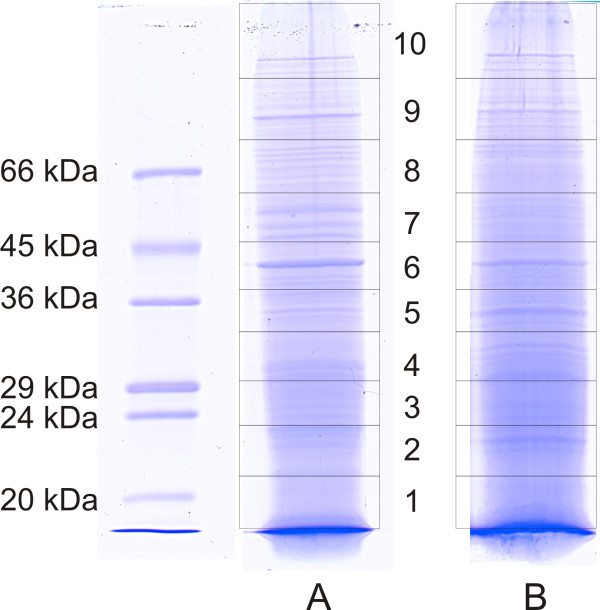
50 μg of total protein from either Daphnia pulex or Daphnia longicephala, was separated by SDS-gel electrophoresis. To evaluate the quality of the electrophoretic separation, the gels were stained with Coomassie. An image of SDS-gels derived from both Daphnia species is shown in Fig. 1. Both samples showed sharp distinct bands, indicating that the performed electrophoreses had good separation strengths. To generate 10 protein fractions of each sample, the corresponding gel lanes were cut into 10 pieces as outlined in Fig. 1. To get samples suitable for LC-MS/MS, each gel slice was subjected to the in-gel digestion procedure described in the Methods chapter.
Figure 1.
Coomassie stained SDS-gels of Daphnia proteins. Scanned image of Coomassie stained SDS-gels. Panel A: 50 μg Daphnia pulex protein; Panel B: 50 μg Daphnia longicephala protein. The rectangles indicate the 10 gel slices which were individually analyzed by LC-MS/MS.
LC-MS/MS analysis of Daphnia pulex proteins
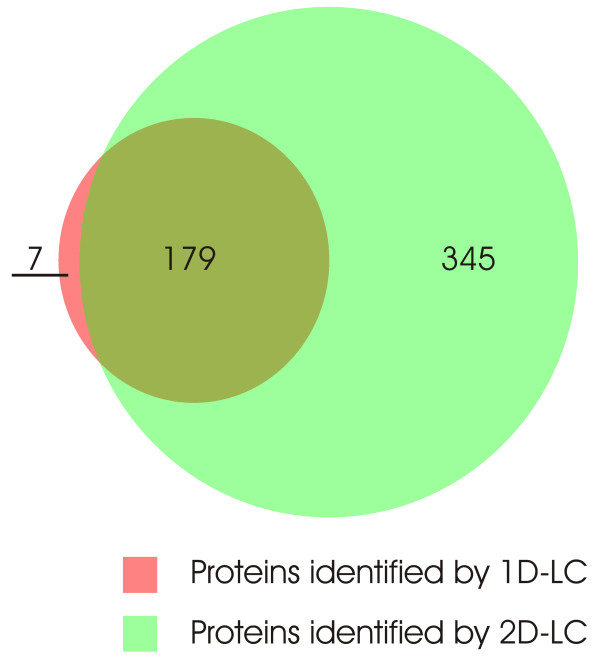
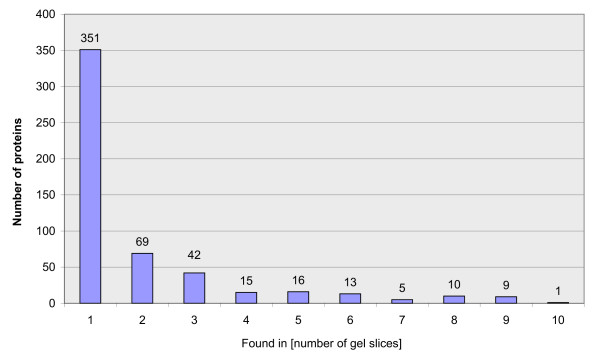
For the qualitative analysis of the Daphnia pulex proteome, two samples were fractionized by SDS-gel electrophoresis (as described in the above paragraph) and subjected to LC-MS/MS analysis. Each of the 10 gel fractions was separated with one-dimensional reversed phase (RP) liquid chromatography (1D-LC) and a combination of strong cation-exchange (SCX) with RP chromatography (2D-LC) respectively. From the 1D-LC-MS/MS runs 100,462 spectra could be collected and from the 2D-LC-MS/MS runs 600,812 spectra were acquired. All MS/MS spectra were searched against the non-redundant filtered models database of Daphnia v1.1 gene builds (July, 2007) http://www.jgi.doe.gov/Daphnia/ and evaluated using the PeptideProphet software. Applying a false discovery rate of = 1%, 7973 MS/MS spectra could be assigned to peptides within the Daphnia database, of which 1654 were unique. The assignment of peptides to proteins using the ProteinProphet algorithm led to the identification of 186 proteins with the 1D-LC-MS/MS approach and 524 proteins with the 2D-LC-MS/MS startegy (false positive discovery rate = 1%). As shown in Fig. 2, all except seven proteins identified in the 1D-LC approach could be found in the 2D-LC-MS/MS dataset as well. Further analysis of the data revealed that a significant fraction of proteins could be identified in more than one gel slice, as summarized in Fig. 3. The overall list of identified proteins and peptides is available as additional file 1.
Figure 2.
Proportional Venn diagram of protein overlap. Proportional Venn diagram demonstrating the degree of overlap of proteins identified by 1D-LC-MS/MS and 2D-LC-MS/MS.
Figure 3.
Identified proteins. Bar chart indicating the number of proteins identified in more than one gel slice. X axis number of gel slices, Y axis number of identified proteins by 2D-LC-MS/MS.
Ontology analysis of the identified proteins
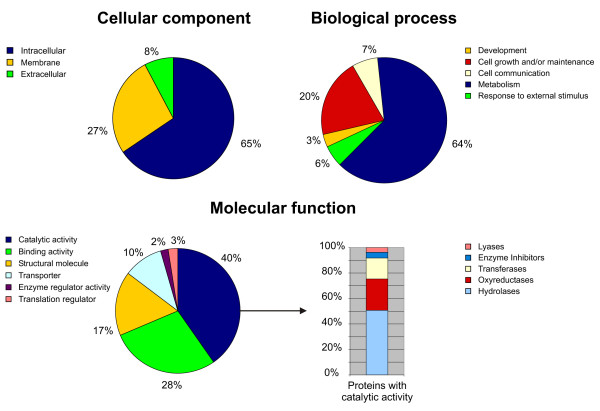
To analyze the ontology of the identified Daphnia pulex proteins the entries of the filtered models database were BLASTp-searched http://www.ncbi.nlm.nih.gov/BLAST/ in the Swiss-Prot database http://www.expasy.ch[15]. We chose the Swiss-Prot database because of its high level of annotation, including entries about protein function, posttranslational modifications as well as a direct link to the Gene Ontology (GO) databases [16]. From the 531 sequences derived from the filtered models database, 499 homologue (E-values < 0.01) protein sequences could be found. The corresponding protein Swiss-Prot IDs were subjected to ontology analysis using the PANDORA server http://www.pandora.cs.huji.ac.il/. The results of this ontology analysis are shown in Fig. 4. In the "cellular component" GO database only 139 proteins of the 499 proteins were listed. Their classification analysis revealed that the majority (65%) are of intracellular origin and the fraction of the particularly interesting class of membrane proteins comprises 27%. The "molecular function" GO revealed 350 proteins the majority of which were classified as proteins with catalytic activity. From these fractions 141 were enzymes from which 68 could be classified as hydrolases, 33 as oxyreductases, 22 as transferases and 5 as lyases. 6 proteins could be classified as enzyme inhibitors. Using the "biological process" database 272 proteins could be classified from which 175 were associated with metabolism, 55 with cell growth and/or maintenance, 18 with cell communication, 15 with response to external stimulus and 9 with developmental processes.
Figure 4.
Ontology analysis. Ontology analysis of 501 identified Daphnia pulex proteins. The classification of the protein set was performed according to the gene ontology terms: "Cellular component", "Biological process" and "Molecular function".
Searches of MS/MS data in the Swiss-Prot and Drosophila melanogaster protein database
To investigate the benefit of the Daphnia pulex filtered models database on the MS based identification of Daphnia proteins, cross-species identification, as suggested by several authors [17,18], was performed using the Metazoa subset of the Swiss-Prot database (Release 54.2, 78,385 entries) and the Drosophila melanogaster database from FlyBase (20,726 entries). Using the MS/MS spectra obtained with the 2D-LC-MS/MS runs of the Daphnia pulex sample, 71 Daphnia proteins could be identified with the Drosophila database and 92 with the Swiss-Prot database with a false-positive identification threshold of = 1%.
LC-MS/MS analysis of Daphnia longicephala proteins
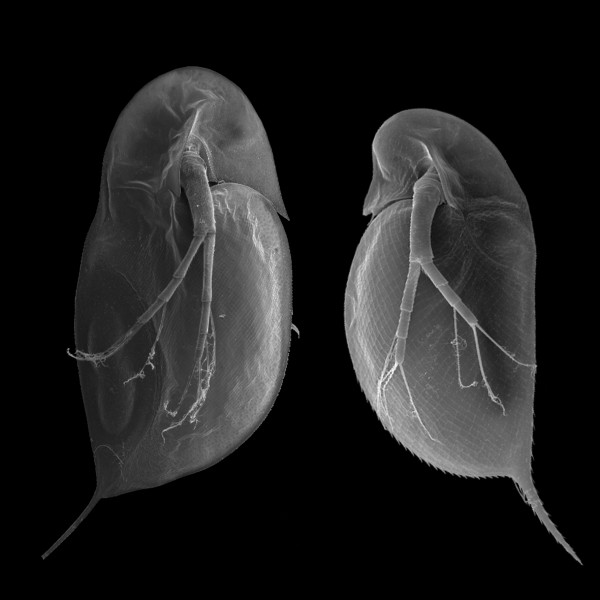
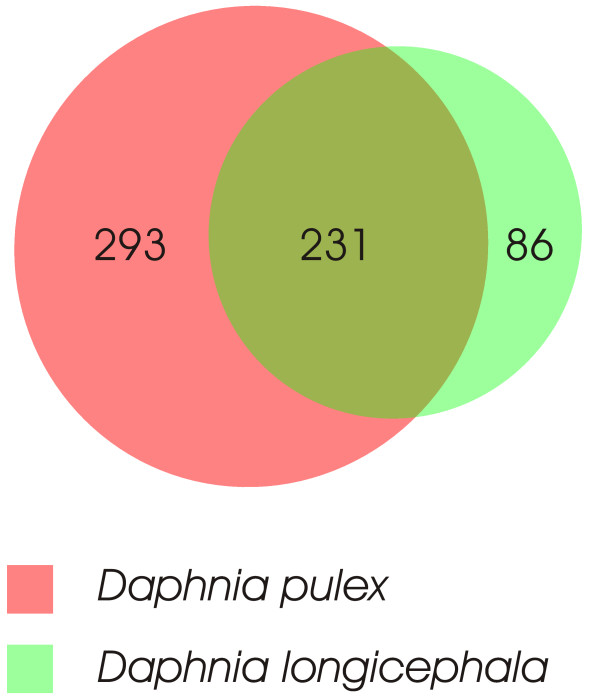
To determine the suitability of the non-redundant filtered models database of putative Daphnia pulex proteins for the MS-based identification of proteins from other Daphnia subgenera, a Daphnia longicephala protein lysate was generated. (A scanning electron micrograph from both, Daphnia pulex and Daphnia longicephala is shown in Fig. 5. For the protein identification exactly the same separation strategy as for D. pulex was used. Using this SDS-PAGE – 2D-LC-MS/MS combination and the non-redundant filtered models database of putative Daphnia pulex proteins, we were able to identify 671 unique peptides (PeptideProphet, false discovery rate = 1%) which could be assigned to 317 Daphnia longicephala proteins (ProteinProphet, false discovery rate = 1%). As shown in Fig. 6, 86 of these proteins could exclusively be identified in Daphnia longicephala samples but not in Daphnia pulex samples.
Figure 5.
Daphnia images. Scanning electron micrograph from Daphnia longicephala (l) and Daphnia pulex (r).
Figure 6.
Proportional Venn diagram of protein overlap. Proportional Venn diagram demonstrating the overlap of proteins identified from Daphnia pulex and Daphnia longicephala.
Discussion
General remarks
For a comprehensive functional and biochemical characterization of organisms, an inventory of their proteins and protein modifications is a prerequisite. In the work presented here, we performed a liquid chromatography – mass spectrometry based qualitative proteome approach with the goal to generate a first protein catalogue of Daphnia pulex, the genome of which is presented in this special issue. To complement gene sequences, the generation of a broad dataset of tandem MS (MS/MS) spectra derived from Daphnia peptides is particularly interesting for two main reasons:
i) High throughput MS/MS protein identification is based on the comparison of experimentally acquired peptide MS/MS spectra with in silico generated theoretical spectra deduced from protein databases. With a large set of MS/MS spectra it can be tested if the Daphnia filtered models protein database is suitable in its current form for proteomics approaches, which are mostly based on protein identification by MS/MS.
ii) The generation of MS/MS spectra derived from Daphnia peptides will lead to the creation of a catalogue of identified daphniid peptides. This will be one of the first datasets giving experimental evidence for a variety of so far only predicted proteins. The Daphnia filtered models protein database in its current form consists of more than 30,000 entries. The corresponding genes were either found by EST sequencing, by homology searches, or ab initio by gene prediction algorithms. However, for the broad majority of database entries, there is so far no experimental evidence that the corresponding genes are in fact translated and the resulting proteins persist in the organism.
Experimental strategy
Among all presently available proteomic techniques, the application of liquid chromatography (LC) as a separation tool combined with electrospray ionization (ESI) [19] tandem mass spectrometry (MS/MS) as an identification tool has the highest performance in terms of protein identifications per time unit. This technique is referred to as LC-MS/MS and has proven its efficiency in many studies [20-22]. Since eukaryotic proteomes consist of highly complex mixtures, the reduction of complexity by pre-fractionation on the level of intact proteins prior to LC-MS/MS analysis is mandatory. The number of identifications usually increases with the overall extent of prefractionation efforts. Because of its high separation strength we choose 1D-SDS-gel electrophoresis for pre-fractionation on the protein level. In this pilot study a number of 10 gel fractions were chosen. To determine the impact of two versus one chromatographic steps on the number of identified peptides, we compared the results obtained with one-dimensional reversed phase (RP) liquid chromatography (1D-LC) versus a combination of strong cation-exchange (SCX) with RP chromatography. The major advantage of the SCX – RP combination is the removal of salt ions from the SCX fractions in the RP step, which would otherwise interfere with the MS-analysis of peptide ions. For reasons of performance, we choose a fully automatic online setup, where SCX fractions are directly eluted onto a RP trap column. This RP trap column is then switched into the RP chromatography system to finally separate the peptides. The SCX flow through as well as 6 salt fractions from each of the 10 gel slices were captured and analyzed by LC-MS/MS; leading to a total number of 80 1D-LC-MS/MS runs (10 gel slices × 1 RP-LC run + 10 gel slices × 7 SCX fractions × 1 run 1 RP-LC run). From this workflow, 701,274 MS/MS spectra were obtained.
Results obtained with LC-MS/MS
Using SDS-PAGE combined with 1D-LC-MS/MS, we identified 186 entries whereas the SDS-PAGE – 2D-LC-MS combination led to the identification of 524 entries from the non-redundant filtered models database of putative Daphnia proteins demonstrating the benefit of a second chromatographic step. In total, we were able to identify 531 non-redundant filtered models database proteins of putative Daphnia pulex proteins. The overall list of identified proteins can be downloaded as additional file 1.
Considering that the main goal of our experiments was to test the benefit of a dedicated Daphnia protein database for LC-MS/MS-based proteomics, this result is promising with respect to the straightforward design of this pilot study. As recently demonstrated by [10], an extensive prefractionation on the level of the biological sample (e.g. selection of different development stages), on the cellular, on the subcellular level as well as on the level of proteins and peptides had to be performed to get a catalogue of thousands of experimentally identified proteins from Drosophila. Our results clearly demonstrate that LC-MS/MS analysis combined with the usage of the Daphnia filtered models database is able to identify hundreds of Daphnia proteins with a high confidence level in a very efficient way. Therefore, this methodology combined with further pre-fractionation steps will lead to an increased analytical depth of the Daphnia proteome.
Determination of false positive ratios
The general strategy to identify peptides by high-throughput MS/MS experiments is a probability based comparison of experimental spectra with theoretical spectra calculated from protein databases deduced from DNA sequences. The software algorithms determine the closest match and a score indicating the reliability of the result. Although this identification strategy has proven its strength in many studies, cut-off values for the obtained scores must be chosen carefully to minimize false-positive identifications [23,24]. Unfortunately, there are no general rules for the confidence of given scores, because their reliability depends on the experimental setup as well as on the database used for the search. In our study, we applied the commonly used Mascot [25] search engine, returning a so called "ions score" for each peptide (for details see http://www.matrixscience.com/. However, special care must be taken when peptides spectra are used as evidence for the existence of corresponding proteins. Since a given peptide sequence can be present in multiple proteins, these shared peptides can lead to an overestimation of the number of identified proteins as well as to an under-estimation of the false discovery rate. An overview of this issue was given by Nesvizhskii et al. [26]. Therefore, to validate the Mascot search results we used the Trans-Proteomic Pipeline [27] downloadable from the Seattle Proteome Center http://tools.proteomecenter.org/TPP.php. This software package includes PeptideProphet http://peptideprophet.sourceforge.net/ to compute probabilities for identified peptides [28] and ProteinProphet http://proteinprophet.sourceforge.net/ to address the issue of shared peptides and to calculate the probabilities of corresponding protein identifications [29]. To further confirm the false positive ratio given by the Trans-Proteomic pipeline we generated a so-called decoy version of the Daphnia pulex filtered models database consisting of random sequences with the same average amino acid composition. This decoy database was attached to the original database and then used to search our MS/MS spectra as proposed by Elias et al. [30]. Any protein hit derived from the decoy part of the combined database was regarded as false-positive identification. The number of four hits from the decoy part of the database is in accordance with the 1% false discovery rate calculated by the Trans-Proteomic Pipeline.
Proteolytic activity
The analysis of the data revealed that a significant fraction (34%) of proteins could be identified in more than one gel slice, as summarized in Fig. 3. A heterogeneity of molecular masses is frequently observed in this kind of approaches [31,32]. and may be caused by posttranscriptional events such as alternative splicing, posttranslational modifications or proteolytic processing. While, inadequate separation strength of the gel can be excluded due to the presence of sharp distinct bands (see Fig. 1), proteolysis of these proteins prior to electrophoresis may contribute to this heterogeneity. Proteolysis can be caused by Daphnia proteases from the intestinal tract. The proteolytic activity of Daphnia magna gut protease was previously described [33,34]. In preliminary studies in which we performed 2D-gel electrophoresis of Daphnia magna and Daphnia longicephala lysates, we tried to eliminate this proteolytic activity with several commercially available protease inhibitor cocktails. The list of tested inhibitors, including the used concentrations, is shown in Table 1. However, the obtained spot patterns of all prepared 2D-gels still reflected significant protein degradation (Data not shown).
Table 1.
List of tested protease inhibitors
| Protease Inhibitor | Concentration |
| Protease Inhibitor Cocktail P8340 (Sigma Aldrich) | 2% |
| Protease Inhibitor Cocktail P2714 (Sigma Aldrich) | 3% |
| Complete Protease Inhibitor Cocktail Tablets (Roche) | 1 tablet in 25 mL extraction buffer |
| APMSF, TLCK, Chymostatin (Sigma Aldrich) | 79 μM; 160 μM; 83 μM |
| TLCK, TPCK (Sigma Aldrich) | 160 μM; 143 μM |
| Soybean Trypsin Inhibitor T 9003 (Sigma Aldrich) | 250 μM |
As the efficient inhibition of Daphnia proteases plays a crucial role in further quantitative proteome studies, we screened our catalogue of identified Daphnia proteins for proteases. In total, we have identified 19 different proteins out of the Daphnia database showing significant homology (BLAST E-value < 0.01) to known proteases with exo- as well as endopepdidase activity (Table 2). In the case of the Daphnia trypsin proteases identified, the masses of the detected peptides did not fit with the theoretical peptide masses of the porcine trypsin used for digestion of the samples. Hence, these peptides clearly originate from Daphnia proteins. The list of Daphnia proteases in Table 2 provides a basis for further sophisticated experiments, e.g. determination of cleavage specificities and screening for protease inhibitors.
Table 2.
List of identified Daphnia proteins homologue to proteins with known exo- or endopeptidase activity
| Dappu Protein ID | Homolgue to [Swiss-Prot Accession] | E-Value | Protein name | Activity |
| 302098 | P13676 | 9E-109 | Acylamino-acid-releasing enzyme (EC 3.4.19.1) (Acyl-peptide hydrolase) (APH) (Acylaminoacyl-peptidase) | acylaminoacyl-peptidase activity |
| 203795 | P15145 | 7E-155 | Aminopeptidase N (EC 3.4.11.2) (pAPN) (Alanyl aminopeptidase) (Microsomal aminopeptidase) (Aminopeptidase M) (CD13) (gp130) | Aminopeptidase activity |
| 301437 | Q27245 | 2E-103 | Putative aminopeptidase W07G4.4 in chromosome V (EC 3.4.11.-) | aminopeptidase activity |
| 307838 | Q10751 | 0.0 | Angiotensin-converting enzyme (EC 3.4.15.1) (Dipeptidyl carboxypeptidase I) (Kininase II) (Fragment) | carboxypeptidase activity |
| 195011 | P04069 | 1E-90 | Carboxypeptidase B (EC 3.4.17.2) | carboxypeptidase activity |
| 300872 | Q95029 | 3E-123 | Cathepsin L precursor (EC 3.4.22.15) (Cysteine proteinase 1) | cysteine-type endopeptidase activity |
| 230618 | Q86GF7 | 1E-36 | Crustapain | cysteine-type endopeptidase activity |
| 126867 | P12955 | 5E-166 | Xaa-Pro dipeptidase (EC 3.4.13.9) (X-Pro dipeptidase) (Proline dipeptidase) (Prolidase) (Imidodipeptidase) | metallocarboxypeptidase D activity |
| 201234 | Q80W54 | 3E-135 | Farnesylated-proteins converting enzyme-1 | metalloendopeptidase activity |
| 200339 | P23004 | 2E-66 | Ubiquinol-cytochrome C reductase complex core protein 2, mitochondrial precursor (EC 1.10.2.2) (Complex III subunit II) | metalloendopeptidase activity |
| 200882 | O16796 | 3E-141 | Neprilysin-2 | Probable cell surface protease |
| 231027 | P00771 | 2E-62 | Brachyurin (EC 3.4.21.32) (Collagenolytic protease) | serine-type endopeptidase activity |
| 230885 | P00765 | 1E-52 | Trypsin I (EC 3.4.21.4) | serine-type endopeptidase activity |
| 26258 | Q00871 | 3E-52 | Chymotrypsin BI precursor (EC 3.4.21.1) | serine-type endopeptidase activity |
| 230174 | P36178 | 3E-49 | Chymotrypsin BII precursor (EC 3.4.21.1) | serine-type endopeptidase activity |
| 231152 | P49275 | 9E-36 | Mite allergen Der f 3 precursor (EC 3.4.21.-) (Der f III) | serine-type endopeptidase activity |
| 231560 | P04814 | 3E-21 | Trypsin alpha precursor (EC 3.4.21.4) | serine-type endopeptidase activity |
| 307138 | P97321 | 3E-119 | Seprase (EC 3.4.21.-) (Fibroblast activation protein alpha) (Integral membrane serine protease) | serine-type endopeptidase activity; exopeptidase activity |
| 303911 | Q9D1A2 | 2E-178 | Cytosolic non-specific dipeptidase (CNDP dipeptidase 2) (Glutamate carboxypeptidase-like protein 1) | Metallopeptidase activity |
Usability of the D. pulex filtered models database for proteome research on other Daphnia subgenera
In phylogenetics, the genus Daphnia is split into three subgenera, Daphnia, Hyalodaphnia and Ctenodaphnia. Sequence divergence between those subgenera indicates an origin in the Mesozoic [35]. Evolution under different environmental conditions such as UV radiation, salinity or predator regimes was certainly a key factor for diversification in this genus. To validate the utility of the Daphnia pulex genome sequence for proteome research on differing Daphnia species, we generated LC-MS/MS data of D. longicephala samples. D. longicephala was chosen due to the fact that it belongs to the taxon of Ctenodaphnia, in contrast to D. pulex which is grouped in the subgenus Daphnia. Moreover, D. longicephala is one of the most prominent examples for morphological plasticity [36] and provides an ideal model organism for future work on the genetic basis of the phenomenon of phenotypic plasticity.
For the proteome analysis of D. longicephala, identical amounts of total protein and the same 2D-LC-MS/MS strategy outlined for D. pulex was used. We were able to identify 317 proteins from the non-redundant filtered models database of putative Daphnia pulex proteins. The difference in number of identified proteins in D. pulex (524 in 2D-LC-MS/MS) may well mirror the genetic divergence between both Daphnia subgenera. This finding reflects the fact that even a single amino acid exchange in a given peptide mostly impairs its automatic identification by MS/MS search algorithms. Nevertheless, the number of identifications obtained from D. longicephala samples demonstrates the suitability of the D. pulex filtered models database for proteome investigations with other Daphnia subgenera.
Another finding is that 86 proteins were exclusively found in the Daphnia longicepha samples as illustrated in Fig. 6. This result might reflect different concentrations of a given protein in lysates of D. pulex and D. longicephala, e.g. through different metabolic activity and/or differences in their cellular assembly. On the other hand, this result may be due to undersampling, i.e., in highly complex samples, the number of co-eluting peptides exceeds the number of MS/MS spectra which can be acquired by the instrument. Therefore in individual LC-MS/MS runs, different low-intensity peptides may be selected for MS/MS analysis by the instrument software. The overall list of identified proteins can be downloaded as additional file 2.
The impact of the D. pulex filtered models database for proteome research of Daphniids
Although several genome projects on crustaceans are in progress, only expressed sequence tag (EST) libraries (e.g. [37]) or the sequence of the mitochondrial genome [38] are available in other crustacean species. In cases where only few protein sequences are known, it is a common strategy to search MS/MS-data against databases of the most related species in order to identify identical peptides within the homologous proteins.
To estimate the impact of the D. pulex filtered models database for high-throughput proteomics of Daphniids, we compared the results obtained with the Daphnia database with the results obtained by searching our MS/MS dataset against two additional databases: As a species specific database we selected the Drosophila melanogaster database from FlyBase [39] (Release 5.2; http://flybase.org/) consisting of 20,726 protein sequences. We chose this species because D. melanogaster, belongs to the taxon of Hexapoda (Insecta and relatives) and is the closest relative of Daphnia pulex with a characterized complete genome sequence [40]. Both arthropod species belong to a group called Pancrustacea, although monophyly of this group is still discussed [41].
The Pancrustacean hypothesis, which is supported by molecular analysis (e.g. [42]), queries that Myriapoda are the closest relatives to Hexapoda but renders crustaceans and hexapods as sister taxa. Given that the latter have likely diverged 550 to 650 million years ago [43] and have evolved in completely different habitats – crustaceans predominantly in aquatic, insects in terrestrial environments – it is expected that protein expression should reflect these evolutionary challenges. Even though some crustacean gene families, such as genes responsible for embryonic development are shared with Hexapoda [44], several Daphnia genes show no sequence similarity to other arthropods [45]. Therefore, gene transcripts different from those of D. melanogaster might reflect adaptations to aquatic habitats such as chemoreception, oxygen uptake or osmoregulation.
As a protein database of a broad variety of species we chose the Metazoa subset of the Swiss-Prot database (Release 54.2, 78,385 entries) providing a minimum of redundancy. To facilitate a comparison of the results obtained with the different databases, searches of MS/MS spectra were performed using exactly the same parameters. Setting a false-positive identification threshold of 1%, only 71 Daphnia proteins matched to the Drosophila database and 92 to the Swiss-Prot database. This finding clearly demonstrates that the D. pulex filtered models database in its current form increases dramatically the number of MS-based identifications and represents an indispensable tool for high-throughput proteome experiments in daphniids. However, many proteins may still be missing in the database. Therefore, yet unassigned spectra in our data set can help to find undisclosed coding regions within the Daphnia genome. Suitable algorithms comprise searching against the entire Daphnia genome sequence or de-novo sequencing – MS BLAST approaches as described by Shevchenko et al. [46]. Finally, the database supports detailed 2D gel analyses to quantify and identify proteins. The application of the latter technique allows the determination of isolelectric points and molecular weights of the proteins and enables the detection of protein isoforms by comparison of experimentally determined IPs with theoretical IPs from database analysis.
Conclusion
Given that Daphnia is an important model organism, for instance to test for deleterious effects of pollutants or environmental changes, the implementation of state of the art techniques in molecular biology such as LC-MS/MS is an auspicious opportunity to unravel mechanisms triggering those critical environmental issues.
Our study is the first applying a LC-MS/MS based proteomic approach in Daphnia that reflects the utility of the Daphnia genome database for molecular works on this multifaceted model organism in several fields of biological research. Since a variety of Daphnia species are used for different scientific approaches, for instance to elucidate the phenomenon of phenotypic plasticity in daphniids [47] at least 20 species have been investigated intensively, it is essential to know the reliability of the Daphnia pulex genome sequence for studies on other species. We give experimental evidence for the translation of a broad variety of predicted coding regions within the Daphnia genome by using high throughput MS/MS protein identification in two Daphnia species. Our data demonstrates the applicability of proteomics research in D. pulex as well as in other Daphnia species. This will stimulate work on hypothetical functions for yet unclassified proteins followed by functional experiments in this new model organism. Moreover, proteomics techniques allow to identify proteins linked to biological phenomena such as induced predator defenses, host parasite-interactions or stress responses to toxic substances.
Methods
Daphnia cultures
We used a laboratory-cultured clonal line of Daphnia pulex and Daphnia longicephala for our experiments. The Daphnia pulex clone "The Chosen One" picked by the Daphnia Genomics Consortium for the sequencing project was isolated from an ephemeral pond in Oregon (USA) whereas Daphnia longicephala was isolated from Lara Pond (Australia).
Age-synchronized cohorts of both Daphnia species were grown prior to the experiments by collecting mothers with freshly deposited eggs. We cultured the latter in 30 L plastic buckets in the laboratory under constant conditions in a temperature-controlled room at 20°C ± 0.5. Fluorescent light was used to simulate a day-night rhythm (16 h day: 8 h night). The daphnids were fed daily with Scenedesmus obliquus at a concentration of 1.5 mg C L-1 to avoid food limitation. A synthetic medium based on ultra-pure water, trace-elements and phosphate buffer, was changed weekly [48]. 300 randomly chosen adult daphnids were collected prior to proteome analysis.
Sample preparation
The medium containing the daphnids was filtered through a fine sieve (mesh aperture 125 μm) and immediately grounded in a pre-cooled ceramic mortar containing liquid nitrogen. For lysis, the following chemicals were added to final concentrations of 8 M urea, 4% CHAPS, 40 mM Tris, 65 mM DTE. If pre-fractionation by SDS PAGE was performed, 400 μM TLCK and 400 μM TCPK protease inhibitors were added.
SDS PAGE
Prior to SDS-PAGE the samples were mixed with 5× sample buffer. SDS-electrophoresis (overall gel size 7 cm (L) × 8.5 cm (W) × 0.75 mm) was performed using a 1.5 cm 4% stacking gel (0.5 M Tris-HCl pH 6.8, 4% acrylamide-/bis-acrylamide (37.5/1), 0.1% w/v SDS, 0.05% w/v APS, 0.1% v/v TEMED) and a 12% separation gel (1.5 M Tris-HCl pH 8.8, 12% acrylamide/bisacrylamide (37.5/1), 0.1% w/v SDS, 0.05% w/v APS, 0.05% v/v TEMED) with a mini-ProteanTM II device (Bio-Rad, Hercules, USA). Gels were run for 15 min at a constant voltage of 100 V and for additional 60 min at 200 V in SDS running buffer (25 mM Tris, 192 mM glycin, 0.1% w/v SDS). The gels were stained overnight (50% v/v methanol, 0.05% w/v Coomassie brilliant blue R-250, 10% v/v acetic acid) and destained for at least 8 h (5% (v/v) Methanol with 7% (v/v) acetic acid).
Gel slicing and tryptic in-gel digest
Prior to gel slicing, the gels were washed twice in water. After washing, each gel line was cut into 10 slices using a scalpel. Each slice was transferred in a 1.5 mL reaction tube and equilibrated twice with 50 mM NH4HCO3 for 10 min. To reduce and block the cystein residues, the gel slices were incubated for 45 min in 50 mM NH4HCO3/10 mM DTE at 65°C, followed by a 30 min incubation step in 50 mM NH4HCO3 with 55 mM iodacetamide. Prior to digestion, gel pieces were washed twice for 15 min in 50 mM NH4HCO3 and minced with a pipette tip. Tryptic hydrolysis was performed overnight at 37°C in 30 μL 50 mM NH4HCO3with 1 μg porcine trypsin (Promega, Madison, USA) per gel slice. The supernatant was collected and preserved. The peptides were further extracted with 50 μL 50 mM NH4HCO3 and a subsequent treatment using 50 μL 80% ACN. Both extraction steps were performed for 5 min under sonification (Sonorex RK100, Bandelin, Berlin, Germany). The ACN supernatant and the NH4HCO3 fractions were combined and concentrated to a volume of 10 μL using a SpeedVac concentrator (Bachover, Vacuum Concentrator). Prior to 2D-LC-MS/MS analysis the peptide were desalted using Pepclean C-18 spin columns (Pierce) as described by the manufacturer.
1D-LC separation
The 1D-nano-LC separation was performed on a multi-dimensional liquid chromatography system (Ettan MDLC, GE Healthcare). Peptides were loaded on a RP trap column with a flow-rate of 6 μL per min (Loading buffer: 0.1% formic acid; Trap column: C18 PepMap 100, 5 μm bead size, 300 μm i.d., 5 mm length, LC Packings) and subsequently separated with an analytical column (C18 PepMap 100, 3 μm bead size, 75 μm i.d.; 15 cm length, LC Packings) with a 72 min linear gradient (A: 0.1% formic acid, B: 84% ACN and 0.1% formic acid) at a flow rate of 260 nL/min.
2D-LC separation
The 2D-nano-LC separation was performed on a multi-dimensional liquid chromatography system (Ettan MDLC, GE Healthcare). An online salt step configuration was chosen, in which 10 μg of the desalted peptide mixture was injected onto a 50 × 0.32 mm SCX column (BioBasic, Thermo Electron) and eluted at a flow rate of 6 μL/min with 6 discrete salt plugs of increasing salt concentration (10, 25, 50, 100, 500 and 800 mM NH4Cl in 0.1% formic acid and 5% ACN). The eluted peptides were bound on a RP trap column (C18 PepMap 100, 5 μm, 300 μm i.d. 5 mm, LC Packings) and subsequently separated on the second-dimension RP column (C18 PepMap 100, 3 μm, 75 μm i.d. 15 cm, LC Packings) with a 72 min linear gradient (A: 0.1% formic acid, B: 84% ACN and 0.1% formic acid) at a flow rate of 260 nL/min.
Mass spectrometry
Mass spectrometry was performed on a linear ion trap mass spectrometer (Thermo LTQ, Thermo Electron) online coupled to a nano-LC system. For electrospray ionization a distal coated SilicaTip (FS-360-50-15-D-20) and a needle voltage of 1.4 kV was used. The MS method consisted of a cycle combining one full MS scan (Mass range: 300–2000 m/z) with three data dependant MS/MS events (35% collision energy). The dynamic exclusion was set to 30 s.
Database search and data analysis
The MS/MS data were searched with Mascot Version: 2.1.03 (Matrix Science, Boston, USA) using the following parameters: i) Enzyme: Trypsin, ii) Fixed Modification: Carbamidomethyl (C), iii) Variable modifications: Oxidation (M); iv) Peptide tol. 2 Da, v) MS/MS tol. 0.8 Da, vi) Peptide charge 1+, 2+ and 3+, vii) Instrument ESI-TRAP and viii) Allow up to 1 missed cleavages. Mascot results were further validated with the open source software "Trans-Proteomic Pipeline" (TPP) V3.5 freely available from the Seattle Proteome Center http://tools.proteomecenter.org/TPP.php. Therefore the Mascot DAT files were first converted to mzXML, merged and evaluated on the peptide level with the built-in PeptideProphet tool. To generate the list of identified proteins (false positive discovery rate of = 1%) the ProteinProphet tool was used. Furthermore, randomized versions of the applied databases were appended to the original databases using the decoy perl script (Matrix Science, Boston, USA) downloadable at http://www.matrixscience.com/help/decoy_help.html. The number of false positive identifications (randomized sequences) using the Mascot/TPP combination and the corresponding probability thresholds was determined.
Ontology analysis
Protein entries from the Daphnia filtered models database v1.1 were BLASTp-searched http://www.ncbi.nlm.nih.gov/BLAST/ in the Swiss-Prot database http://www.expasy.ch. Homologue protein entries (E-values < 0.01) were subjected to ontology analysis using the PANDORA server http://www.pandora.cs.huji.ac.il/.
Abbreviations
1D: one-dimensional; 2D: two-dimensional; DGC: Daphnia Genomics Consortium; ESI: electrospray ionization; EST: expressed sequence tag; GO: gene ontology; LC: liquid chromatography; LC: liquid chromatography; MS/MS: tandem mass spectrometry; MS: mass spectrometry; PTMs: post-translational modifications; RP: reversed phase; SCX: strong cation-exchange.
Authors' contributions
TF participated in the design of the study, performed the LC-MS/MS experiments, data analysis as well as data interpretation and contributed to the writing of the manuscript. CL initiated and coordinated the study and participated in its design; supervised the biological part of the study; performed sample preparation; performed critical reading and writing of the paper. RF and TB carried out Daphnia cultivation, preparation of samples for mass spectrometry and contributed to the bioinformatic processing of the data. GJA supervised the proteomic part of the work and contributed to project conception and manuscripts writing.
Supplementary Material
Identified Daphnia pulex proteins. Contains Daphnia pulex proteins, ProteinProphet error graph, a list of identified Daphnia pulex peptides and proteins with corresponding PeptideProphet, ProteinProphet and Mascot scores and results of BLAST searches.
Identified Daphnia longicephala proteins. Contains Daphnia longicephala proteins, ProteinProphet error graph, a list of identified Daphnia longicephala peptides and proteins with corresponding PeptideProphet, ProteinProphet and Mascot scores and results of BLAST searches.
Acknowledgments
Acknowledgements
We thank Patrick Bolbrinker and Erik Dülsner for critical reading of the manuscript, Piers Napper for linguistic improvement and, Mechthild Kredler for helping with the Daphnia cultivation and sample preparation. Research work in our laboratory is granted by the "Deutsche Forschungsgemeinschaft", DFG research unit FOR 478 AR 362/1-4, DFG clinical research unit KFO 128 1–2, BMBF FUGATO project AZ 0313388, DFG research unit FOR 585 AR 362/3-2. The sequencing and portions of the analyses were performed at the DOE Joint Genome Institute under the auspices of the U.S. Department of Energy's Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Livermore National Laboratory under Contract No. W-7405-Eng-48, Lawrence Berkeley National Laboratory under Contract No. DE-AC02-05CH11231, Los Alamos National Laboratory under Contract No. W-7405-ENG-36 and in collaboration with the Daphnia Genomics Consortium (DGC) http://daphnia.cgb.indiana.edu. Additional analyses were performed by wFleaBase, developed at the Genome Informatics Lab of Indiana University with support to Don Gilbert from the National Science Foundation and the National Institutes of Health. Coordination infrastructure for the DGC is provided by The Center for Genomics and Bioinformatics at Indiana University, which is supported in part by the METACyt Initiative of Indiana University, funded in part through a major grant from the Lilly Endowment, Inc. Our work benefits from, and contributes to the Daphnia Genomics Consortium.
Contributor Information
Thomas Fröhlich, Email: frohlich@lmb.uni-muenchen.de.
Georg J Arnold, Email: arnold@lmb.uni-muenchen.de.
Rainer Fritsch, Email: fritsch@zi.biologie.uni-muenchen.de.
Tobias Mayr, Email: mayr@zi.biologie.uni-muenchen.de.
Christian Laforsch, Email: laforsch@bio.lmu.de.
References
- Lockhart DJ, Winzeler EA. Genomics, gene expression and DNA arrays. Nature. 2000;405:827–836. doi: 10.1038/35015701. [DOI] [PubMed] [Google Scholar]
- Schena M, Shalon D, Davis RW, Brown PO. Quantitative Monitoring of Gene-Expression Patterns with A Complementary-Dna Microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekirian TL, Thomas SN, Yang A. Advancing signaling networks through proteomics. Expert Rev Proteomics. 2007;4:573–583. doi: 10.1586/14789450.4.4.573. [DOI] [PubMed] [Google Scholar]
- Omenn GS, States DJ, Adamski M, Blackwell TW, Menon R, Hermjakob H, Apweiler R, Haab BB, Simpson RJ, Eddes JS, Kapp EA, Moritz RL, Chan DW, Rai AJ, Admon A, Aebersold R, Eng J, Hancock WS, Hefta SA, Meyer H, Paik YK, Yoo JS, Ping P, Pounds J, Adkins J, Qian X, Wang R, Wasinger V, Wu CY, Zhao X, Zeng R, Archakov A, Tsugita A, Beer I, Pandey A, Pisano M, Andrews P, Tammen H, Speicher DW, Hanash SM. Overview of the HUPO Plasma Proteome Project: results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics. 2005;5:3226–3245. doi: 10.1002/pmic.200500358. [DOI] [PubMed] [Google Scholar]
- Hamacher M, Apweiler R, Arnold G, Becker A, Bluggel M, Carrette O, Colvis C, Dunn MJ, Frohlich T, Fountoulakis M, van Hall A, Herberg F, Ji J, Kretzschmar H, Lewczuk P, Lubec G, Marcus K, Martens L, Palacios Bustamante N, Park YM, Pennington SR, Robben J, Stuhler K, Reidegeld KA, Riederer P, Rossier J, Sanchez JC, Schrader M, Stephan C, Tagle D, Thiele H, Wang J, Wiltfang J, Yoo JS, Zhang C, Klose J, Meyer HE. HUPO Brain Proteome Project: summary of the pilot phase and introduction of a comprehensive data reprocessing strategy. Proteomics. 2006;6:4890–4898. doi: 10.1002/pmic.200600295. [DOI] [PubMed] [Google Scholar]
- He F. Human liver proteome project: plan, progress, and perspectives. Mol Cell Proteomics. 2005;4:1841–1848. doi: 10.1074/mcp.R500013-MCP200. [DOI] [PubMed] [Google Scholar]
- Paik YK, Jeong SK, Lee EY, Jeong PY, Shim YH. C. elegans: an invaluable model organism for the proteomics studies of the cholesterol-mediated signaling pathway. Expert Rev Proteomics. 2006;3:439–453. doi: 10.1586/14789450.3.4.439. [DOI] [PubMed] [Google Scholar]
- Brunner E, Ahrens CH, Mohanty S, Baetschmann H, Loevenich S, Potthast F, Deutsch EW, Panse C, de Lichtenberg U, Rinner O, Lee H, Pedrioli PG, Malmstrom J, Koehler K, Schrimpf S, Krijgsveld J, Kregenow F, Heck AJ, Hafen E, Schlapbach R, Aebersold R. A high-quality catalog of the Drosophila melanogaster proteome. Nat Biotechnol. 2007;25:576–583. doi: 10.1038/nbt1300. [DOI] [PubMed] [Google Scholar]
- Fermin D, Allen BB, Blackwell TW, Menon R, Adamski M, Xu Y, Ulintz P, Omenn GS, States DJ. Novel gene and gene model detection using a whole genome open reading frame analysis in proteomics. Genome Biol. 2006;7:R35. doi: 10.1186/gb-2006-7-4-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner S, Shen Z, Ng J, Florea L, Guigo R, Briggs SP, Bafna V. Improving gene annotation using peptide mass spectrometry. Genome Res. 2007;17:231–239. doi: 10.1101/gr.5646507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CEIII, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Gene Ontology (GO) project in 2006. Nucleic Acids Res. 2006;34:D322–D326. doi: 10.1093/nar/gkj021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Brophy PM, Hamilton JV. Analysing proteomic data. Int J Parasitol. 2005;35:543–553. doi: 10.1016/j.ijpara.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Biron DG, Brun C, Lefevre T, Lebarbenchon C, Loxdale HD, Chevenet F, Brizard JP, Thomas F. The pitfalls of proteomics experiments without the correct use of bioinformatics tools. Proteomics. 2006;6:5577–5596. doi: 10.1002/pmic.200600223. [DOI] [PubMed] [Google Scholar]
- Mann M, Wilm M. Electrospray mass spectrometry for protein characterization. Trends Biochem Sci. 1995;20:219–224. doi: 10.1016/S0968-0004(00)89019-2. [DOI] [PubMed] [Google Scholar]
- Frohlich T, Arnold GJ. Proteome research based on modern liquid chromatography–tandem mass spectrometry: separation, identification and quantification. J Neural Transm. 2006;113:973–994. doi: 10.1007/s00702-006-0509-3. [DOI] [PubMed] [Google Scholar]
- Ishihama Y. Proteomic LC-MS systems using nanoscale liquid chromatography with tandem mass spectrometry. J Chromatogr A. 2005;1067:73–83. doi: 10.1016/j.chroma.2004.10.107. [DOI] [PubMed] [Google Scholar]
- Shen Y, Smith RD. Advanced nanoscale separations and mass spectrometry for sensitive high-throughput proteomics. Expert Rev Proteomics. 2005;2:431–447. doi: 10.1586/14789450.2.3.431. [DOI] [PubMed] [Google Scholar]
- Wilkins MR, Appel RD, Van Eyk JE, Chung MC, Gorg A, Hecker M, Huber LA, Langen H, Link AJ, Paik YK, Patterson SD, Pennington SR, Rabilloud T, Simpson RJ, Weiss W, Dunn MJ. Guidelines for the next 10 years of proteomics. Proteomics. 2006;6:4–8. doi: 10.1002/pmic.200500856. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Vitek O, Aebersold R. Analysis and validation of proteomic data generated by tandem mass spectrometry. Nat Methods. 2007;4:787–797. doi: 10.1038/nmeth1088. [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Aebersold R. Interpretation of shotgun proteomic data: the protein inference problem. Mol Cell Proteomics. 2005;4:1419–1440. doi: 10.1074/mcp.R500012-MCP200. [DOI] [PubMed] [Google Scholar]
- Keller A, Eng J, Zhang N, Li XJ, Aebersold R. A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Mol Syst Biol. 2005;1 doi: 10.1038/msb4100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Elias JE, Haas W, Faherty BK, Gygi SP. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat Methods. 2005;2:667–675. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
- Ahmad QR, Nguyen DH, Wingerd MA, Church GM, Steffen MA. Molecular weight assessment of proteins in total proteome profiles using 1D-PAGE and LC/MS/MS. Proteome Sci. 2005;3:6. doi: 10.1186/1477-5956-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KH, Park GW, Kim JY, Lee SK, Lee JH, Kim YH, Kim SY, Park YM, Yoo JS. Island clustering analysis for the comparison of the membrane and the soluble protein fractions of human brain proteome. Proteomics. 2008;8:1149–1161. doi: 10.1002/pmic.200700756. [DOI] [PubMed] [Google Scholar]
- Agrawal MK, Zitt A, Bagchi D, Weckesser J, Bagchi SN, von Elert E. Characterization of proteases in guts of Daphnia magna and their inhibition by Microcystis aeruginosa PCC 7806. Environ Toxicol. 2005;20:314–322. doi: 10.1002/tox.20123. [DOI] [PubMed] [Google Scholar]
- von Elert E, Agrawal MK, Gebauer C, Jaensch H, Bauer U, Zitt A. Protease activity in gut of Daphnia magna: evidence for trypsin and chymotrypsin enzymes. Comp Biochem Physiol B Biochem Mol Biol. 2004;137:287–296. doi: 10.1016/j.cbpc.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Hebert PD. The systematics of North American Daphnia (Crustacea: Anomopoda): a molecular phylogenetic approach. Philos Trans R Soc Lond B Biol Sci. 1996;351:349–360. doi: 10.1098/rstb.1996.0028. [DOI] [PubMed] [Google Scholar]
- Tollrian R, Dodson SI. Inducible defenses in Cladocera: constraints, costs and multipredator environments. In: Tollrian R, Harvell CD, editor. The Ecology and Evolution of inducible Defenses. Princeton, New Jersey: Princeton University Press; 1999. pp. 177–202. [Google Scholar]
- Shafer TH, McCartney MA, Faircloth LM. Identifying exoskeleton proteins in the blue crab from an expressed sequence tag (EST) library. Integr Comp Biol. 2006;46:978–990. doi: 10.1093/icb/icl022. [DOI] [PubMed] [Google Scholar]
- Ogoh K, Ohmiya Y. Complete mitochondrial DNA sequence of the sea-firefly, Vargula hilgendorfii (Crustacea, Ostracoda) with duplicate control regions. Gene. 2004;327:131–139. doi: 10.1016/j.gene.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Crosby MA, Goodman JL, Strelets VB, Zhang P, Gelbart WM. FlyBase: genomes by the dozen. Nucleic Acids Res. 2007;35:D486–D491. doi: 10.1093/nar/gkl827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Nardi F, Spinsanti G, Boore JL, Carapelli A, Dallai R, Frati F. Hexapod origins: monophyletic or paraphyletic? Science. 2003;299:1887–1889. doi: 10.1126/science.1078607. [DOI] [PubMed] [Google Scholar]
- Giribet G, Edgecombe GD, Wheeler WC. Arthropod phylogeny based on eight molecular loci and morphology. Nature. 2001;413:157–161. doi: 10.1038/35093097. [DOI] [PubMed] [Google Scholar]
- Pisani D, Poling LL, Lyons-Weiler M, Hedges SB. The colonization of land by animals: molecular phylogeny and divergence times among arthropods. BMC Biol. 2004;2:1. doi: 10.1186/1741-7007-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GK, D'Alessio JA, Patel NH. Pax3/7 genes reveal conservation and divergence in the arthropod segmentation hierarchy. Dev Biol. 2005;285:169–184. doi: 10.1016/j.ydbio.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Eads BD, Shaw J, Bohuski E, Bauer DJ, Andrews J. Sampling Daphnia's expressed genes: preservation, expansion and invention of crustacean genes with reference to insect genomes. BMC Genomics. 2007;8:217. doi: 10.1186/1471-2164-8-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waridel P, Frank A, Thomas H, Surendranath V, Sunyaev S, Pevzner P, Shevchenko A. Sequence similarity-driven proteomics in organisms with unknown genomes by LC-MS/MS and automated de novo sequencing. Proteomics. 2007;7:2318–2329. doi: 10.1002/pmic.200700003. [DOI] [PubMed] [Google Scholar]
- Laforsch C, Ngwa W, Grill W, Tollrian R. An acoustic microscopy technique reveals hidden morphological defenses in Daphnia. Proc Natl Acad Sci USA. 2004;101:15911–15914. doi: 10.1073/pnas.0404860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke JM, Tollrian R. Density-dependent effects of prey defences. Oecologia. 2000;123:391–396. doi: 10.1007/s004420051026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identified Daphnia pulex proteins. Contains Daphnia pulex proteins, ProteinProphet error graph, a list of identified Daphnia pulex peptides and proteins with corresponding PeptideProphet, ProteinProphet and Mascot scores and results of BLAST searches.
Identified Daphnia longicephala proteins. Contains Daphnia longicephala proteins, ProteinProphet error graph, a list of identified Daphnia longicephala peptides and proteins with corresponding PeptideProphet, ProteinProphet and Mascot scores and results of BLAST searches.