Abstract
Maternal nutrient restriction (NR) from early to midgestation has marked effects on endocrine sensitivity and organ function of the resulting offspring. We hypothesized that early NR may reset the expression profile of genes central to myocardial energy metabolism, influencing ectopic lipid deposition and cardiac function in the obese adult offspring. NR offspring were exposed to an “obesogenic” environment, and their cardiac function and molecular indexes of myocardial energy metabolism were assessed to explore the hypothesis that an obese individual's risk of heart disease may be modified after maternal NR. Pregnant sheep were fed 100% (control) or 50% (NR) energy requirement from days 30 to 80 of gestation and 100% energy requirement thereafter. At weaning, offspring were exposed to an obesogenic environment or remained lean. At ∼1 yr of age, the hemodynamic response of these offspring to hypotension, together with left ventricular expression profiles of fatty acid-binding protein 3 (FABP3), peroxisome proliferator-activated receptor-γ (PPARγ) and its coactivator (PGC)-1α, acetyl-CoA carboxylase (ACC), AMP-activated protein kinase (AMPK)-α2, and voltage-dependent anion channel 1 (VDAC1), was determined. Obesity produced left ventricular hypertrophy in all animals, with increased ectopic (myocardial) lipid in NR offspring. Obesity per se significantly reduced myocardial transcript expression of PGC-1α, AMPKα2, VDAC1, and ACC and increased expression of PPARγ and FABP3. However, although NR animals were similarly obese, their transcript expression of ACC, PPARγ, and FABP3 was similar to that of lean animals, indicating altered cardiac energy metabolism. Indeed, blunted tachycardia and an amplified inotropic response to hypotension characterized cardiac function in obese NR offspring. The results suggest that maternal NR during early organogenesis can precipitate an altered myocardial response to hypotension and increased myocardial lipid deposition in the adult offspring after adolescent-onset obesity, potentially rendering these individuals more at risk of early heart failure as they age.
Keywords: lipid infiltration, hypotensive challenge, myocardial energy metabolism, ectopic lipid deposition, insulin resistance
obesity is a significant risk factor for diabetes, hypertension, cardiovascular disease, and dyslipidemia (56). Sedentary lifestyle, together with increased caloric intake, is the main cause of obesity and related onset of the metabolic syndrome (27, 42). Obesity is well known to increase the risk of a broad spectrum of noncommunicable diseases, including renal (11) and cardiovascular disease, through changes in sympathetic activation (2), an upregulated renin-angiotensin system (40), and cardiac hypertrophy, atherosclerosis, and local inflammatory responses (1, 28). Specifically, obesity has been shown to have a substantial inhibitory effect on cardiac function, partly as a consequence of excess fat accumulation in and around the heart (29). Increased peri- and epicardial fat is associated with a fetal pattern of myocardial energy utilization, i.e., a predominantly glucose, rather than fatty acid, dependence and changes in expression of the key intracellular molecular energy “switches,” e.g., peroxisome proliferator-activated receptor (PPAR)-γ coactivator (PGC)-1α (31), acetyl-CoA carboxylase (ACC), and AMP-activated protein kinase (AMPK)-α2 (30). At the same time, obesity results in significant changes in left ventricular (LV) and diastolic function (13) that are exacerbated by the concomitant sympathetic overdrive (24, 25), leading to a myocardium that is refractory to β-adrenergic signaling (8).
Global changes in maternal macronutrient intake targeted at defined stages of pregnancy can have pronounced effects on cardiovascular control in the resulting offspring (21). Such adaptations are usually accompanied by significant changes in the density of expression of organ receptor populations and relevant enzyme activities and, hence, endocrine responsiveness and substrate transport and utilization (for review see Ref. 34). For example, maternal nutrient restriction in early pregnancy is associated with increased tissue-specific glucocorticoid receptor (GR) expression in the newborn (52), which results in increased blood pressure later in life, but only in a prefeeding basal state (23). Maternal nutrient restriction coincident with early heart development, however, has no obvious long-term effects on cardiac function when the offspring are maintained under a natural “free-living” environment (22, 23). Importantly, the long-term programming outcomes appear to be amplified when the offspring are exposed to an obesogenic environment (44, 45).
The extent to which the prenatal nutritional environment may influence the expression of genes associated with cardiac hypertrophy has been examined previously (26), but potential effects on energy metabolism in the heart, especially with an early onset of obesity, have not been studied. This is important, since cardiac hypertrophy is associated with suppressed insulin-dependent GLUT4 expression and heart-type fatty acid-binding protein (FABP)-3 (43) but enhanced insulin-independent GLUT1 transport (1) and ectopic lipid accumulation (47). These changes in myocardial energy metabolism with obesity may also impact other “nutrient sensors” within cardiac tissue, such as expression of PPARγ and its coactivator (PGC-1α) (14). Adaptations of this type could also determine, in part, progression toward heart disease with obesity, as a consequence of changes in myocardial substrate supply and utilization, particularly fatty acids, since these account for more than half of ATP production in the heart.
Although the prenatal nutritional environment has been shown in many studies to alter specific aspects of growth, development, and physiology per se, it is evident that the physical expression of a “programmed” end point is dependent, in part, on the postnatal environment. Specifically, the degree of physical activity, food intake, and energy density of the food, i.e., the degree of exposure and active engagement in an “obesogenic” lifestyle, may influence the levels of hypertension and/or obesity (39, 51, 53). In the present study, we hypothesized that programming by prenatal nutrient restriction may reset the expression profile of genes that are central to myocardial energy metabolism, influencing lipid deposition in this tissue and, ultimately, affecting cardiac function under duress. In allowing these “programmed” animals to become overweight, we sought to model a sedentary, Westernized culture, characterized by reduced physical activity and increased access to energy-dense food. The effects of obesity on myocardial function were then assessed in vivo during a hypotensive challenge followed by a molecular characterization of myocardial energy metabolism. This included a direct comparison of the effects of obesity per se by further comparison of cardiovascular function and cardiac outcomes between lean and obese individuals born to normally fed mothers.
MATERIALS AND METHODS
Animals and Experimental Design
All procedures were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986, and approved by the local ethics committee of the University of Nottingham at Sutton Bonington. At day 30 of gestation, 16 twin-bearing ewes were randomly allocated to receive a control [∼7–8 MJ/day of metabolizable energy (ME), n 6] or nutrient-restricted (50% of control, n = 10) diet until day 80 of gestation. Thereafter, all sheep were fed to 100% calculated ME requirements to full term (12–13 MJ/day near to term) (2). Offspring were delivered spontaneously and reared by their mothers as singletons (i.e., 1 twin was euthanized) from day 7 to weaning (10 wk). There were three males in the control group and two males in the nutrient-restricted groups. After birth, all mothers were fed a diet of hay ad libitum, together with a fixed amount of concentrate pellets sufficient to fully meet their own ME requirements plus that needed to maintain lactation. All diets contained adequate minerals and vitamins. From weaning to 12 mo of age, all offspring were group housed in a barn (50 m2; i.e., restricted activity) with ad libitum access to hay and concentrate pellets (140 g/kg crude protein, 3% oil, 12.7 MJ/kg dry matter; Manor Farm Feeds) to promote increased fat deposition; these sheep were designated “obese” (O) or “nutrient-restricted obese” (NRO), as previously described (48). Another group of sheep (n = 8) was incorporated into the experimental design to control for the postnatal treatment structure, i.e., adolescent-onset obesity. These sheep were contemporaneous to control animals; i.e., the ewes were fed 100% ME requirements throughout gestation and gave birth to twin female offspring, with only one of each twin pair used for study. At weaning, however, these sheep lived in an environment that encouraged unlimited low-to-moderate physical activity (a field as opposed to a barn), with supplemental feed provided as required (53). These sheep were designated “lean” (L). At 1 yr of age, i.e., as young adults, arterial (carotid) and venous catheters (jugular) were inserted into all sheep. Before surgery, all food, but not water, was withdrawn from the animals for 24 h. Anesthesia was induced with propofol (Rapinovet; 6 mg/kg) and maintained with 3–4% isoflurane in 3–4 l/min O2. All sheep received a course of antibiotic (10 mg/kg im procaine penicillin; Duphapen, Fort Dodge Animal Health, Southampton, UK) and analgesia (2 mg/kg im flunixin meglumine; Finadyne, Schering-Plough, Kenilworth, UK) for 3 days postoperatively. Catheter patency was maintained by daily flushing with heparinized saline (50 IU heparin/ml). All sheep had established normal feeding patterns within 1 h after surgery and showed no visible signs of discomfort for the duration of the experimental period.
Experimental Protocols
Cardiovascular experiments.
No experiment was performed until 2–4 days after postoperative recovery. The investigator was blinded to the dietary origin of the sheep before any experiment was performed. All sheep were prehabituated to the experimental conditions before the same experiment was conducted with three different treatments on 3 separate days: 1) with a background infusion of saline, 2) with pretreatment and infusion of the muscarinic antagonist atropine sulfate, and 3) with pretreatment and infusion of the mixed β-antagonist propranolol.
Cardiovascular responses to sodium nitroprusside infusion.
Sheep were habituated to a metabolic crate, and after ≥1 h, the arterial catheter was connected to precalibrated pressure transducers (SensorNor 840; S 4925) attached at heart level and linked to a data acquisition system (Po-Ne-Mah, version 3, Gould Instrument Systems), and baseline data were recorded over 1 h. Analog signals for real-time systolic, diastolic, and mean arterial pressure and heart rate were recorded at 1-s intervals, digitized, and then stored on an Excel spreadsheet for further analysis (18). Resting cardiovascular data (baseline systolic, diastolic, and mean arterial pressure and heart rate) for these animals has been described elsewhere (53). The first derivative of the positive and negative change in pressure associated with each heartbeat (+dP/dt and −dP/dt) was calculated automatically and taken to reflect the strength of myocardial contractility and relaxation, respectively. The rate-pressure product [(mmHg·min−1)/103] was used as an assessment of myocardial work.
Saline infusion.
On a background of saline infusion (1 ml/min), the sheep were infused intravenously (2.5 μg·kg−1·min−1) with the endothelium-dependent vasodilator sodium nitroprusside (SNP; Abbott Laboratories, Maidenhead, UK) for 5 min, with a further 5-min recording of the recovery period.
Atropine infusion.
On a separate day, the protocol for saline infusion was followed exactly, except the sheep received a bolus dose of atropine intravenously (2.4 mg) followed by a constant infusion of 1 mg/ml. Further bolus doses (1.2 mg) were given, and each failed to elicit an increase in heart rate, confirming complete muscarinic blockade, as previously described in detail (55).
Propranolol infusion.
On a separate day, the protocol for saline infusion was again followed, except the sheep received a bolus dose of propranolol intravenously (20 mg) followed by a constant infusion of 1 mg·ml−1·min−1, as previously described in detail (55).
Blood and Molecular Analyses
Biochemical analyses.
Whole blood was withdrawn from the jugular catheter into pretreated [50 μl of glutathione-EGTA solution (4.75 g of EGTA and 3.00 g of glutathione dissolved in 50 ml of deionized water)] heparinized tubes at 5 min before and 4 min into the SNP infusion with saline. Plasma concentrations of catecholamines were measured by HPLC with electrochemical detection, as previously described in detail (19). At the end of all experimental protocols, all sheep were humanely euthanized with electrocortical stunning and exsanguination. A representative sample of LV tissue was flash frozen in liquid nitrogen and stored at −80°C until analysis.
Lipid extraction and tissue triglyceride assay.
Lipids in frozen LV (∼500 mg) were first extracted with 1:1 chloroform-methanol and dissolved in 1:1 (vol/vol) tert-butyl alcohol-Triton X-100 before enzymatic measurement (Infinity Triglycerides Liquid Stable Reagent, Thermo Electron) (12, 18).
Total RNA isolation and reverse transcription.
RNA was extracted from a fixed quantity of the LV (∼100–200 mg) using TriReagent (catalog no. T9424, Sigma). RNA concentration was measured spectrophotometrically, and its purity was confirmed by measurement of the ratio of absorbance at 260 nm to absorbance at 280 nm. RNA concentration was adjusted to 3 μg/μl using nuclease-free water (Ambion), and samples were stored at −80°C. First-strand cDNAs were reverse transcribed in a reaction containing 3 μg of total RNA, 200 U of SuperScript II reverse transcriptase (catalog no. 18064-014, Invitrogen), first-strand buffer [250 nM Tris·HCl (pH 8.3), 375 mM KCl, and 15 mM MgCl2], 125 ng of pd(N)6 random hexamer 5′-phosphate (catalog no. 27-2166-01, GE Healthcare), 10 mM dNTP mix (catalog no. 28-4065-64, GE Healthcare), and 40 U of RNaseOUT recombinant RNase inhibitor (catalog no. 10777-019, Invitrogen). The conditions of synthesizing cDNA were therefore in accordance with the manufacturer's protocol.
PCR for standard curve generation.
PCR was performed in the presence of Thermo-Start Master Mix (AB-0938-DC-MM, ABgene). The forward and reverse primers (Sigma Genosys) are listed in Table 1. The amplification parameters were as follows: 95°C (15 min); 35–40 cycles at 94°C (45 s), annealing temperature was 72°C for 15 min. Annealing temperature was 55°C for the α/β-adrenergic receptors (ARs) PGC-1α and AMPKα2 and 60°C for ACC. The amplified PCR products were extracted from agarose gels by the QIAquick gel extraction method (catalog no. 28706, Qiagen). The extracted amplicons were sequenced to confirm the target region of amplification and serially diluted to generate standard curves for real-time PCR assay.
Table 1.
Summary of ovine specific oligonucleotide primers for myocardial genes determined using real-time PCR
| Gene | Accession No. |
Primer Sequences |
||
|---|---|---|---|---|
| Forward | Reverse | |||
| Adrenergic receptors | ||||
| α1- | NM_174498 | 5′-atc cac acc atc tcc ctc ag-3′ | 5′-tcg tct cta agc cct acc tct g-3′ | |
| α2- | NM_174499 | 5′-ctg cac gtc ttc cat agt gc-3′ | 5′-tcc tgc ctc tct tct tct cg-3′ | |
| β1- | NM_194266 | 5′-cgc tca cca acc tct tca tc-3′ | 5′-cac aca ggg tct caa tgc tg-3′ | |
| β2- | NM_174231 | 5′-att gcc tcc tcc att gtg tc-3′ | 5′-cat cct gct cca ctt gac tg-3′ | |
| Energy metabolism | ||||
| PGC-1α | NM_177945 | 5′-gtg tgt gtt tgc ctg gtt tg-3′ | 5′-gcc tgt ggt tgg ttg gtt ag-3′ | |
| AMPKα2 | NM_214266 | 5′-gct gga ttt tga atg gaa gg-3′ | 5′-cag cac ctc atc atc aat gc-3′ | |
| ACC | NM_001009256 | 5′-gct atg gaa gtc ggc tgt gga ag-3′ | 5′-tcg tca gga aga ggc gga tgg-3′ | |
| GR | X70407.1 | 5′-act gcc cca agt gaa aac aga-3′ | 5′-atg aac aga aat ggc aga cat ttt att-3′ | |
| GLUT1 | U89029.1 | 5′-gca gga gat gaa gga gga gag c-3′ | 5′-gca gca cca cgg aaa tga g-3′ | |
| GLUT4 | AB005283.1 | 5′-agt atg tgg cgg atg cta tgg g-3′ | 5′-cgg cgg aag acg gct gag-3′ | |
| IR | AY157728.1 | 5′-ctg cac cat cat caa cgg aa-3′ | 5′-cgt aac ttc cgg aag aag ga-3′ | |
| FABP3 | NM_174313 | 5′-aga tgg ttg acg gga aac tc-3′ | 5′-cac aat gag aac gga act gg-3′ | |
| VDAC1 | NM_001126352 | 5′-cct ccc acg tat gct gat ctt-3′ | 5′-aga tca agt ttt att aag cca aat cca tag-3′ | |
| PPARγ | NM_001100921 | 5′-acg gga aag acg aca gac aaa tc-3′ | 5′-cac gga gcg aaa ctg aca cc-3′ | |
| 18S | NR_002170 | 5′-ctc ctg gtg gtg ccc ttc c-3′ | 5′-gat gcg gcg gcg tta ttc c-3′ | |
ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; FABP, fatty acid-binding protein; GR, glucocorticoid receptor; IR, insulin receptor; PGC, peroxisome proliferator-activated receptor (PPAR) coactivator; VDAC, voltage-dependent anion channel.
Quantitative real-time PCR assay.
The relative abundance of each gene was measured by quantitative real-time PCR using the Quantica Real-Time Nucleic Acid Detection System (Techne, Barloworld Scientific) with QuantiTect SYBR Green PCR Master Mix (catalog no. 204145, Qiagen), diluted RT reactions, and 10–15 pmol of the forward and reverse primers (Table 1). A reverse transcribed-negative control was used to ensure absence of genomic DNA contamination. The amplification parameters were as follows: 95°C (15 min); 45 cycles at 94°C (30 s), annealing temperature described above (see PCR for standard curve generation). Melt curve analysis was performed to ensure reaction specificity. Primers were designed to amplify small fragments (150–250 bp) and ensure ∼98% amplification efficiency. 18S was chosen as the housekeeping gene, inasmuch as the alternatives (e.g., GAPDH and β-actin) are nutritionally sensitive (49).
Statistical Analyses
Data were first analyzed for an effect of treatment group (L, O, and NRO) by a univariate general linear model procedure with treatment and, where appropriate, sex as a fixed effect using SPSS version 14 (SPSS, Chicago, IL). Specific contrasts selected a priori to test for effects of postnatal obesity (L vs. O) or prenatal diet (O vs. NRO) were also examined. Estimated marginal means are presented, together with their respective SE, unless otherwise stated. For cardiovascular responses, data were analyzed as area under the response curve (Prism version 5, GraphPad, San Diego, CA). For repeated measures (e.g., catecholamines before and after SNP), all data were compared by paired t-test followed by a repeated- measures general linear model to test for any treatment effects (SPSS version 14). For all comparisons, statistical significance was accepted when P < 0.05.
RESULTS
Body Composition and Metabolic Status
The overall effect of exposure to an obesogenic environment [∼65% reduced physical activity, ∼30% increased food intake (53)] from after weaning (3 mo of age) to young adulthood (1 yr of age) has been reported previously (44, 53). Obese sheep were, by definition, significantly heavier [∼90 vs. 58 kg (±1.8 SE)] and fatter [∼7.0 vs. 1.5 kg (±0.3 SE) visceral fat mass] than lean sheep at 1 yr of age, with marginally increased fasting plasma glucose concentration [5.47 vs. 4.47 mmol/l (±0.57 SE)] and significantly higher plasma nonesterified fatty acid [0.60 vs. 0.31 mmol/l (±0.08 SE)], leptin [20 vs. 3 ng/ml (±1 SE)], and insulin [1.34 vs. 0.56 ng/ml (±0.20 SE)] concentrations.
LV Triglyceride and Transcript Expression for Cardiac Energy Metabolism
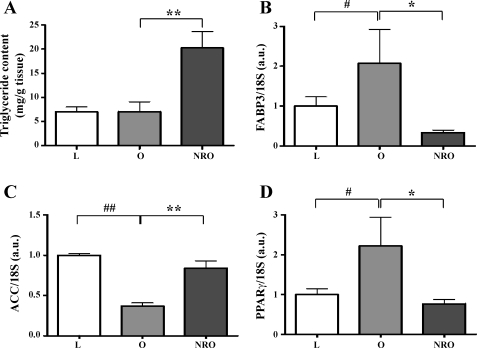
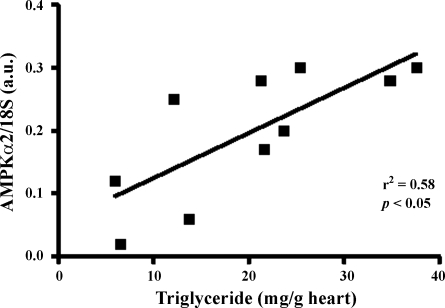
The triglyceride content of the LV was unaffected by postnatal obesity per se but was significantly increased (∼3-fold) in the nutrient-restricted obese offspring (Fig. 1A). Myocardial expression of FABP3 and PPARγ2 was raised by obesity, a response that was not seen in the nutrient-restricted obese offspring (Fig. 1, B and D). However, mRNA abundance for ACC was reduced with postnatal obesity, but this was not observed in nutrient-restricted obese offspring (Fig. 1C). Gene expression of PGC-1α, AMPKα2, and voltage-dependent anion channel 1 (VDAC1) were reduced by postnatal obesity per se, again an adaptation not observed in nutrient-restricted obese offspring (Table 2). Gene expression of AMPKα2 was positively correlated with triglyceride content in the nutrient-restricted obese offspring only (r2 = 0.57, P < 0.05; Fig. 2).
Fig. 1.
Left ventricular (LV) triglyceride and transcript expression for cardiac energy metabolism. A: triglyceride content in LV. B–D: mRNA abundance of fatty acid-binding protein 3 (FABP3), acetyl-CoA carboxylase (ACC), and peroxisome proliferator-activated receptor-γ (PPARγ). AU, arbitrary units. Values are means ± SE for lean (L, n = 8), obese (O, n = 6), and obese offspring born to nutrient-restricted mothers (NRO, n = 10) at 1 yr of age. #P < 0.05 and ##P < 0.01, L vs. O. *P < 0.05 and **P < 0.01, O vs. NRO.
Table 2.
Mean expression of genes involved in cardiac energy metabolism in LV of lean, obese, or prenatally nutrient-restricted obese offspring
| Gene |
mRNA Abundance in LV, AU |
P
|
|||
|---|---|---|---|---|---|
| L (n = 8) | O (n = 6) | NRO (n = 11) | L vs. O | O vs. NRO | |
| PGC-1α | 1.00±0.08 | 0.55±0.10 | 0.62±0.10 | <0.05 | NS |
| AMPKα2 | 1.00±0.25 | 0.44±0.10 | 0.56±0.08 | <0.05 | NS |
| VDAC | 1.00±0.19 | 0.59±0.14 | 0.36±0.04 | <0.05 | NS |
| GR | 1.00±0.14 | 1.46±0.52 | 0.52±0.09 | NS | <0.05 |
| GLUT1 | 1.00±0.37 | 1.21±0.49 | 0.13±0.04 | NS | <0.05 |
| GLUT4 | 1.00±0.19 | 0.65±0.25 | 0.35±0.01 | NS | NS |
| IR | 1.00±0.39 | 0.63±0.14 | 0.45±0.08 | NS | NS |
Values are means ± SE. AU, arbitrary units; LV, left ventricle; L, lean; O, obese; NRO, nutrient-restricted obese; NS, not significant.
Fig. 2.
Positive correlation between AMP-activated protein kinase-α2 (AMPKα2) and triglyceride content in the heart of obese offspring born to mothers nutrient restricted between early and midgestation.
There was no effect of obesity on gene expression for GLUT4, the insulin receptor (IR), or the GR, although GR and GLUT1 mRNA abundance were reduced in the nutrient-restricted obese offspring (Table 2). However, GLUT1 gene expression was raised in the obese group compared with the lean group (Table 2).
Plasma Catecholamines and Myocardial AR Gene Expression
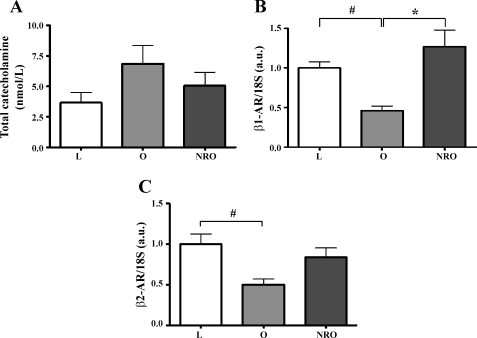
Resting plasma catecholamines were almost twofold greater in obese than in lean animals, but because of individual variation, this difference did not achieve statistical significance (Fig. 3A). With hypotension, obese animals exhibited a significantly greater increment in total plasma catecholamines (−0.153 ± 0.716, 0.538 ± 1.523, and 1.516 ± 0.946 nmol/l for L, O, and NRO, respectively, P < 0.05, L vs. O). Gene expression for the β1- and β2-ARs was reduced with obesity, but not in nutrient-restricted obese offspring (Fig. 3, B and C). There was no difference between dietary groups in the expression of mRNA for the α1- or α2-ARs (data not shown).
Fig. 3.
Effects of obesity and maternal nutrient restriction on total plasma catecholamine concentration (A) and cardiac gene expression of β1- and β2-adrenergic receptors (β1- and β2-AR, B and C). Values are means ± SE of lean (n = 8), obese (n = 6), and obese offspring born to mothers nutrient restricted between early and midgestation (n = 10) sampled at 1 yr of age. #P < 0.05, L vs. O. *P < 0.05, O vs. NRO.
Resting Cardiovascular Status and Response to SNP
Saline infusion.
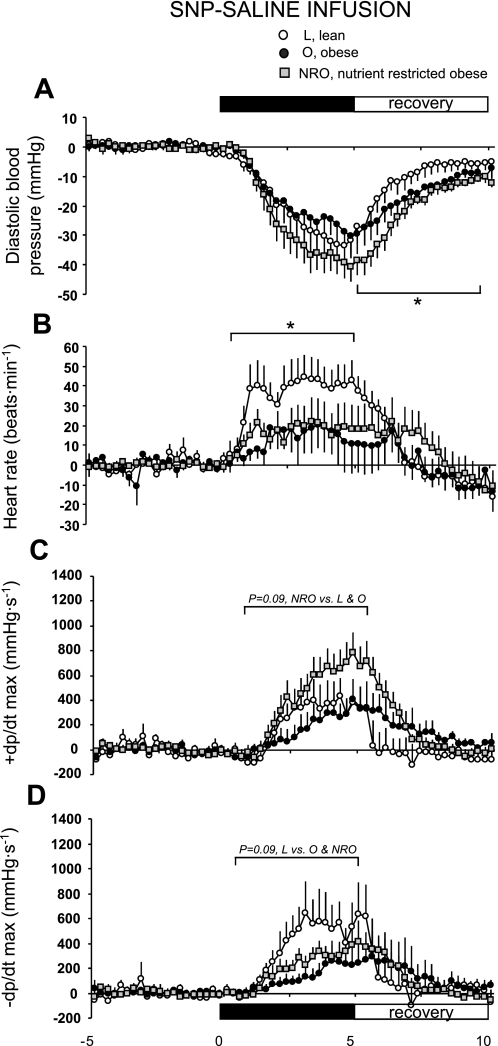
Before saline infusion, mean arterial pressure was higher in obese (O and NRO) than in lean sheep (97 ± 2 and 99 ± 2 mmHg in O and NRO, respectively, vs. 89 ± 1 mmHg in L, P = 0.03 by 1-way ANOVA), with no interaction with prenatal diet. These results are similar to those published previously for systolic and diastolic pressures in these animals (53). Resting rate-pressure product was significantly higher in obese sheep [8.62 ± 0.87, 10.07 ± 0.66, and 11.96 ± 0.82 (mmHg·min−1)/10−3 in L, O, and NRO, respectively], but +dP/dt (800 ± 117, 739 ± 113, and 803 ± 103 mmHg/s in L, O, and NRO, respectively) and −dP/dt (545 ± 74, 328 ± 72, and 458 ± 66 mmHg/s in L, O, and NRO, respectively) were similar in obese and lean sheep. SNP infusion elicited a rapid and significant decline in blood pressure (Fig. 4A), provoking a significant increment in heart rate (Fig. 4B) during the challenge that was significantly greater (0 to 5 min; P < 0.05, F = 4.25) in lean than in obese sheep (33 ± 8, 12 ± 8, and 12 ± 7 beats/min in L, O, and NRO, respectively). Although there was no effect of obesity per se on +dP/dt (Fig. 4C), there was a trend (P = 0.09, F = 3.16) for the increment to be greater in the nutrient-restricted obese group [2,689 ± 2,008 and 7,208 ± 1,555 area-under-the-curve (AUC) units in O and NRO, respectively]. In addition, there was a trend (P = 0.09, F = 3.15) for the rate of cardiac relaxation (−dP/dt) to be slower in obese sheep (6,935 ± 1,819 and 3,232 ± 1,017 AUC units in L and O, respectively; Fig. 4D). During the 5-min recovery period, the return of diastolic pressure (−137 ± 47 and −362 ± 26 AUC units in L and O, respectively, P = 0.001, F = 17.2) and +dP/dt (−487 ± 1,228 and 3,113 ± 686 AUC units in L and O, respectively, P = 0.01, F = 6.54) toward baseline was significantly blunted in obese compared with lean sheep. There were no effects of prenatal diet on the recovery of cardiac function in obese sheep.
Fig. 4.
Cardiovascular response to sodium nitroprusside (SNP) during saline infusion in lean (n = 8), obese (n = 6), and obese offspring born to mothers nutrient restricted between early and midgestation (n = 10) at 1 yr of age. +dP/dt and −dP/dt, 1st derivative of positive and negative change in blood pressure. Values are means ± SE. *P < 0.05, L vs. O.
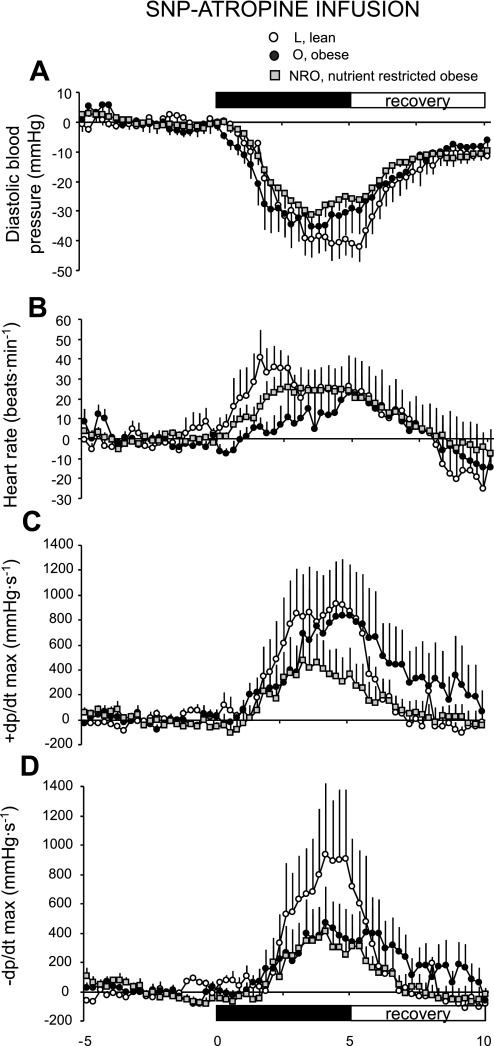
Atropine infusion.
There were no significant effects of obesity or prenatal diet on the cardiac response to SNP during atropine infusion (Fig. 5); however, the differences in response during saline infusion were abolished when the experiment was conducted on a background of muscarinic blockade (Fig. 4A vs. Fig. 5A). Overall, the cardiac response to SNP as reflected in heart rate (Fig. 5B), +dP/dt (Fig. 5C), and −dP/dt (Fig. 5D) after atropine was similar to that observed during saline infusion (i.e., a comparison of equivalent results presented in Figs. 4 and 5) in all groups.
Fig. 5.
Basal blood pressure response to SNP during atropine infusion in lean (n = 8), obese (n = 6), and obese offspring born to mothers nutrient restricted between early and midgestation (n = 10) at 1 yr of age. Values are means ± SE.
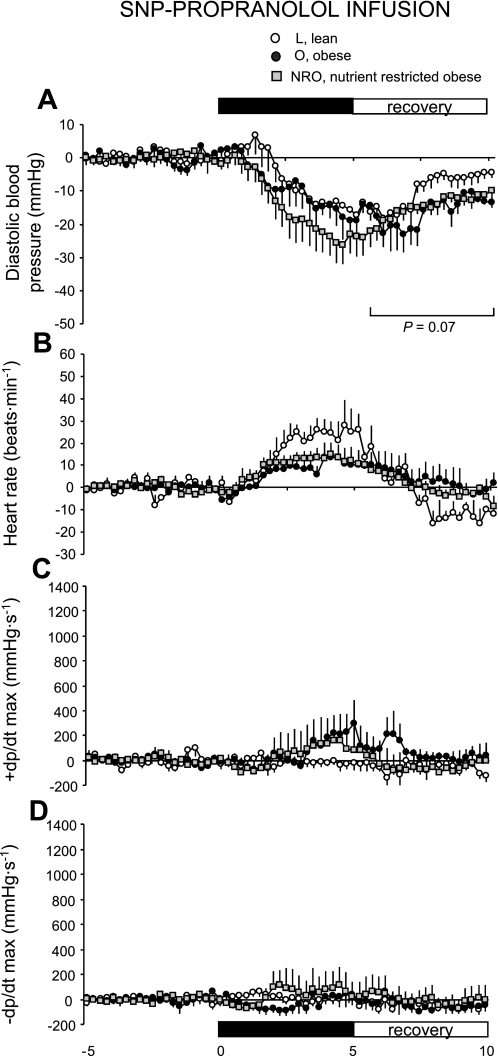
Propranolol infusion.
There were no significant effects of obesity or prenatal diet on the cardiac response to SNP during propranolol infusion (Fig. 6A). As expected, propranolol significantly blunted the increment in heart rate (Fig. 6B) and cardiac contractility (+dP/dt; Fig. 6C) and relaxation (−dP/dt; Fig. 6D) in all groups relative to results observed during saline infusion (i.e., a comparison of equivalent results presented in Figs. 4 and 6). Consistent with the response observed during saline infusion, the recovery of diastolic pressure after withdrawal of SNP tended to be blunted in obese compared with lean sheep (Fig. 6A).
Fig. 6.
Basal blood pressure response to SNP during propranolol infusion in lean (n = 8), obese (n = 6), and obese offspring born to mothers nutrient restricted between early and midgestation (n = 10) at 1 yr of age. Values are means ± SE.
DISCUSSION
The major finding of the present study is that fetal exposure to maternal nutrient restriction during early gestation increased ectopic lipid deposition in the LV and reset cardiac energy metabolism when the offspring became obese. This was particularly striking, since levels of myocardial ectopic lipid levels in contemporaneous nutritional controls, which were equally obese, were as low as those in lean animals. Myocardial lipid infiltration is normally related to current diet, aging, and diastolic dysfunction and clinically is often seen in heart failure patients with diabetes or obesity (5, 29, 47). Our findings in prenatally programmed animals thus suggest that they are potentially more at risk from earlier heart failure. However, despite having higher blood pressure (53), they did not show any obvious clinically significant metabolic or other health problems at ∼1 yr of age (44). Therefore, the molecular changes that accompanied raised lipid within the hearts of these in utero nutrient-restricted obese animals may provide important early evidence of the developmental mechanisms by which cardiac function can become impaired with obesity.
Obesity is the most significant and potentially preventable risk factor for coronary events, increasing the incidence of other significant risk factors such as hypertension (35, 36, 50). Defined obesity is increasingly observed in childhood and, subsequently, tracks into adulthood, giving these individuals an exacerbated cardiovascular risk (4). In the present study, juvenile-onset obesity per se led to LV hypertrophy (LVH), most likely through increased afterload, myocardial workload, and increased peripheral vascular resistance (49). Indeed, in the present study, rate-pressure product was significantly greater in obese animals, indicative of increased myocardial functional stress, which, together with the increase in LV wall thickness, but without clinical symptoms, suggests that these offspring are at increased risk of heart failure (1, 5, 41, 54). Functionally, in all obese sheep, the heart was compromised, as indicated by delayed myocardial relaxation after a hypotensive stimulus. Although it is accepted that the greater increment in plasma catecholamines during hypotension in obese animals may, in part, contribute to this response, the failure of propranolol infusion to eliminate the effect suggests deficits in cardiac contractility per se as the primary mechanism.
Chronic sympathetic activation by systemic β-AR agonist administration results in cardiac hypertrophy, blunted parasympathetically mediated cardiovascular reflexes, and suppressed β-AR-mediated inotropic responses and sensitivity (10, 37, 38). We also report reduced gene expression of β1/β2-AR (but not α1/α2-AR) in hypertrophied myocardium with obesity. Desensitization of adrenergic signaling occurs in chronic heart failure that is characterized by a rapid increase in sympathetic activation and a decrease in β1-AR abundance (7, 20). In the present study, there was a nonsignificant trend for higher circulating catecholamine concentrations in obese than in lean animals and, in the obese group only, downregulated β1/β2-AR. Maternal nutrient restriction combined with postnatal obesity, however, resulted in protected β1/β2-AR gene expression and, consequently, a strong and sustained increase in systolic contractility with hypotension that was abolished by pretreatment with propranolol.
In combination with reduced β1/β2-AR signaling in the obese heart, we also observed significant changes in the molecular machinery influencing myocardial energy metabolism, as reflected by lower transcript levels of β1/β2-AR, AMPKα2, ACC, and PGC-1α. Myocardial energetic deficiency is the likely mechanism responsible for an apparent inability to increase cardiac work under physiological stimuli, such as hypoxia, sympathetic activation, and ischemia (3). Myocardial PGC-1α has been shown to be downregulated by obesity induced through consumption of a high-fat diet and to underpin the cardiac dysfunction in this model (15) and in an LVH model unrelated to obesity (31). Thus, in the present study, we propose that low PGC-1α in both groups of obese animals is a major factor contributing to LVH (53). In addition, further myocardial energetic controls are mediated by AMPK, of which α2 is the primary isoform mediating its catalytic activity in the heart (9). AMPKα2 is activated by a high AMP-to-ATP ratio, which enables phosphorylation of ACC, which, in turn, favors myocardial fatty acid utilization. Again, we have confirmed that obesity downregulates AMPKα2 and ACC mRNA abundance. However, importantly, in prenatally nutrient-restricted obese animals (NRO group), there was dissociation between the two enzymes, i.e., low AMPKα2 but normalized ACC. This suggests that early-life exposure to a reduced maternal intake of a balanced diet entrains a myocardial energetic imbalance, when coupled with excess weight gain postnatally. Therefore, based on these data, we speculated that the myocardium of nutrient-restricted obese animals was more susceptible to fatty acid infiltration. Indeed, this proved to be the case. Triglyceride content was nearly threefold greater in the hearts of prenatally nutrient-restricted, but postnatally overnourished, animals than in contemporaneous overweight controls, in which myocardial triglyceride content was similar to that in lean animals.
The primary change in oxidative metabolism in the failing heart is due to a reliance on the β-oxidation of nonesterified fatty acids to glycolysis for metabolic energy, i.e., a return to a fetal pattern of oxidative metabolism (6, 17). Indirect evidence that an adaptation of this type was beginning to occur in the previously nutrient-restricted obese offspring is provided by the relative changes in transcript expression for the enzymes governing myocardial energy metabolism (ACC, AMPKα2, and FABP3). Maintenance of elevated ACC in conjunction with reduced AMPKα2 in the hearts of offspring born to nutrient-restricted mothers could be indicative of a shift away from fatty acid toward carbohydrate oxidation (17). Indeed, removal of the normal inhibitory effect of ACC on AMPKα2, which is the catalytic subunit in the heart, could explain the strong positive correlation between this gene and triglyceride content that was only seen in the obese animals born to nutrient-restricted mothers. In addition, the apparent failure of nutrient-restricted offspring to increase gene expression of FABP3 with obesity would further suppress fatty acid oxidation in the heart. Alternatively, it may be that, in these animals, the primary source of substrate utilization is ectopic stores, i.e., stored triglycerides, rather than circulating metabolites, inasmuch as GLUT1 gene expression was also substantially suppressed in these individuals. Given the resetting of substrate metabolism in the obese animals born to nutrient-restricted mothers, we indirectly determined the potential for ATP and ADP transport by measuring gene expression of the inner mitochondrial membrane protein VDAC1 (33). Its expression was suppressed in all obese animals, and, given that VDAC1 may also influence glucose metabolism (33), our findings suggest potential metabolic inefficiency in the hearts of previously nutrient-restricted but obese offspring (16, 48). Clearly, future studies should be focused on examining the regulation of cardiac energy metabolism in nutritionally programmed animals that subsequently become obese.
Perspectives and Significance
We have shown, for the first time in a relevant large-animal model, that maternally nutrient-restricted offspring, as obese adults, develop dysfunction in their myocardial energy metabolism, resulting in a tendency for elevated ectopic lipid deposition in their myocardial tissue. It is hypothesized that these individuals would therefore have an increased likelihood of heart failure as they age, an adverse outcome that is likely exacerbated by their accompanying insulin resistance (44) and changes in adipose tissue biology (45).
GRANTS
This work was supported by a British Heart Foundation Lectureship to D. S. Gardner (BS/03/001) and the Early Nutrition Programming Project of the European Union Sixth Framework Programme for Research and Technical Development of the European Community (FOOD-CT-2005-007036).
REFERENCES
- 1.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 88: 389–419, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533–2536, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35–46, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 357: 2329–2337, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee S, Peterson LR. Myocardial metabolism and cardiac performance in obesity and insulin resistance. Curr Cardiol Rep 9: 143–149, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci 318: 36–42, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Carroll JF, Jones AE, Hester RL, Reinhart GA, Cockrell K, Mizelle HL. Reduced cardiac contractile responsiveness to isoproterenol in obese rabbits. Hypertension 30: 1376–1381, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Carvajal K, Zarrinpashneh E, Szarszoi O, Joubert F, Athea Y, Mateo P, Gillet B, Vaulont S, Viollet B, Bigard X, Bertrand L, Ventura-Clapier R, Hoerter JA. Dual cardiac contractile effects of the α2-AMPK deletion in low-flow ischemia and reperfusion. Am J Physiol Heart Circ Physiol 292: H3136–H3147, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Chang HY, Klein RM, Kunos G. Selective desensitization of cardiac β-adrenoceptors by prolonged in vivo infusion of catecholamines in rats. J Pharmacol Exp Ther 221: 784–789, 1982. [PubMed] [Google Scholar]
- 11.Cignarelli M, Lamacchia O. Obesity and kidney disease. Nutr Metab Cardiovasc Dis 17: 757–762, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Danno H, Jincho Y, Budiyanto S, Furukawa Y, Kimura S. A simple enzymatic quantitative analysis of triglycerides in tissues. J Nutr Sci Vitaminol (Tokyo) 38: 517–521, 1992. [DOI] [PubMed] [Google Scholar]
- 13.de las Fuentes L, Brown AL, Mathews SJ, Waggoner AD, Soto PF, Gropler RJ, Davila-Roman VG. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur Heart J 28: 553–559, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Dewald O, Sharma S, Adrogue J, Salazar R, Duerr GD, Crapo JD, Entman ML, Taegtmeyer H. Downregulation of peroxisome proliferator-activated receptor-α gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species and prevents lipotoxicity. Circulation 112: 407–415, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Dong F, Li Q, Sreejayan N, Nunn JM, Ren J. Metallothionein prevents high-fat diet-induced cardiac contractile dysfunction: role of peroxisome proliferator-activated receptor-γ coactivator 1α and mitochondrial biogenesis. Diabetes 56: 2201–2212, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Essop MF, Razeghi P, McLeod C, Young ME, Taegtmeyer H, Sack MN. Hypoxia-induced decrease of UCP3 gene expression in rat heart parallels metabolic gene switching but fails to affect mitochondrial respiratory coupling. Biochem Biophys Res Commun 314: 561–564, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Finck BN, Lehman JJ, Barger PM, Kelly DP. Regulatory networks controlling mitochondrial energy production in the developing, hypertrophied, and diabetic heart. Cold Spring Harbor Symp Quant Biol 67: 371–382, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 19.Forster CD, Macdonald IA. The assay of the catecholamine content of small volumes of human plasma. Biomed Chromatogr 13: 209–215, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Francis GS, Cohn JN. The autonomic nervous system in congestive heart failure. Annu Rev Med 37: 235–247, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Gardner DS, Bell RC, Symonds ME. Fetal mechanisms that lead to later hypertension. Curr Drug Targets 8: 894–905, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Gopalakrishnan GS, Gardner DS, Dandrea J, Langley-Evans SC, Pearce S, Kurlak LO, Walker RM, Seetho IW, Keisler DH, Ramsay MM, Stephenson T, Symonds ME. Influence of maternal pre-pregnancy body composition and diet during early-mid pregnancy on cardiovascular function and nephron number in juvenile sheep. Br J Nutr 94: 938–947, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gopalakrishnan GS, Gardner DS, Rhind SM, Rae MT, Kyle CE, Brooks AN, Walker RM, Ramsay MM, Keisler DH, Stephenson T, Symonds ME. Programming of adult cardiovascular function after early maternal undernutrition in sheep. Am J Physiol Regul Integr Comp Physiol 287: R12–R20, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G Sympathetic overdrive and cardiovascular risk in the metabolic syndrome. Hypertens Res 29: 839–847, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Grassi G Adrenergic overdrive as the link among hypertension, obesity, and impaired thermogenesis: lights and shadows. Hypertension 49: 5–6, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Han HC, Austin KJ, Nathanielsz PW, Ford SP, Nijland MJ, Hansen TR. Maternal nutrient restriction alters gene expression in the ovine fetal heart. J Physiol 558: 111–121, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill JO Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev 27: 750–761, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab 91: 4689–4695, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Lee CH, Olson P, Evans RM. Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144: 2201–2207, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol 29: 339–345, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator—thinking outside the box. Biochim Biophys Acta 1762: 181–190, 2006. [DOI] [PubMed] [Google Scholar]
- 34.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85: 571–633, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Morricone L, Malavazos AE, Coman C, Donati C, Hassan T, Caviezel F. Echocardiographic abnormalities in normotensive obese patients: relationship with visceral fat. Obes Res 10: 489–498, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima T, Fujioka S, Tokunaga K, Matsuzawa Y, Tarui S. Correlation of intra-abdominal fat accumulation and left ventricular performance in obesity. Am J Cardiol 64: 369–373, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Nomura Y, Kajiyama H, Segawa T. Alteration in sensitivity to isoproterenol and acetylcholine in the rat heart after repeated administration of isoproterenol. J Pharmacol Exp Ther 220: 411–416, 1982. [PubMed] [Google Scholar]
- 38.Osadchii OE Cardiac hypertrophy induced by sustained β-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev 12: 66–86, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature 427: 411–412, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Pantanetti P, Garrapa GG, Mantero F, Boscaro M, Faloia E, Venarucci D. Adipose tissue as an endocrine organ? A review of recent data related to cardiovascular complications of endocrine dysfunctions. Clin Exp Hypertens 26: 387–398, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Powell BD, Redfield MM, Bybee KA, Freeman WK, Rihal CS. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol 98: 116–120, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Rizzo NS, Ruiz JR, Oja L, Veidebaum T, Sjostrom M. Associations between physical activity, body fat, and insulin resistance (homeostasis model assessment) in adolescents: the European Youth Heart Study. Am J Clin Nutr 87: 586–592, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Schaap FG, Binas B, Danneberg H, van der Vusse GJ, Glatz JF. Impaired long-chain fatty acid utilization by cardiac myocytes isolated from mice lacking the heart-type fatty acid binding protein gene. Circ Res 85: 329–337, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Sebert SP, Hyatt MA, Chan LL, Patel N, Bell RC, Keisler D, Stephenson T, Budge H, Symonds ME, Gardner DS. Maternal nutrient restriction between early-to-mid gestation and its impact upon appetite regulation following juvenile obesity. Endocrinology 150: 634–641, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharkey D, Gardner DS, Fainberg HP, Sebert S, Bos P, Wilson V, Bell R, Symonds ME, Budge H. Maternal nutrient restriction during pregnancy differentially alters the unfolded protein response in adipose and renal tissue of obese juvenile offspring. FASEB J. In press. [DOI] [PubMed]
- 47.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 18: 1692–1700, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation 114: 2130–2137, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Vasan RS Cardiac function and obesity. Heart 89: 1127–1129, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vetta F, Cicconetti P, Ronzoni S, Rizzo V, Palleschi L, Canarile G, Lupattelli MR, Migliori M, Morelli S, Marigliano V. Hyperinsulinaemia, regional adipose tissue distribution and left ventricular mass in normotensive, elderly, obese subjects. Eur Heart J 19: 326–331, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279: E83–E87, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11β-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin II receptor in neonatal sheep. Endocrinology 142: 2854–2864, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Williams PJ, Kurlak LO, Perkins AC, Budge H, Stephenson T, Keisler D, Symonds ME, Gardner DS. Hypertension and impaired renal function accompany juvenile obesity: the effect of prenatal diet. Kidney Int 73: 279–289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med 4: 436–443, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Yu ZY, Lumbers ER. Effect of cold on fetal heart rate and its variability. Clin Exp Pharmacol Physiol 27: 607–611, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 117: 1658–1667, 2008. [DOI] [PubMed] [Google Scholar]